www.elsevier.com / locate / bres
Research report
Effects of subtypes alpha- and beta-adrenoceptors of the lateral
hypothalamus on the water and sodium intake induced by angiotensin
II injected into the subfornical organ
a ,
*
b˜
Luiz Antonio de Arruda Camargo
, Wilson Abrao Saad ,
a
Gabriela Pavan de Arruda Camargo
a
´
Department of Physiology, School of Dentistry, Paulista State University, UNESP, 1680 Humaita Street, Araraquara, Sao Paulo 14801-903, Brazil
b
´ ´
Department of Odontology, Taubate University, UNITAU, Taubabe, Sao Paulo, Brazil
Accepted 8 August 2000
Abstract
The present experiments were conducted to investigate the role of thea1A-,a1B,b1- andb2-adrenoceptors of the lateral hypothalamus (LH) on the water and salt intake responses elicited by subfornical organ (SFO) injection of angiotensin II (ANG II) in rats. 5-methylurapidil (ana1A-adrenergic antagonist), cyclazosin (ana1B-adrenergic antagonist) and ICI-118,551 (ab2-adrenergic antagonist) injected into the LH produced a dose-dependent reduction, whereas efaroxan (ana2-antagonist) increased the water intake induced by administration of ANG II into the SFO. These data show that injection of 5-methylurapidil into the LH prior to ANG II into the SFO increased the water and sodium intake induced by the injection of ANG II. The present data also show that atenolol (ab1-adrenergic antagonist), ICI-118,551, cyclazosin, or efaroxan injected into the LH reduced in a dose-dependent manner the water and sodium intake to angiotensinergic activation of SFO. Thus, the a1- and b-adrenoceptors of the LH are possibly involved with central mechanisms dependent on ANG II and SFO that control water and sodium intake. 2000 Elsevier Science B.V. All rights reserved.
Theme: Neural bases of behaviors
Topic: Ingestive behaviors
Keywords: a-adrenergic antagonists;b-adrenergic antagonists; Water intake; Sodium intake; Subfornical organ; Lateral hypothalamus
1. Introduction [25,37,43]. The lateral hypothalamus (LH) also is an area of CNS closely involved in the regulation of water and It is well known that circulating angiotensin II (ANG II) sodium [13,14,28].
has several physiological effects mediated through actions It has been postulated that ANG II acts as a neuro-on the central nervous system (CNS) like water and modulator in the CNS because of its interactions with sodium appetite [20,38]. Reductions of extracellular fluid neurotransmitters, especially the catecholamines [9,31,34]. induces an increase in water and sodium ingestion in part The extensive neural pathways from the SFO to the due to the actions of ANG II originated within the CNS hypothalamus have been implicated in the control of body [16,26] and are limited to stimulation of receptors that are fluid intake regulation. Several lines of evidence indicate situated in regions outside the blood–brain barrier, such as that fibers from the SFO converge on the nucleus medianus those in the circumventricular organs [12,33]. The sub- and also project to the supraoptic nucleus, paraventricular fornical organ (SFO) is a circumventricular structure that nucleus and throughout the lateral preoptic area–LH [36]. participates in the regulation of body fluid homeostasis The central part of the SFO, which binds circulating ANG II [39], also contains an ANG II-immunoreactive terminal field that appears to arise from cells in the LH [24]. *Corresponding author. Tel.: 155-16-201-6488; fax: 1
55-16-222-Considering the importance of the SFO and LH for the 4823.
E-mail address: [email protected] (L.A.d.A. Camargo). regulation of water and sodium in rats, the present
experiments were designed to determine the participation of thea1A,a1B,b1,b2anda2-adrenoceptors of the LH in the thirst and sodium appetite induced by SFO application of ANG II.
