www.elsevier.com/locate/ibmb
Considerations on the structural evidence of a ligand-binding
function of ultraspiracle, an insect homolog of vertebrate RXR
Grace Jones
a,*, Davy Jones
baSchool of Biological Sciences, University of Kentucky, Lexington, KY 40506, USA
bGraduate Center for Toxicology, Chandler Medical Center, University of Kentucky, Lexington, KY 40506, USA
Received 31 October 1999; received in revised form 31 December 1999; accepted 25 January 2000
Abstract
This analysis considers the structural evidence of a ligand-binding function of the nuclear receptor ultraspiracle (USP). The positions and nature of residues in the ligand-binding domain of USP from six higher insects is evaluated in comparison to the function of conserved residues vertebrate receptors that have been co-crystallized with ligand. USP appears to conserve residues that in vertebrate receptors (1) form the hydrophobic ligand-binding pocket, (2) contact oxygen-containing moieties on ligands, such as hydroxyl, keto and carboxyl groups, and (3) in response to ligand-binding conformationally change to form a multi-helix hydrophobic groove for recruitment of transcriptional co-activators. These structural features are consistent with the recent report that USP can bind the epoxymethylfarnesoates (juvenile hormones) and thereupon is induced to change conformation. 2000 Published by Elsevier Science Ltd. All rights reserved.
Keywords: Ultraspiracle; USP; Nuclear receptor; RXR; Juvenile hormone
During the last decade, there has perhaps been no problem in insect biochemistry more exasperating than the molecular identification of juvenile hormone recep-tors (Jones, 1995; Riddiford, 1996; Feyereisen, 1998). A number of potential candidates for nuclear JH receptors have been offered with various levels of supporting evi-dence, but there is not yet consensus on whether the field has actually arrived at its long sought destination (Jones and Sharp, 1997; Palli et al., 1994; Ashok et al., 1998; Harmon et al., 1995).
The structural relationship between juvenile hormone and retinoic acid prompts the consideration of whether a retinoid-type of receptor in insects may serve as a juv-enile hormone receptor. The last several years have seen an increasing number of reports on the primary structure of invertebrate homologs of vertebrate RXR. The ligand-binding domain of jellyfish, crustacean, tick and grass-hopper homologs have been shown to have closer sequence identity to the vertebrate RXR than to the USP
* Corresponding author. Tel.:+1-606-257-2105; fax:+ 1-606-323-1059.
E-mail addresses: [email protected] (G. Jones), [email protected] (D. Jones).
0965-1748/00/$ - see front matter2000 Published by Elsevier Science Ltd. All rights reserved. PII: S 0 9 6 5 - 1 7 4 8 ( 0 0 ) 0 0 0 3 8 - 2
of higher insects such as Diptera and Lepidoptera (Chung et al., 1998; Guo et al., 1998; Kostrouch et al., 1998; Hayward et al., 1999). While the jellyfish RXR does bind 9-cis retinoic acid (RA), the tick RXR did not support 9-cis RA activation of transcription under the cell line transfection system used, and the binding properties of the grasshopper and crustacean RXRs have yet not been reported. The USP of higher insects has been considered to be an orphan receptor (Thummel, 1995; Buszczak and Segraves, 1998), and hypotheses have been advanced that the ligand-binding function of higher insect USP has been lost (Kapitskaya et al., 1996; Hayward et al., 1999). However, recently Jones and Sharp (1997) reported that D. melanogaster USP (dmUSP) can bind to JH III and JH III bisepoxide, which are natural JHs of that insect. The occasion of the VII International Symposium on Juvenile Hormones pro-vides an appropriate occasion to consider what structural evidence exists that USP of higher insects is a nuclear receptor that binds one or more endogenous ligands.
