252 (2000) 75–84
www.elsevier.nl / locate / jembe
Fast repetition rate (FRR) fluorometry: variability of
chlorophyll a fluorescence yields in colonies of the corals,
Montastraea faveolata (w.) and Diploria labyrinthiformes (h.)
recovering from bleaching
a b ,* c
Michael R. Lombardi , Michael P. Lesser , Maxim Y. Gorbunov
a
Biology Program, University of New Hampshire, Durham, NH 03824, USA
b
Department of Zoology and Center for Marine Biology, University of New Hampshire, Durham, NH03824, USA
c
Environmental Biophysics and Molecular Ecology Program, Institute of Marine and Coastal Sciences, Rutgers University, 71 Dudley Road, New Brunswick, NJ 08901, USA
Received 31 December 1999; received in revised form 29 March 2000; accepted 6 June 2000
Abstract
Recently, an underwater version of a fast repetition rate fluorometer (FRRF) was developed for the non-destructive study of fluorescence yields in benthic photoautotrophs. We used an FRRF to study bleached colonies of the corals, Montastraea faveolata and Diploria labyrinthiformes at sites surrounding Lee Stocking Island, Exuma, Bahamas, to assess their recovery from bleaching (|1 year after the initial bleaching event) induced by elevated temperatures. The steady state
9
quantum yields of chlorophyll a fluorescence (DF9/F ) from photosystem II (PSII) within coralm colonies were separated into three categories representing visibly distinct degrees of bleaching
9
ranging from no bleaching to completely bleached areas. Differences in DF9/Fm were sig-nificantly different from bleached to unbleached regions within colonies. Dark, unbleached regions
9
within colonies exhibited significantly higher DF9/Fm values (0.43860.019; mean6S.D.) when compared to lighter regions, and occupied a majority of the colonies’ surface area (46–73%).
9
Bleached regions exhibited significantly lowerDF9/Fm (0.33760.014) and covered only 7–25% of the colonies’ surface area. The observations from this study suggest that zooxanthellae in bleached regions of a colony exhibit reduced photosynthetic activity as long as one year after a bleaching event and that in situ fluorescence techniques such as FRRF are an effective means of studying coral responses and recovery from natural or anthropogenic stress in a non-destructive manner. 2000 Elsevier Science B.V. All rights reserved.
Keywords: Chlorophyll a; Coral bleaching; FRRF; Photosystem II; Zooxanthellae
*Corresponding author. Tel.:11-603-862-3442; fax:11-603-862-3784. E-mail address: [email protected] (M.P. Lesser).
1. Introduction
In recent years, much attention in tropical marine ecosystems has been directed towards studying a phenomenon in zooxanthellate corals known as ‘‘bleaching’’. Bleaching results from the expulsion of symbiotic dinoflagellates (5zooxanthellae) from the animal host due to one or several environmental stressors such as elevated seawater temperatures, excess visible or ultraviolet radiation, or water pollution (Glynn, 1996). As the zooxanthellae leave, the coral progressively whitens since the remaining polyp is translucent and the coral skeleton is highly reflective. This discoloration is a common indicator of stress in corals (Brown and Howard, 1985). Many investigators now believe that the zooxanthellae are the primary target of these environmental perturbations (Iglesias-Prieto et al., 1992; Lesser, 1997; Warner et al., 1996, 1999), and a number of mechanisms have been suggested to explain the mechanism by which zooxanthellae leave the host. Among these are exocytosis and host cell detachment (Gates et al., 1992). Zooxanthellae can contribute over 100% of the carbon requirements of the host, via translocated photosynthate, as their contribution to the symbiosis (Falkowski et al., 1990). This relationship permits the assessment of stress or damage on the intact symbiosis through the analysis of parameters associated with the photosynthetic competency of the zooxanthellae. Common procedures include changes in cell counts, the aerial or per cell concentration of chlorophyll a (Iglesias-Prieto et al., 1992), and indirect calorimetry using oxygen electrodes (Lesser, 1997) to measure photosynthesis and respiration, therefore inferring the energetic costs and benefits associated with the symbiosis. All of these methods require some destructive sampling of the reef community by collecting pieces of coral that also results in damage to neighboring organisms due to the destructive nature of the collection.