2. Materials and methods
2.1. Animals
Male Holtzman rats weighing 260–300 g at the begin-ning of the experiments were housed in individual
meta-1
bolic cages. Standard Purina pellets (Na content 5 nmols /
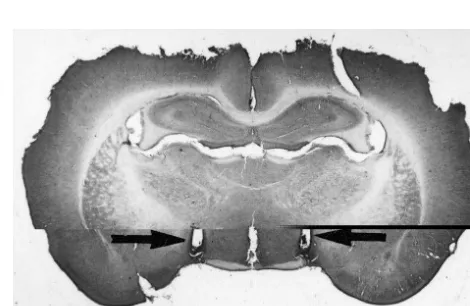
Fig. 1. Photomicrograph of a hematoxylin-stained transverse section of 100 g), tap water and 3% NaCl solution were available ad
the rat brain showing the site of injection into the SFO (arrow). libitum unless otherwise noted. Temperature in the animal
colony was maintained at approximately 238C. The
12:12-h lig12:12-ht–dark cycle began wit12:12-h lig12:12-hts on at 7:00 A.M. All 2.4. Drugs experiments began between 10:00 A.M. and 2:00 P.M.
5-methylurapidil, cyclazosin hydrochloride, efaroxan hydrochloride, (6)-atenolol, ICI-118,551 hydrochloride
2.2. Brain surgery (RBI, Natick, MA, USA) and ANG II (Sigma Chemical
Co., St. Louis, MO, USA). After an acclimatization period of 7 days, the animals
were maintained under tribromoethanol (Aldrich) (20 mg /
100 g b.wt., intraperitoneal [i.p.]) anesthesia throughout 2.5. Histology surgery. A stainless steel guide-cannula (1030.7 mm O.D.)
was stereotaxically implanted into the brain with its At the end of the experiments, the animals were opening protruding into the top of the SFO (coordinates: anesthetized with ether and given a 2-ml injection of fast AP51.3 mm caudal to the bregma; V54.2 mm from the green dye via the intracranial cannula, followed by perfu-dura mater; L50.0 mm from the sagittal line). For LH sion with saline and buffered formalin. The brains were cannulation the cannula (1430.7 mm O.D.) was positioned removed, fixed in 10% formalin, frozen to2258C and cut bilaterally as follows: AP51.6 mm posterior to the into 20–30-mm section. The presence or absence of dye in bregma; V57.5 mm below the dura mater; L51.5 mm the ventricles was observed at this time. Only animals in from the sagittal midline. For cannula implantation the which the presence of dye was noted to be restricted to the stereotaxic incisor bar was positioned 2.5 mm above the SFO and in which the injection was placed in the LH as interaural line. The cannula was secured to the top of the described in the atlas of Paxinos [27] were used in this skull with dental cement and fastened with two screws. study. Figs. 1 and 2 present the site of the injection into the The insertion of a close fitting stylet kept the lumen free of SFO and the LH, respectively.
debris and clots. A prophylactic dose of penicillin (30.000 I.U.) was given intramuscularly (i.m.) presurgically.
2.3. Intracerebral injection techniques
Single pulse intracranial injections were made after gently removing the animal from its cage, replacing the stylet with an injector that protruded 0.2 and 1.0 mm beyond the tip of the guide-cannula for SFO and LH, respectively, and was connected by PE-10 tubing to a 10-ml microsyringe, and injecting a total volume of 0.5ml over a period of 30 s. Stylet and injector were always wiped with cotton soaked with 70% alcohol. After the injection, the injector was removed and replaced with the
2.6. Statistical analysis
The results are reported as mean6S.E.M. and were analyzed by one-way analysis of variance. Values were considered to be statistically significant when P,0.05. The Newman–Keuls post-hoc test was used to assess the difference between individual means.