1. Primary structure
Dip-672 G. Jones, D. Jones / Insect Biochemistry and Molecular Biology 30 (2000) 671–679
tera and Lepidoptera (e.g., Henrich et al., 1990; Oro et al., 1990; Shea et al., 1990; Tzertzinis et al., 1994; Kapit-skaya et al., 1996; Jindra et al., 1997; Perera et al., 1998; Vogtli et al., 1999). The sequence identity between these USPs versus the vertebrate and invertebrate RXRs is in the range of 40–50%, while that between invertebrate RXRs and vertebrate RXRs is around 70%. However, these statistics in and of themselves do not provide a basis for inferring whether USPs bind ligands and what those ligands might be. For example, vertebrate RAR and RXR both bind the same ligand 9-cis RA, yet the percent identity between them is only around 27% (Mangelsdorf et al., 1990). The relevant indicators are whether the residues that have been retained identically or with conservative substitution are those residues necessary to preserve the secondary structures for basic architecture of the ligand-binding domain, and those necessary to preserve a functional ligand-binding pocket within the ligand-binding domain. Thus, in the analyses below, we do not confer importance to a residue in USP merely because it is conserved in USPs, but rather also because of its location in USP relative to the functional location of ligand- or co-activator-associated residues conserved in other nuclear receptors.
2. Secondary and tertiary structure
The crystal structure for unliganded human RXRα (hRXRα) has been reported, including the residues part-icipating in the various secondary structures (Bourguet et al., 1995). It is possible to gauge the accuracy of algorithms for prediction of secondary structure by com-paring the consensus predictions of several methods for hRXRα against the secondary structure arrangement actually observed in crystallized hRXRα. Using the vari-ous secondary structure prediction alogithms available at the http://pbil.ibcp.fr/NPSA web site, we performed such an analysis. Fig. 1 shows the consensus locations of pre-dicted secondary structures for hRXRαvs those actually observed in the crystal structure, for the regions of the ligand-binding domain that contain corresponding resi-dues known to line the ligand-binding pocket of hRARγ. In general, the analyses reasonably predict for hRXRα the locations ofα-helices. We then applied those analyti-cal methods to Drosophila melanogaster USP (dmUSP). The predictions of α-helical secondary structure for dmUSP generally parallel the predictions for hRXRα (Fig. 1). These data on the predicted arrangement of sec-ondary structures in dmUSP support the hypothesis that the general tertiary architecture of the ligand-binding domain (LBD) of dmUSP is similar to that of the LBD for hRXRα. The remaining analyses below are rested on this hypothesis that the general tertiary architecture of secondary structures of the LBD of dmUSP is similar to that of the LBD for hRXRα.
3. Receptor residues contacting oxygen-containing moieties
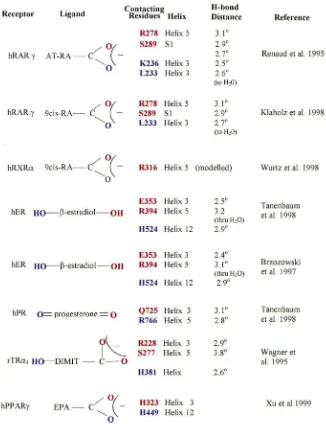
The crystal structure of the ligand-binding domain has been reported for apo-hRXRα, holo-hRARγ, holo-rat thyroid hormone receptor (rTRβ1), holo-human estrogen receptor (hER), and both apo- and holo-human pro-gesterone receptor (hPPARγ) (Bourguet et al., 1995; Renaud et al., 1995; Wagner et al., 1995; Brzozowski et al., 1997; Nolte et al., 1998; Shiau et al., 1998; Tanen-baum et al., 1998; Uppenberg et al., 1998; Williams and Sigler, 1998; Xu et al., 1999). The ligand-binding pock-ets of these LBDs are lined primarily with hydrophobic amino acid side chains (Fig. 1). Those residues in the pocket that are charged are in most cases those residues that hydrogen bond with hydroxyl, keto or carboxyl groups of the respective ligands (Fig. 2). In every case examined thus far of a crystallized (or modelled, for hRXRα) nuclear receptor complexed with an endogen-ous ligand containing a ‘terminal’ hydroxyl, keto or car-boxyl group at its ‘leading end’, that group is hydrogen bonded to two residues which each are placed on differ-ent secondary structures; either one onα-helix 3 and the other onα-helix 5, or one onα-helix 5 and the other on aβ-turn (S1–S2) (Figs. 1 and 2). Thus far, the hydrogen bonds of the hydroxyl-, keto- and carboxyl-containing moieties always include at least a basic residue (arginine or histidine) on either α-helix 3 or 5, and a nonbasic, but hydrogen-bonding residue (glutamine, glutamic acid or serine) on either the other helix or on theβ-turn (Fig. 2). hPPARγ, which has some additional secondary struc-tures and a tertiary arrangement most different from the other nuclear receptors, also binds the carboxyl group of its ligand through histidine on α-helix 5, but is excep-tional in that a histidine on α-helix 11 also hydrogen bonds with that same carboxylate group. On the basis of the above pattern of the nature and locations of hydro-gen-bonding residues in a variety of nuclear receptors, we make the following hypothesis: if USP binds to an endogenous ligand containing an oxygen-substituted end-group, that binding will be by hydrogen-bond of that end-group through at least arginine or histidine contacts at one of either α-helices 3 or 5, and perhaps through a second contact on the other respective α-helix (3 or 5) or on the β-turn.