Recent methodologies used to assess stress in corals involve the analysis of the biochemical and biophysical aspects of photosynthesis within the algal symbiont itself, more specifically of the PSII reaction center. Photosynthetically active radiation (PAR:
22 21
400–700 nm inmmol quanta m s ) reaches the ocean and is attenuated by scattering and absorption within the water column with the remaining quanta absorbed by photosynthetic pigments within the zooxnthellae (Falkowski and Kolber, 1993). In the PSII reaction center a primary electron donor chlorophyll molecule called P680 is present, 680 denoting the wavelength (nm) of peak absorbance for chlorophyll a (Campbell, 1996). As photons are absorbed by chlorophyll molecules charge separation occurs and causes the photochemical oxidation of H O to O (Kolber and Falkowski,2 2
1993). The absorbed light energy is converted to chemical energy via photochemical charge separation, dissipated as thermal energy, or re-emitted as fluorescence (Kolber and Falkowski, 1993; Campbell et al., 1998). Chlorophyll fluorescence, and the changes in fluorescence yields, within PSII can be used to assess photosynthetic activity since changes in fluorescence yields reflect changes in de-excitation pathways such as electron transfer and heat dissipation (Campbell et al., 1998).
(FRR) fluorescence methods (Kolber et al., 1998). New instruments have been developed that incorporate protocols to measure the multiple photochemical turnover (PAM), and single photochemical turnover of PSII (FRR) in the laboratory and in the field (Schreiber et al., 1986; Gorbunov et al., 2000). In particular, PAM measurements of fluorescence in corals have become widespread (Brown et al., 1999; Ralph et al., 1999; Warner et al., 1996, 1999). Previous studies using PAM fluorescence protocols have indicated that corals exposed to bleaching conditions, such as elevated seawater
9
temperature, yield progressively lowerDF9/Fm of PSII fluorescence values before they bleach (Warner et al., 1999). Similar results have been obtained with FRR fluorometers (Lesser and Gorbunov, in press).
Since the quantum yield of PSII fluorescence is an important indicator of functional PSII units we examined the association of color variability corresponding with
9
zooxanthellae loss, or decrease of photosynthetic pigment, with changes in theDF9/Fm
of PSII fluorescence. In this paper we describe the use of a newly described in situ FRR fluorometer (Gorbunov et al., 2000), to examine the fluorescence yields of zooxanthellae in hospite on corals recovering from a bleaching event due to elevated seawater temperatures.
2. Materials and methods
This study was conducted at the Caribbean Marine Research Center on Lee Stocking Island (LSI), Bahamas during June, 1999. Preliminary dives were made on a number of reef sites to determine the most appropriate methodology to conduct the work described below. Montastraea faveolata and Diploria labyrinthiformes were selected as the coral species to be studied due to the variability in bleaching observed within their respective populations. Horseshoe Reef (depth 10–12 m) and Rainbow Gardens (depth 3–4 m) were chosen as research sites, as they hosted populations of both coral species. Both of these sites were affected by a bleaching event that occurred in May 1998 by the intrusion of warm (maximum temperature 34–358C) Bahama Bank water onto the Exuma island chain (Lesser, personal observation). Both of these coral species bleached around LSI and at the two study sites in June of 1998. At that time the pattern of bleaching observed was that whole colonies were bleaching with very little hetero-geneity in color. That is, colonies largely conformed to the light coloration pattern described below (Lesser, personal observation).
bleaching and was categorized into three groups, light, intermediate, and dark colored regions. This simple breakdown encompassed a much more variable color palette, however three regions allowed for an easier analysis of the relationship between the variability of color and fluorescence yields within coral colonies. All measurements were
9
taken between 9 and 10 AM (EST) to avoid the mid-day depression in DF9/Fm
measured in these corals using FRR fluorescence protocols (Lesser and Gorbunov, in press).