2.7. Experimental protocol
2.7.1. Water or NaCl 3% intake
Five days after brain surgery, different groups of animals for water or sodium intake were submitted to
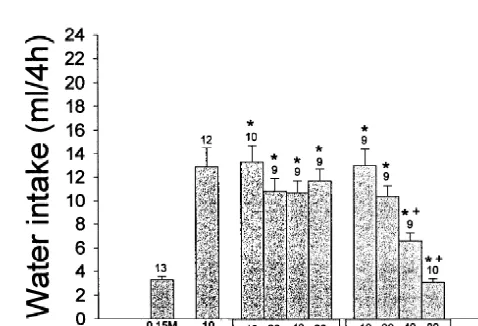
experimental sessions. Each animal was submitted to four Fig. 3. Effect of pretreatment with 5-methylurapidil, cyclazosin, and efaroxan into the LH on water intake induced by injection of ANG II into or five experimental sessions at 3-day intervals. An a
-the SFO. Data are reported as mean6S.E.M. The number of animals is adrenergic antagonist (5-methylurapidil, cyclazosin or
indicated at the top of each column. *P,0.05, compared with saline efaroxan) and the b-adrenergic antagonists (atenolol or 1
(control); P,0.05, compared with ANG II. ICI-118,551) were injected into the LH 20 min before
injection of ANG II into the SFO. Recording of water or
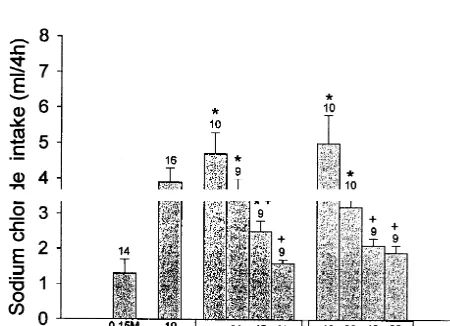
3.2. Effects of 5-methylurapidil, cyclazosin, efaroxan, sodium intake started immediately after ANG II injection
atenolol, or ICI-118,551 into the LH on the sodium and continued for 4 h. Before the salt appetite test the next
intake induced by ANG II injected into the SFO morning, the overnight water intake was measured, and
graduated cylinders were replaced with glass burettes
Control rats (0.15 M NaCl into the SFO) consumed a containing 3% NaCl. The burettes were calibrate to 0.1 ml
mean amount of 1.360.4 ml of sodium over a period of 4 and fitted with drinking spouts. The presence of sodium
h. ANG II (10 pmol) injected into the SFO led to ingestion was announced to the drinking rats by sprinkling a few
of 4.060.4 ml of sodium over the same period. Previous drops of the saline solution on their lips and whiskers.
treatment with 5-methylurapidil (40 and 80 nmol) into the LH potentiated the effect on 3% NaCl intake induced by ANG II injected into the SFO [F(3.33)55.00, P,0.05]. 3. Results Pretreatment with cyclazosin and efaroxan (40 and 80 nmol) into the LH reduced the sodium intake induced by 3.1. Effects of 5-methylurapidil, cyclazosin, efaroxan, ANG II [F(3.34)54.70, P,0.05 and F(3.35)57.30, P, atenolol, or ICI-118,551 into the LH on the water intake 0.05, respectively] (Fig. 5). Previous treatment with induced by ANG II injected into the SFO atenolol and ICI-118,551 (40 and 80 nmol) into the LH
The water intake observed during a period of 4 h in the control experiment (0.15 M NaCl into the SFO) was 3.360.3 ml. Injection of ANG II (10 pmol) into the SFO produced an increase in water ingestion (12.961.6 ml / 2 h). Previous injection of 5-methylurapidil (20, 40, and 80 nmol) and cyclazosin (40 and 80 nmol) into the LH decreased the water intake induced by ANG II administra-tion into the SFO [F (3,30)517.16, P,0.05 and F (3.35)517.45, P,0.05, respectively], whereas efaroxan (40 and 80 nmol) increased this effect [F(3.34)53.33, P,0.05] (Fig. 3). Previous treatment with ICI-118,551 (40 and 80 nmol) into the LH elicits a potentiation in water intake induced by ANG II injected into the SFO [F(3.33)5 23.96, P,0.05]. No changes in the dipsogenic effect of ANG II were observed after previous treatment with
Fig. 4. Effect of pretreatment with atenolol and ICI-118,551 into the LH atenolol [F(3.33)51.03, P.0.05]. (Fig. 4).