In hRXRα, the apparent closest relative of dmUSP that has been crystallized, the modelled structure of 9-cis RA in the RXR ligand-binding pocket places I268 and Q275 on α-helix 3 as straddling on each side of the ligand, near the backbone double bond positioned between theαandβcarbons respective to the carboxyl group (C14 and C15, Fig. 2). Those two residues are conserved in higher insect USPs as an invariant L and the correspondng invariant Q, respectively.
674 G. Jones, D. Jones / Insect Biochemistry and Molecular Biology 30 (2000) 671–679
Fig. 1. Conservation of primary, secondary and tertiary structure of portions of ligand-binding domain of vertebrate nuclear receptors in comparison with primary and inferred secondary and tertiary structure of portions of ligand-binding domain of ultraspiracle (USP) proteins from higher insects. For the vertebrate receptors, theα-helices (barrels) orβ-sheet (block arrows) contain the primary sequence shown by crys-tallography to constitute that secondary structure in the respective receptor. Open rectangles around hRXRαand around the sequence for dmUSP show the consensus of secondary structure predictions. Resi-dues coded in orange are present in the ligand-binding pocket of the crystallized receptor. Residues coded in dark brown touch oxygen-con-taining moieties on the ligand in the ligand-receptor cocrystal. Blue bars connecting residues in two receptors are positions in which differ-ences in hydrophobic side chains discriminate between ligands of the two receptors. Insect USP and dmE75A sequences are shown in align-ment below the vertebrate receptors. Light brown coded residues denote highly conserved insect sequences at selected positions. Purple coded dmE75 residues denote positions in which the dmE75 residue is a non-conservative change from the conserved series at the respect-ive USP position. Dark brown underlining forα-helices 3, 10/11 and theβ-turn indicate narrower regions in vertebrate receptors that contact oxygen-containing moieties on the respective ligands. Primary sequences and alignments for vertebrate receptors are from Wurtz et al. (1996). Sources for secondary and tertiary structure designations and receptor acronyms are as follows: hPR=human progesterone recep-tor (Williams and Sigler, 1998); hER=human estrogen receptor (Brzozowski et al., 1997); hRARγ=human retinoic acid receptor γ (Renaud et al., 1995); hRXRα=human retinoid X receptorα(Bourguet et al., 1995); rTRβ1=rat thryoid hormone receptorβ1; dm=Drosophila melanogaster (Oro et al., 1990); aa=Aedes aegypti (Kapitskaya et al., 1996); ct=Chironomus tetans (Vogtli et al., 1999); bm=Bombyx mori (Tzertzinis et al., 1994); cf=Choristoneura fumiferana (Perera et al., 1998); ms=Manduca sexta (Jindra et al., 1997).
leading end of ligands of a variety of crystalized ligand-receptor complexes leads to additional hypotheses on candidate residues in dmUSP that may also contact an oxygen-substituted endogenous ligand. The residues on
α-helix 3 reported to hydrogen-bond with the hydroxyl-, keto-, or carboxyl moiety of the respective ligands are all in positions within 5 amino acids of each other on helix 3 (Fig. 1). In this immediate area on hRXRαare also the I268 and Q275 that are modelled as just distal to the leading carboxyl group. The only invariant resi-dues in that region for the USPs are those in the motif LXXXXXKQ, where L and Q correspond to I268 and Q275 of hRXRα. Further, on α-helix 5, hPR, hER, hRARγand hRXRαall possess a conserved arginine that participates in hydrogen-bonding to the hydroxl-, keto or carboxyl- moiety of their ligand. However, in several of the USPs, this residue is neither arginine nor another basic residue. Based on the nature and locations of con-served residues in USP α-helix 3 relative to the corre-sponding placement of ligand-associated residues in other crystallized receptors, we hypothesize that should higher insect USPs bind an endogenous ligand with ter-minal oxygen-substitution, then either the conserved K or Q on helix 3 participates in hydrogen-bonding to that moiety.