The FRRF exposes the coral to a sequence of flashlets that gradually close PSII reaction centers, resulting in an increase in chlorophyll fluorescence (Kolber and Falkowski, 1992). The fluorescence yields during the time of an FRRF excitation
9
protocol while illuminated is defined by F9 (steady state) and Fm (maximum) components of the yield (Kolber et al., 1998). From these values the quantum yield of
9
PSII fluorescence (DF9/F ) can be calculated (Gorbunov et al., 2000). The instrumentm
enables the diver to monitor and focus the target organism with a Marshall Electronics black and white video camera. A pair of IR laser diodes is incorporated into the LCD display to aid in focussing and controlling distance to target. A piezo key trigger allows the diver to ‘‘shoot’’ the target. Upon shooting, an image is captured and stored (Fig. 1), as well as the associated fluorescence data. This instrument is described in greater detail by Gorbunov et al. (2000).
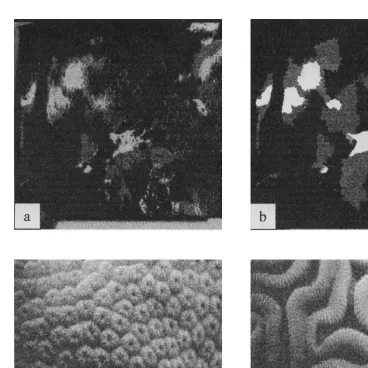
Each quadrat photograph was scanned using a Nikon slidescanner at 300-dpi resolution. The images’ gamma curves were adjusted using the visually segregated regions of light, intermediate, and dark color variability within the colony. The same procedure was performed on all images. Using the PVC quadrat as a reference, each image was cropped to the equivalent of 25325 cm. These cropped images were then resized to 4003400 pixels in Adobe Photoshop 5.0. The images were then highlighted using Jasc Paint Shop Pro. The best representation of light, intermediate, and dark areas were isolated and highlighted to yield an image containing four colors including black backdrop representing non-coral cover. Next, all highlighted images were adjusted to grayscale and saved (Fig. 1). Finally, the three areas representing the different shades of coral cover were quantified as percent cover using NIH Image v1.61.
A three-way ANOVA using species, site, and color as treatments was run to examine
9
differences in DF9/Fm at the 5% level. When significant treatment effects occurred, a post hoc test (Student–Newman–Keuls [SNK]) was used to determine differences in
9
DF9/Fm between the three shades of coral cover. A two-way ANOVA was run for each of the color regions to examine the effects of site and species on the percent cover of each color region (light, intermediate, and dark) occupied on the coral colony surface.
9
Both DF9/Fm and percent cover were arcsine transformed before analysis.
3. Results
9
The three way ANOVA of log transformed fluorescence data (DF9/F ) revealedm 9
Fig. 1. Top: (a) Cropped (4003400 pixels), grayscale quadrat photo of Montastraea faveolata at the Horseshoe Reef site. (b) Highlighted light, intermediate, and dark regions of M. faveolata. Bottom: Grayscale images captured from CX-100-30 frame grabber (Imagination Vision Systems Specialists) CCD camera portion of the FRR fluorometer. (c) Montastraea faveolata and (d) Diploria labyrinthiformes.
9
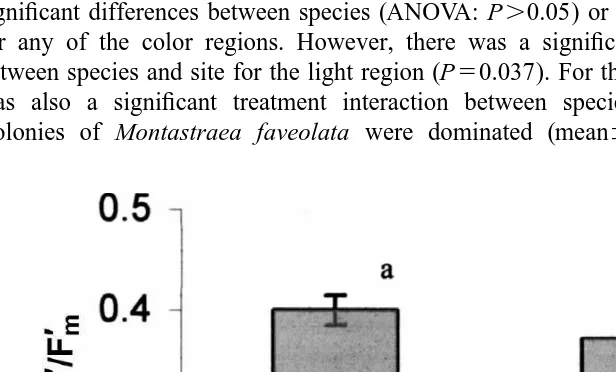
light regions contain zooxanthellae with lower DF9/Fm of PSII fluorescence (0.33760.014, mean6S.E.), followed by intermediate (0.38260.012), and dark (0.43860.019) regions (Fig. 2).