on water intake induced by injection of ANG II into the SFO. Data are Injection of only 5-methylurapidil, cyclazosin, efaroxan, reported as mean6S.E.M. The number of animals is indicated at the top
1
atenolol, or ICI-118,551 into the LH produced no altera- of each column. *P,0.05, compared with saline (control); P,0.05,
Several lines of evidence suggest that the adrenergic receptors in the LH are involved in the control of water intake [13,14,18,21,22]. A role of forebrain catechola-minergic systems in the mediation of ANG II-elicited drinking and blood pressure responses has also been suggested [4–6,32]. Interaction of ANG II with brain angiotensinergic receptors initiates a cascade of cellular events that results in stimulation of the NET system and catecholaminergic synthesis and turnover [1,30,35].
The central noradrenergic system has different effects on central ANG II-inducing drinking behavior. Water intake induced by central administration of ANG II is inhibited by a1-, a2- or b-antagonists depending on the site of injection. Prazosin and phentolamine, but not yohimbine, injected into the rostral hypothalamus reduced the water Fig. 5. Effect of pretreatment with 5-methylurapidil, cyclazosin, and
intake induced by intracerebroventricular (icv) ANG II efaroxan into the LH on sodium intake induced by injection of ANG II
into the SFO. Data are reported as mean6S.E.M. The number of animals [19]. The previous administration of prazosin and propran-is indicated at the top of each column. *P,0.05, compared with saline olol into the medial preoptic area (MPOA) inhibits the
1
(control); P,0.05, compared with ANG II.
dipsogenic effect of ANG II injected into the same area [3]. Bilateral lesions of the LH reduced the dipsogenic antagonized the sodium intake induced by ANG II ad- effects of ANG II and noradrenaline injected into the ministration into the SFO [F(3.33)511,73, P,0.05 and median preoptic area (MnPO) [28]. Prazosin and yohim-F(3.34)59.50, P,0.05, respectively] (Fig. 6). bine injected into the MnPO reduced the water intake Injection of only 5-methylurapidil, cyclazosin, efaroxan, induced by ANG II [29]. More recently it was demon-atenolol, or ICI-118,551 into the LH produced no altera- strated that the pretreatment with prazosin and propranolol tions in sodium intake. into the LH enhanced, whereas yohimbine antagonized the water intake produced by ANG II injected into the SFO [7]. Taken together, these results show that the central 4. Discussion injection of anaorb-adrenergic antagonist can disrupt the dipsogenic effect of ANG II in rats and suggest that the The present results show that the a1A-adrenoceptor adrenergic pathways of LH can produce a dual effect on antagonist 5-methyl urapidil, thea1B-adrenoceptor antago- water intake. The release of noradrenaline may be an nist cyclazosin and the b2-adrenoceptor antagonist ICI- excitatory step along the ANG II-inducing thirst pathway 118,551 injected into the LH reduces, whereas the a2- and if we use an adrenergic antagonist we can block the adrenoceptor antagonist efaroxan increases the water in- dipsogenic response. In spite of this excitatory effect, the take induced by ANG II injected into the SFO. adrenergic pathways could also be involved in an inhib-itory system for water intake. The present results show that the blockade of thea1A-,a1B-, orb2-adrenergic receptors into the LH increases, whereas the blockade of the a2 -adrenergic receptors decreases the water intake induced by ANG II. Recent data demonstrated that the action of noradrenaline on hypothalamus-brain stem areas of rats brain on angiotensinergic receptors (AT1 receptors) [42] involves thea1A-adrenergic receptors [40] and pharmaco-logical findings indicate that activation of postsynaptic a1-adrenoceptors potentiates b-adrenoceptors [15].
The present data also show that 5-methylurapidil in-jected into the LH causes an increase, whereas cyclazosin, atenolol, ICI-118,551 and efaroxan causes a decrease in sodium intake induced by administration of ANG II into the SFO.