More problemmatic is conjecturing which residues are
candidates for a second hydrogen bond with the oxygen-containing moiety of a putative USP ligand. The short region between S1 and S2 of hRARγand rTRβ1 contains in those receptors the serine residue making the second hydrogen bond to the leading-end oxygen-containing moiety. It is likely of significance that this short region has been substituted for a larger and more variable region in the higher insect USPs (Fig. 1). This region in each USP apparently contains an invariant aspartic acid, in addition to at least one serine.
Also relevant to this analysis is comparison of the status of these hypothesized ligand-associated residues of USP with the corresponding residues in dmE75A. This latter regulator has also been hypothesized to be an orphan receptor, or, at least, there have been no reports of it binding to juvenile hormones or JH-like molecules (Thummel, 1995; Buszczak and Segraves, 1998). dmE75A does not conserve the invariant Q on
Fig. 2. Summary of specific hydrogen bonding contacts between specific ligand-binding pocket residues and oxygen-containing motifs of the respective ligands, as shown by co-crystallography. The residue of a particular color moiety of the same color for each receptor-ligand combination shown. Also provided are the hydrogen-bonding distances from the indicated residue to the respective site on the ligand, or to a water molecule that in turn h-bonds with the oxygen-containing moiety. EPA=eicosapentaenoic acid; AT-RA=all trans retinoic acid; DIMIT=3,5-dimethyl-39 -isopropylthryonine. Each specific oxygen-containing moiety shown is a part of the indicated ligand and not an extra moiety added to it. All other abbreviations as in Fig. 1.
imply that E75A does not have a ligand, but rather inquires as to why the USPs, but not E75A, have been selected to conserve residues that in RXR provide a hydrophobic ligand-binding environment.
The end of the ligand-binding pocket that is near the ligand entrance point shows more divergence among receptors. However, a narrow region on α-helix 11 is the location of residues that in some crystallized recep-tors make additional hydrogen-bonds, again with other hydroxyl or keto moieties that are also present on the opposite end of the respective ligand (e.g., progesterone and thryoid hormone, Figs. 1 and 2). In both hER and rTRb1, it is a basic histidine that makes the respective hydrogen-bond (Fig. 2). In the same narrow respective
676 G. Jones, D. Jones / Insect Biochemistry and Molecular Biology 30 (2000) 671–679
4. Conservation of residues participating in ligand-induced coactivator-binding
There has been intense investigation of the nature of transcriptional coactivators that are recruited to bind to the new conformations adopted by vertebrate receptors upon binding with their respective ligand (Glass et al., 1997; McKenna et al., 1999). Thus far, three different transcriptional coregulators that are recruited to RXR of the heterodimers PPAR/RXR, RAR/RXR and BOR/RXR are only recruited to the RXR partner after RXR binds its ligand 9-cis RA (Lee and Wei, 1999; Monden et al., 1999; Wiebel et al., 1999). Recently, coactivator binding to the ecdysone receptor has been demonstrated (Tsai et al., 1999). Several vertebrate receptors have been cocrystalized in complexation with such a recruited coactivator, giving precise identification of receptor residues that constitute the surface that binds the coactivator. Part of this surface is contributed by resi-dues in α-helix 12, which upon receptor binding of agonist ligand moves to contact α-helices 3 and 4. Two differently functioning parts of this combined, coacti-vator-binding surface have been identified. One is a hydrophobic groove in which the floor is lined with hydrophobic residues and the rim with polar residues. The other is a ‘charge clamp’ where a basic residue con-tributed by α-helix 3 and an acidic residue contributed by α-helix 12 assist to hold in place the recruited co-activator that has inserted its LXXLL motif into the hydrophobic groove (Darimont et al., 1998; Nolte et al., 1998; Shiau et al., 1998).