There was also a significant difference (ANOVA: P50.04) between sites (all samples
9
pooled), where DF9/Fm of the shallower site (Rainbow Gardens) (0.37260.014) were significantly less than that of the deeper site (Horseshoe Reef) (0.40060.015) (Fig. 3).
9
9
Fig. 2. Mean (6S.D.) quantum yield of PSII fluorescence (DF9/F ) values for light (Nm 516, 0.33760.014), intermediate (N516, 0.38260.012), and dark (N516, 0.43860.019) colored regions. Treatment groups with similar superscripts are not significantly different from one another.
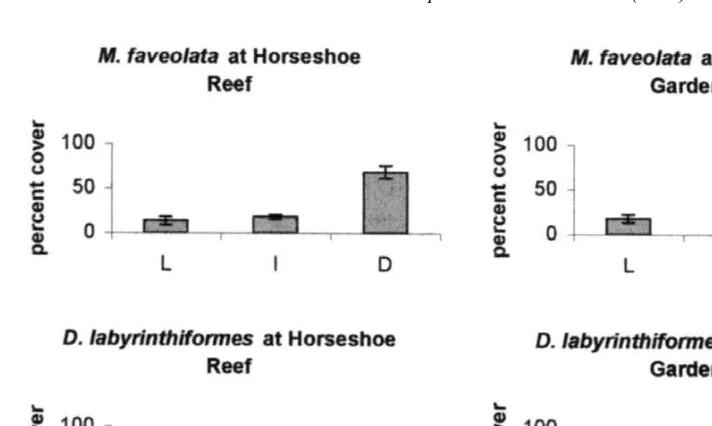
A two-way ANOVA of arcsine transformed percent cover data did not reveal significant differences between species (ANOVA: P.0.05) or sites (ANOVA: P.0.05) for any of the color regions. However, there was a significant treatment interaction between species and site for the light region (P50.037). For the dark color region there was also a significant treatment interaction between species and site (P50.021). Colonies of Montastraea faveolata were dominated (mean6S.D., 68.567.00%) by
9
Fig. 4. Percent cover (Mean6S.D.) of light, intermediate, and dark regions at for Montastraea faveolata and Diploria labyrinthiformes at each site. Dark regions are significantly greater in percent cover suggesting that the coral colonies are in a state of recovery despite having variable quantum yields from bleached to unbleached regions.
darker, ‘‘healthier’’, color at Horseshoe Reef compared with light (13.764.33%) and intermediate (17.865.95%) regions (Fig. 4). Although the dark color region also covers a higher percentage of corals at Rainbow Gardens (49.367.47%), there is an increased percentage of light (17.464.60%) and intermediate (33.367.81%) regions of color compared to Horseshoe Reef. Diploria labyrinthiformes was also dominated by the darker color regions at both sites, however, the distribution of dark color at Rainbow Gardens (73.4616.37%) was greater than at Horseshoe Reef (45.761.63%) showing a different pattern compared to that observed for M. faveolata (Fig. 4).
4. Discussion
The results of this study support the hypothesis that bleached areas of coral harbor
9
zooxanthellae yielding lower DF9/Fm of PSII fluorescence than visibly darker areas of coral long after the temperature perturbation and bleaching has ended. There are
9
significant differences in theDF9/Fm of PSII fluorescence with increasing values in a progression from bleached to unbleached regions of coral colonies. There were no
9
ambient irradiance increased, quantum yields of photochemistry decrease. Each site, also representing a different depth, exhibits differences in visible irradiances (PAR;
Horse-22 21 22 21
shoe Reef: 500 mmol quanta m s , Rainbow Garden: 1100mmol quanta m s ). Therefore, one would expect higher quantum yields at the deeper site. Horseshoe Reef
9
(10–12 m) has higherDF9/Fm(0.40060.015, mean6S.D.) than Rainbow Gardens (3–4 m) (0.37260.014). Taking measurements of fluorescence yields at different depths in Montastraea faveolata reveals a bathymetric trend of increasing quantum yields of PSII fluorescence with increasing depth (Lesser and Gorbunov, in press).