Saline intake is also inhibited [11,40] or activated by Fig. 6. Effect of pretreatment with atenolol and ICI-118,551 into the LH noradrenaline. Thus, this double mediation of the control on sodium intake induced by injection of ANG II into the SFO. Data are
of water and sodium intake by noradrenaline is explained reported as mean6S.E.M. The number of animals is indicated at the top
1 by the dual-role hypothesis which proposes that central
of each column. *P,0.05, compared with saline (control); P,0.05,
Camargo, Role of adrenergic pathway of the medial preoptic area in of both behaviors [10,14,41]. A relationship between
ANG II-induced water and renal excretion in rats, Brain Res. 636 noradrenaline and ANG II facilitation of sodium intake
(1994) 81–86.
was first shown by Chiaraviglio and Taleisnik [8], who [4] I.B. Bellin, R.K. Bhatnagar, A.K. Johnson, Periventricular norad-obtained 1% NaCl intake in normovolemic animals with renergic systems are critical for angiotensin-induced drinking and noradrenaline injected into the third ventricle close to the blood pressure responses, Brain Res. 403 (1987) 105–112.
[5] I.B. Bellin, S.K. Landas, A.K. Johnson, Localized injections of columns of the fornix. Other authors reported the same
6-hydroxydopamine into lamina terminalis-associated structures: kind of increase after noradrenaline was injected icv [2] or
effects on experimentally induced drinking and pressor responses, in the paraventricular nucleus [23]. However, it has been Brain Res. 416 (1987) 75–83.
proposed [17] that the organum vasculosum laminae [6] I.B. Bellin, S.K. Landas, A.K. Johnson, Selective catecholamine terminalis (OVLT) is the circumventricular organ site for a depletion of structures along the ventral lamina terminalis: effects on experimentally-induced drinking and pressor responses, Brain Res. peripheral action of salt appetite because infusions of ANG
456 (1988) 9–16. II into the OVLT, but no SFO, elicit salt appetite and
[7] L.A.A. Camargo, W.A. Saad, Effects of the alpha antagonists and because lesions of the OVLT suppress salt appetite aroused agonists injected into the lateral hypothalamus on the water and either by a low dose of oral captopril or by sodium sodium intake induced by angiotensin II injection into the subforni-depletion. On the other hand, lesions of the SFO reduce cal organ, Brain Res. Bull. 48 (1999) 521–525.
[8] E. Chiaraviglio, S. Taleisnik, Water and salt intake induced by salt appetite aroused by sodium depletion [38,43].
hypothalamic implants of cholinergic and adrenergic agents, Am. J. The injection procedure to localize brain areas receptive
Physiol. 216 (1969) 1148–1422.
for the effects of ANG II on water and sodium intake has [9] J.T. Cunningham, A.K. Johnson, Decreased norepinephrine in the some limitations. A major problem is diffusion. Due to ventral lamina terminalis region is associated with angiotensin II dose and volume utilized in the present work, it is possible drinking response deficits following local 6-hydroxydopamine
in-jections, Brain Res. 480 (1989) 65–71. that when ANG II was administered into the SFO, neurons
[10] L.A. De Luca Jr., L.A.A. Camargo, J.V. Menani, A. Renzi, W.A. in the MnPO as well as neurons in the adjacent
periven-Saad, On a possible dual role for central noradrenaline in the control tricular parts of the SFO and the OVLT were activated as a of hydromineral fluid intake, Brazilian J. Med. Biol. Res 27 (1994) result of the hormone being transported to these sites by 905–914.
cerebroventricular systems. [11] P.M. De Paula, M.A. Sato, J.V. Menani, L.A. De Luca Jr, Effects of central a-adrenergic agonists on hormone-induced 3% NaCl and The present results demonstrate that injection of
5-water intake, Neurosci. Lett. 214 (1996) 155–158. methylurapidil, cyclazosin or ICI-118,551 into the LH
[12] M.D. Evered, Investigating role of angiotensin II in thirst: interac-reduces, whereas efaroxan increases the water intake tions between arterial pressure and the control of drinking, Can. J. induced by ANG II administration into the SFO. Previous Physiol. Pharmacol. 70 (1992) 791–797.