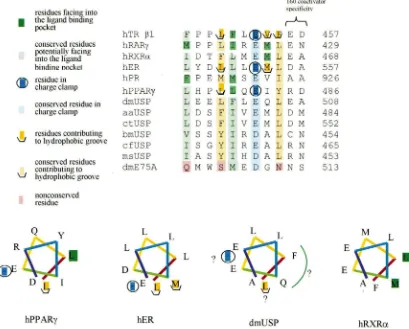
As shown in Fig. 3, higher insect USPs have invariantly conserved on α-helix 3 the presence of a basic arginine at the same location as is conserved the charge clamp lysine residue in the vertebrate receptors. dmE75A instead has a glycine at this position (E75A G454). The insect USPs have also conserved a number of the corresponding hydrophobic residues on α-helices 3 and 4 that in the vertebrate receptors contribute to the
Fig. 3. Conservation in insect USPs ofα-helix 3 and 4 residues contributing in vertebrate receptors to the hydrophobic groove for ligand-induced coactivator binding. Dark brown coded residues are those observed in co-crystals to form part of the hydrophobic groove. Light brown are those residues in other vertebrate receptors and USPs that conserve the residues forming the hydrophobic groove. Dark blue is the basic lysine residue that constitutes one side of the “charge clamp” holding the co-activator in place as seen in cocrystals, while light blue coding shows the conservation of a basic residue at this location in other receptors. Pink-coded residues are not conserved. Abbreviations as in Fig. 1. Sources for sites of co-activator binding deduced by crystallography are: rTRβ1, Darimont et al. (1998); hPPARγ, Nolte et al. (1998); hER, Shiau et al. (1998).
formation of the hydrophobic groove. Crystallography has also shown that a glutamine invariantly conserved in the vertebrate RXRs and the insect USPs is an integral part of the framework for the coactivator binding region (Shiau et al., 1998). That glutamine is not conserved in dmE75A (K467).
ligand/co-Fig. 4. Structural analysis of AF-2 region ofα-helix 12 in vertebrate and invertebrate nuclear receptors. Dark brown coded hydrophobic residues with trough symbol were observed to contribute to the hydrophobic groove that binds co-crystallized co-activators, while light brown coded residues are those conserved hydrophobic residues at the corresponding position in the indicated other receptors. Dark blue, encircled acidic residues are those observed to form part of charge clamp with the co-crystallized coactivator, while light blue, coded residues are those conserved acidic residues at the corresponding position in the other indicated receptors. Dark green hydrophobic residues are those that face into the hydrophobic ligand-binding pocket in the ligand-receptor cocrystal, while light green residues are those conserved hydrophobic residues at the corresponding position. Pink encoded residues are not conserved. Shown below the alignment is a helical wheel representation of the amphipathicα-helix observed for this region in crystalized holo-hPPARγ and holo-hER, and apo-hRXRα, and a modeled representation of the potential similar structure of the corresponding region in dmUSP. Colors on different segments of the wheel have no structural significance and are included solely to aid in visualization of the direction of the spiral. For dmUSP, the green arc offers a possible face of the amphipathic helix that would face into the hydrophobic pocket in the putative ligand-induced conformation. The last two rows of residues shown are residues that vary between receptors and receptor isoforms that appear to contribute to receptor-specific binding responses to 160 coactivator family proteins. Sources for residues contributing to the site of co-activator binding, as deduced by crystallography are: rTRβ1, Darimont et al. (1998); hPPARγ, Nolte et al. (1998); hER, Shiau et al. (1998). Abbreviations are as in Fig. 1.
activator-associated residues, we hypothesize that if insect USPs bind an endogenous ligand, and then change conformation (Jones and Sharp, 1997), part of that con-formational change is movement of α-helix 12 into a position that forms with helices 3 and 4 a hydrophobic groove constituting a co-activator binding site, complete with charge clamp.