Since this study occurred approximately one-year following a major bleaching event colonies dominated by dark color regions appear to be approaching a state of full recovery. Had this study occurred during or immediately following a bleaching event,
9
one would predict a lowerDF9/Fmand an increase in percent cover of the light colored regions. Dark color regions dominate the surface of Montastraea faveolata colonies at both sites, although more so at Horseshoe Reef. Horseshoe Reef might be expected to be less prone to intense bleaching conditions, since it a deeper site, and consequently may also require a shorter recovery period. Although photosynthetic performance within the color regions didn’t differ between species, the distribution of color regions in Diploria
labyrinthiformes is significantly different than that of M. faveolata. It was noted during
preliminary dives that colonies of D. labyrinthiformes at Rainbow Gardens appeared visually more ‘‘healthy’’ than at Horseshoe Reef. Fig. 4 shows that the percent cover of darker regions were significantly greater in this species at both sites, however there was a significantly higher percent cover of dark color regions at Rainbow Gardens. Since Rainbow Gardens is the shallower site, one would have expected results similar to that observed for M. faveolata. The significant interaction term in the analysis of percent cover for light and dark regions suggests that these factors (site, species) are not independent of one another.
This study, utilizing light, intermediate, and dark color regions as measurements of recovery from bleaching, is representative of the extent of bleaching damage observed due to zooxanthellae or photosynthetic pigment loss. For example, the darkest region of Diploria labyrinthiformes may not have been the same color as the darkest region of Montastraea faveolata, however, both regions host the zooxanthellae with the highest
9
DF9/Fmof PSII fluorescence within each species. Improvements in this type of analysis for the future might include using high-resolution digital photographic techniques with high intensity strobes that would better define the color range complexity on a per species and per colony basis. These photographs could also be used to correlate regional color variation with aerial pigment content or cell concentration.
As expected, light (0.33760.014, mean6S.E.), intermediate (0.38260.012), and dark
9
9
differences inDF9/Fm suggesting that photosynthetic performance of the zooxanthellae in the bleached regions is still compromised compared to unbleached areas. The
9
functional relationship between the differences inDF9/Fm and color are similar to those seen in newly bleached corals (Warner et al., 1999) but may not have universal applicability to all bleached corals. Additional studies on corals recovering from bleaching at different time intervals are needed to test the generality of these results.
Gorbunov et al. (2000) showed that zooxanthellae in healthy corals generally have a low quantum yield of 0.39060.07 (N5350, mean6S.E.) because of nutrient limitation. Although the data presented here exhibits a similar mean of 0.38660.010 (N548), there
9
was a much greater range ofDF9/Fm values in the data from 0.221 to 0.655 because of the varying degrees of recovery from bleaching. Since there is variability from bleached to unbleached regions in recovering corals, the health of these colonies is uniquely represented by the simultaneous use of fluorescence yields and the quantification of the variation in color regions. This analysis provides information regarding the state of recovery one year after a severe bleaching event and has shown that using FRR fluorescence to monitor the recovery of corals from episodes of bleaching could be a valuable tool for future research in this field. Long term monitoring of the variation of
9
color in corals and the associated DF9/Fm of PSII fluorescence can potentially provide predictive information regarding the health and stability of corals after bleaching caused by elevated seawater temperatures.