treatment with 5-methylurapidil into the LH increases, [13] A.C. Ferrari, L.A.A. Camargo, W.A. Saad, A. Renzi, L.A. De Luca Jr, J.V. Menani, Clonidine and phenylephrine injected into the lateral whereas pretreatment with cyclazosin, atenolol,
ICI-hypothalamus inhibit water intake in rats, Brain Res. 522 (1990) 118,551 or efaroxan reduces the salt ingestion produced by
125–130.
ANG II administration into the SFO. These findings show [14] A.C. Ferrari, L.A.A. Camargo, W.A. Saad, A. Renzi, L.A. De Luca that the water and sodium intakes to ANG II injected into Jr, J.V. Menani, Role of the alpha1- and alpha2-adrenoceptors of the the SFO of rats are mediated bya1A-,a1B-, anda2as well lateral hypothalamus in the dipsogenic response to central
angioten-sin II in rat, Brain Res. 560 (1991) 291–296. as b1- and b2-adrenoceptor subtypes.
[15] B. Ferry, B. Roozendaal, J.L. McGaugle, Basolateral amygdala influences on memory storage are mediated by an interaction between beta- and alpha -adrenoceptors, J. Neurosci. 19 (1999)1
Acknowledgements 5119–5123.
[16] D.A. Fitts, D.B. Masson, Forebrain sites of action for drinking and salt appetite to angiotensin or captopril, Behav. Neurosci. 103 The authors appreciate the technical assistance of
Re-(1989) 865–872.
´ ´
ginaldo C. Queiroz, Silas P. Barbosa, Silvia Foglia and
[17] D.A. Fitts, D.B. Masson, Preoptic angiotensin and salt appetite, Silvana A. D. Malavolta. They also thank Ana V. Oliveira Behav. Neurosci. 104 (1990) 63–650.
for animal care. Research supported by CNPq and FAP- [18] S.F. Grossman, Direct adrenergic and cholinergic stimulation of
ESP. hypothalamic mechanisms, Am. J. Physiol. 202 (1962) 872–882.
[19] D.L. Jones, Hypothalamic alpha-adrenergic blockade modifies drink-ing and blood pressure responses to central angiotensin II in conscious rats, Can. J. Physiol. Pharmacol. 66 (1988) 1270–1277. References [20] Y. Kawano, R.T. Sudo, C.M. Ferrario, Effects of chronic
intraven-tricular sodium on blood pressure and fluid balance, Hypertension 17 (1991) 28–35.
[1] R.H. Alpers, M.R. Steel, W.F. Ganong, Angiotensin II increases
[21] S.F. Leibowitz, Pattern of drinking and feeding produced by catecholamine synthesis in select hypothalamic nuclei, Soc.
Neuro-hypothalamic norepinephrine injection in the satiated rat, Physiol. sci. Abstr. 8 (1982) 421.
Behav. 14 (1975) 731–742. [2] J. Antunes-Rodrigues, S.M. McCann, Water, sodium chloride, and
[22] S.F. Leibowitz, Ingestion in the satiated rat: role of alpha and beta food intake, produced by injections of cholinergic and adrenergic
receptors in mediating effects of hypothalamic adrenergic stimula-drugs into the third ventricle of the rat brain, Proc. Soc. Exp. Biol.
tion, Physiol. Behav. 14 (1975) 743–754. Med. 133 (1970) 1464–1470.
gane, J. Panksepp (Eds.), Behavioral Studies of the Hypothalamus, [32] W.B. Severs, J. Summy-Long, A. Daniel-Severs, J.D. Connor, Vol. 3, Marcel Dekker, New York, 1980, pp. 299–437. Influence of adrenergic blocking drug on central angiotensin effects, [24] W.A. Lind, L.W. Swanson, D. Ganten, Organization of angiotensin II Pharmacology 5 (1971) 205–214.
immunoreactive cells and fibers in the rat central nervous system, [33] J.B. Simpson, The circumventricular organs and the central actions Neuroendocrinology 40 (1990) 2–24. of angiotensin, Neuroendocrinology 32 (1981) 248–256.