5. Additional structural and functional considerations
We have considered thus far in these analyses the fol-lowing evidences from the structures of higher insect
678 G. Jones, D. Jones / Insect Biochemistry and Molecular Biology 30 (2000) 671–679
charge clamp, (5) Jones and Sharp (1997) have demon-strated that dmUSP can bind to certain end-oxygenated terpenoids (epoxymethylfarnesoates) and be thereby induced to undergo conformational change. We feel that there is a sufficient body of structural evidence to reasonably support the working model that the structure of higher insect USPs is such that (a) they are capable of binding an endogenous, terpenoid-derived ligand(s), (b) that ligand possesses at least one terminal oxygen-containing moiety, and (c) upon binding of this endogen-ous ligand USP is induced to undergo a vertebrate recep-tor-type conformational change for coactivator recruit-ment.
Particularly enigmatic at this time is the functional significance of the insertion of a 15–27 amino acid sequence in the region of the β-turn between α-helices 5 and 6 of USPs (Fig. 1). This situation may be remi-niscent of the additional 20 amino acid α-helix 29 inserted into the sequence of hPPARγ (Nolte et al., 1998). This additional insert into hPPARγcontributes in part to a larger volume of ligand-binding pocket than is found in other nuclear receptors. Intriguingly, it has been shown that the ligand pocket of hPPARγ is capable of binding the partially unsaturated fatty acid (eicosopentanoic acid) in two different configurations, where for each the ‘head’ carboxyl group of the ligand is in the same position, but the ‘tail’ of the ligand is bent into either of two configurations at the point of unsatu-ration (Xu et al., 1999). Some partially unsaturated ses-quiterpenoids and related structures, such as JH, would have similar flexibility. The ligand-binding properties of hPPARγand the conformations it adopts in binding vari-ous ligands has prompted the suggestion that PPAR has evolved to bind to several different endogenous ligands. This property would explain its affinity for several ligands each with Kdin the micromolar range in contrast to the other vertebrate receptors that bind a single ligand with nanomolar Kd(Nolte et al., 1998). Jones and Sharp (1997) demonstrated that dmUSP can bind at least three natural ligands, JH III, JH III bisepoxide and JH III acid, with a Kdfor the first two near 1µM. Perhaps addition-ally relevant in this regard is the report of yet additional natural JH structures, including ‘hydroxy-JHs’ (Darrouzet et al., 1997). Recent reports have also described competitive binding of JH and vertebrate thy-roid hormone to the same binding site (Kim et al., 1999). Although that latter report deals with a cell surface receptor, the prospect of two structures as different as JH and thyroxine binding to the same receptor again evokes a theme potentially similar to the binding of dif-ferent ligand structures by PPAR. In any event, it is now possible to envision specific molecular hypotheses on nuclear receptor-mediated JH action, framed on the basis of the structure and behavior of insect molecules. The field’s frustrated, agonizing and seemingly endless search for molecular mechanisms of JH action may be
drawing nearer to a close, with the prospect at hand of a prototypical nuclear receptor that can bind endogenous JH or JH-like structures.
Acknowledgements
This study was supported in part by NIH grants DK39197, AI43342 (GJ) and CA81537 (DJ) and NSF grant 9818433 (GJ). We appreciate the opportunity extended by the organizers of the JH VII Workshop to contribute this paper. DJ and GJ contributed equal responsibility and effort to this paper.
References
Ashok, M., Turner, C., Wilson, T.G., 1998. Insect juvenile hormone resistance gene homology with the bHLH-PAS family of transcrip-tional regulators. Proc. Natl. Acad. Sci. USA 95, 2761–2766. Bourguet, W., Ruff, M., Chambon, P., Gronemeyer, H., Moras, D.,
1995. Crystal structure of the ligand-binding domain of the human nuclear receptor RXR-alpha. Nature 375, 377–382.
Buszczak, M., Segraves, W.A., 1998. Drosophila metamorphosis: the only way is USP? Curr. Biol. 8, 879–882.
Brzozowski, A.M., Pike, A.C., Dauter, Z., Hubbard, R.E., Bonn, T., Engstrom, O., Ohman, L., Greene, G.L., Gustafsson, J.A., Carlqu-ist, M., 1997. Molecular basis of agonism and antagonism in the oestrogen receptor. Nature 389, 753–758.