Acknowledgements
Thank you to Dr. Chris Neefus (University of New Hampshire) for suggestions and assistance with statistical analyses and to an anonymous reviewer whose comments improved this manuscript. This work was funded by grants to MPL from the Office of Naval Research (ONR) – Environmental Optics Program, Coastal Benthic Optical Properties (CoBOP) Program and from the NOAA National Undersea Research Program administered through the Caribbean Marine Research Center. The views expressed herein are those of the author and do not necessarily reflect the views of ONR or NOAA or any of its subagencies. Finally, we’d like to thank the staff of CMRC for logistic support during this study. [SS]
References
Brown, B.E., Howard, L.S., 1985. Assessing the effects of stress on coral reefs. Advances in Marine Biology 22, 1–63.
Brown, B.E., Ambarsari, I., Warner, M.E., Fitt, W.K., Dunne, R.P., Gibb, S.W., Cummings, D.G., 1999. Diurnal changes in photochemical efficiency and xanthophyll concentrations in shallow water reef corals: evidence for photoinhibition and photoprotection. Coral Reefs 18, 99–105.
Campbell, N.A., 1996. In: Biology, 4th Edition. Benjamin / Cummings Publishing Co. Inc, Menlo Park, CA, pp. 189–193.
Falkowski, P.G., Jokiel, P.L., Kinzie, III R.A., 1990. Irradiance and corals. In: Dubinsky, Z. (Ed.), Ecosystems of the World: Coral Reefs, pp. 89–107.
Falkowski, P., Kolber, Z., 1993. Estimation of phytoplankton photosynthesis by active fluorescence. ICES Marine Sci. Symp. 197, 92–103.
Gates, R.D., Baghdasarian, G., Muscatine, L., 1992. Temperature stress causes host cell detachment in symbiotic cnidarians: implications for coral bleaching. Biol. Bull. 182, 324–332.
Glynn, P.W., 1996. Coral reef bleaching: facts, hypotheses and implications. Global Change Biology 2, 495–509.
Gorbunov, M.Y., Falkowski, P.G., Kolber, Z.S., 2000. Photosynthetic parameters in the benthos measured in situ with a SCUBA-based fast repetition rate fluorometer. Limnology and Oceanography 45, 242–245. Iglesias-Prieto, R., Matta, J.L., Robins, W.A., Trench, R.K., 1992. Photosynthetic response to elevated
temperature in the symbiotic dinoflagellate Symbiodinium microadriaticum in culture. Proc. Natl. Acad. Sci. USA 89, 10302–10305.
Kolber, Z., Falkowski, P.G., 1993. Use of active fluorescence to estimate phytoplankton photosynthesis in situ. Limnol. Oceanogr. 38 (8), 1646–1665.
Kolber, Z., Falkowski, P.G., 1992. Fast Repetition Rate (frr) Fluorometer For Making in Situ Measurements of Primary Productivity. In: Proceedings of Ocean ’92 Conference, Newport, RI, Oct. 26–29, pp. 637–641. Kolber, Z.S., Prasil, O., Falkowski, P.G., 1998. Measurements or variable chlorophyll fluorescence using fast repetition rate techniques: defining methodology and experimental protocols. Biochim. Biophys. Acta. 1367, 88–106.
Lesser, M.P., 1997. Oxidative stress causes coral bleaching during exposure to elevated temperatures. Coral Reefs 16, 187–192.
Ralph, P.J., Gademann, R., Larkum, A.W.D., Schreiber, U., 1999. In situ underwater measurements of photosynthetic activity of coral zooxanthellae and other reef-dwelling dinoflagellate endosymbionts. Mar. Ecol. Prog. Ser. 180, 139–147.
Schreiber, U., Schliwa, U., Bilger, W., 1986. Continuous recording of photochemical and non-photochemical chlorophyll fluorescence quenching with a new type of modulation fluorometer. Photosynth. Res. 10, 51–62.
Warner, M.E., Fitt, W.K., Schmidt, G.W., 1996. The effect of elevated temperature on the photosynthetic efficiency of zooxanthellae in hospite from four different species of reef coral: a novel approach. Plant Cell Environ. 19, 291–299.