Chung, A.C., Durica, D.S., Clifton, S.W., Roe, B.A., Hopkins, P.M., 1998. Cloning of crustacean ecdysteroid receptor and retinoid-X receptor gene homologs and elevation of retinoid-X receptor mRNA by retinoic acid. Mol. Cell. Endocrinol. 139, 209–227. Darimont, B.D., Wagner, R.L., Apriletti, J.W., Stallcup, M.R.,
Kushner, P.J., Baxter, J.D., Fletterick, R.J., Yamamoto, K.R., 1998. Structure and specificity of nuclear receptor-co-activator interac-tions. Genes Dev. 12, 3343–3356.
Darrouzet, E., Mauchamp, B., Prestwich, G.D., Kerhoas, L., Ujvary, I., Couillaud, F., 1997. Hydroxy juvenile hormones: new putative juvenile hormones biosynthesized by locust corpora allata in vitro. Biochem. Biophys. Res. Commun. 240, 752–758.
Feyereisen, R., 1998. Juvenile hormone resistance:! no PASaran! Proc. Natl. Acad. Sci. USA 95, 2725–2726.
Glass, C.K., Rose, D.W., Rosenfeld, M.G., 1997. Nuclear receptor co-activators. Curr. Opin. Cell. Biol. 9, 222–232.
Guo, X., Xu, Q., Harmon, M.A., Jin, X., Laudet, V., Mangelsdorf, D.J., Palmer, M.J., 1998. Isolation of two functional retinoid X receptor subtypes from the Ixodid tick Amblyomma americanum (L.). Mol. Cell. Endocrinol. 139, 45–60.
Harmon, M.A., Boehm, M.F., Heyman, R.A., Mangelsdorf, D.J., 1995. Activation of mammalian retinoid X receptors by the insect growth regulator methoprene. Proc. Natl. Acad. Sci. USA 92, 6157–6160. Hayward, D.C., Bastiani, M.J., Trueman, J.W., Truman, J.W., Riddi-ford, L.M., Ball, E.E., 1999. The sequence of Locusta RXR, hom-ologous to Drosophila Ultraspiracle, and its evolutionary impli-cations. Dev. Genes Evol. 209, 564–571.
Jones, G., 1995. Molecular mechanisms of action of juvenile hormone. A. Rev. Entomol. 40, 147–169.
Kapitskaya, M., Wang, S., Cress, D.E., Dhadialla, T.S., Raikhel, A.S., 1996. Mol. Cell. Endocrinol. 121, 119–132.
Kim, Y., Davari, E.D., Sevala, V., Davey, K.G., 1999. Functional bind-ing of a vertebrate hormone, L-3,5,39-triiodothryonine (T3) on insect follicle cell membranes. Insect Biochem. Molec. Biol. 29, 943–950.
Kostrouch, Z., Kostrouchova, M., Love, W., Jannini, E., Piatigorsky, J., Rall, J.E., 1998. Retinoic acid X receptor in the diploblast Trip-edalia cystophora. Proc. Natl. Acad. Sci. USA 95, 13442–13447. Lee, C.H., Wei, L.N., 1999. Characterization of receptor-interacting protein 140 in retinoid receptor activities. J. Biol. Chem. 274, 31320–31326.
Mangelsdorf, D.J., Ong, E.S., Dyck, J.A., Evans, R.M., 1990. Nuclear receptor that identifies a novel retinoic acid response pathway. Nat-ure 345, 224–229.
McKenna, N.J., Xu, J., Nawaz, Z., Tsai, S.Y., Tsai, M.J., O’Malley, B.W., 1999. Nuclear receptor coactivators: multiple enzymes, mul-tiple complexes, mulmul-tiple functions. J. Steroid Biochem. Mol. Biol. 69, 3–12.
Monden, T., Kishi, M., Hosoya, T., Satoh, T., Wondisford, F.E., Hol-lenberg, A.N., Yamada, M., Mori, M., 1999. p120 acts as a specific coactivator for 9-cis-retinoic acid receptor (RXR) on peroxisome proliferator-activated receptor-gamma/RXR heterodimers. Mol. Endocrinol. 13, 1695–1703.
Nolte, R.T., Wisely, G.B., Westin, S., Cobb, J.E., Lambert, M.H., Kurokawa, R., Rosenfeld, M.G., Willson, T.M., Glass, C.K., Mil-burn, M.V., 1998. Ligand-binding and coactivator assembly of the peroxisome proliferator-activated receptor-gamma. Nature 395, 137–143.
Oro, A.E., McKeown, M., Evans, R.M., 1990. Relationship between the product of the Drosophila ultraspiracle locus and the vertebrate retinoid x receptor. Nature 347, 298–301.
Palli, S.R., Touhara, K., Charles, J.P., Bonning, B.C., Atkinson, J.K., Trowell, S.C., Hiruma, K., Goodman, W.G., Kyriakides, T., Prestwich, G.D., 1994. A nuclear juvenile hormone-binding protein from larvae of Manduca sexta: a putative receptor for the metamor-phic action of juvenile hormone. Proc. Natl. Acad. Sci. USA 91, 6191–6195.
Perera, S.C., Palli, S.R., Ladd, T.R., Krell, P.J., Retnakaran, A., 1998. The ultraspiracle gene of the spruce budworm, choristoneura fumi-ferana: cloning of cDNA and developmental expression of mRNA. Dev. Genetics 22, 169–179.
Renaud, J.P., Rochel, N., Ruff, M., Vivate, V., Chambon, P., Groneme-yer, H., Moras, D., 1995. Crystal structure of the RAR-gamma ligand-binding domain bound to all-trans retinoic acid. Nature 378, 681–689.
Riddiford, L.M., 1996. Juvenile hormone: the status of its “status quo” action. Arch. Insect Biochem. Physiol. 32, 271–286.
Shea, M.J., King, D.L., Conboy, M.J., Mariani, B.D., Kafatos, F.C., 1990. Proteins that bind to Drosophila chorion cis-regulatory elements: a new C2H2 zinc finger protein and a steroid receptor-like component. Genes Dev. 4, 1128–1140.
Shiau, A.K., Barstad, D., Loria, P.M., Cheng, L., Kushner, P.J., Agard, D.A., Greene, G.L., 1998. The structural basis of estrogen receptor/coactivator recognition and the antagonism of this interac-tion by tamoxifen. Cell 95, 927–937.
Tanenbaum, D.M., Wang, Y., Williams, S.P., Sigler, P.B., 1998. Crys-tallographic comparison of the estrogen and progesterone recep-tor’s ligand-binding domains. Proc. Natl. Acad. Sci. USA 95, 5998–6003.
Thummel, C.S., 1995. From embryogenesis to metamorphosis: the regulation and function of Drosophila nuclear receptor superfamily members. Cell 83, 871–877.
Tsai, C.C., Kao, H.Y., Yao, T.P., McKeown, M., Evans, R.M., 1999. SMRTER, a Drosophila nuclear receptor coregulator, reveals that EcR-mediated repression is critical for development. Mol. Cell 4, 175–186.
Tzertzinis, G., Malecki, A., Kafatos, F.C., 1994. BmCF1, a Bombyx mori RXR type receptor related to the Drosophila ultraspiracle. J. Mol. Biol. 238, 479–486.
Uppenberg, J., Svensson, C., Jaki, M., Bertilsson, G., Jendeberg, L., Berkenstam, A., 1998. Crystal structure of the ligand-binding domain of the human nuclear receptor PPAR? J. Biol. Chem. 273, 31108–31112.
Vogtli, M., Imhof, M.O., Brown, N.E., Rauch, P., Spindler-Barth, M., Lezzi, M., Henrich, V.C., 1999. Functional characterization of two ultraspiracle forms (CtUSP-1 and Ct-USP-2) from Chironomus ten-tans. Insect Biochem. Molec. Biol. 29, 931–942.
Wagner, R.L., Apriletti, J.W., McGrath, M.E., West, B.L., Baxter, J.D., Fletterick, R.J., 1995. A structural role for hormone in the thyroid hormone receptor. Nature 378, 690–697.
Wiebel, F.F., Steffensen, K.R., Treuter, E., Feltkamp, D., Gustafsson, J.A., 1999. Ligand-independent coregulator recruitment by the tri-ply activatable OR1/retinoid X receptor-alpha nuclear receptor het-erodimer. Molec. Endocrinol. 13, 1105–1118.
Williams, S.P., Sigler, P.B., 1998. Atomic structure of progesterone complexed with its receptor. Nature 393, 392–396.