[25] M.L. Mangiapane, J.B. Simpson, Subfornical organ: forebrain site of [34] C. Summers, Norepinephrine increases angiotensin II binding in rat pressor and dipsogenic action of angiotensin II, Am. J. Physiol. 239 brain synaptosomes, Brain Res. Bull. 28 (1992) 411–415. (1980) R382–R389. [35] C. Summers, M.K. Rizada, Angiotensin II Receptor Subtypes in [26] D.B. Masson, D.A. Fitts, Subfornical organ connectivity and drink- Neuronal Cells, in: M.K. Raizada, M.I. Phillips, C. Summers (Eds.),
ing to captopril or carbachol in rats, Behav. Neurosci. 103 (1989) CRC Press, Boca Raton, Fla, 1993, pp. 379–411.
873–880. [36] T.N. Thrasher, Role of forebrain circumventricular organs in body [27] G. Paxinos, C. Watson, The Rat Brain in Stereotaxic Coordinates, fluid balance, Acta Physiol. Scand. 583 (Suppl.) (1989) 141–150.
Academic Press, San Diego, 1986. [37] R.L. Thunhorst, K.J. Erlich, J.B. Simpson, Subfornical organ [28] R.K.P. Pereira daSilva, W.A. Saad, A. Renzi, J.V. Menani, L.A.A. participates in salt appetite, Behav. Neurosci. 104 (1990) 637–642. Camargo, Effect of lateral hypothalamus lesions on the water and [38] R.L. Thunhorst, D.A. Fitts, Peripheral angiotensin causes salt salt intake, and sodium and urine excretion induced by activation of appetite in rats, Am. J. Physiol. 36 (1994) R171–R177.
the median preoptic nucleus in conscious rats, J. Auton. Nerv. Syst. [39] M. Van Houtten, E.L. Schiffrin, J.F.E. Mann, B.I. Posner, R. 53 (1995) 195–204. Boucher, Radioautographic localization of specific binding sites for [29] R.K.P. Pereira da Silva, J.V. Menani, W.A. Saad, A. Renzi, J.E.N. blood born angiotensin II in rat brain, Brain Res. 186 (1980)
Silveira, A.C. Luiz, L.A.A. Camargo, Role of the alpha1-, alpha2- 480–485.
and beta-adrenoceptors of the median preoptic area on the water [40] M.M. Yada, P.M. De Paula, J.V. Menani, L.A. De Luca Jr, Central intake, renal excretion, and arterial pressure induced by ANG II, adrenergic agonists and need-induced 3% NaCl and water intake, Brain Res. 717 (1996) 38–43. Pharmacol. Biochem. Behav. 57 (1997) 137–143.
[30] M.K. Raizada, D. Lu, C. Summers, AT Receptors and Angiotensin1 [41] M.M. Yada, P.M. De Paula, J.V. Menani, A. Renzi, L.A.A. Camargo, Actions in the Brain and Neural Cultures of Normotensive Actions L.A. De Luca Jr, Receptor-mediated effects of clonidine on need-in the Braneed-in and Neural Cultures of Normotensive and Hypertensive induced 3% NaCl and water intake, Brain Res. Bull. 42 (1997) Rats, in: A. Mukhopadhyau, M.K. Raiada (Eds.), Current Concepts: 205–209.
Tissue Renin-angiotensin as Local Regulators in Reproductive and [42] H. Yang, D. Lu, M.K. Raizafa, Lack of cross talk between a1 -Endocrine Organs, Plenum Publishing Corp, New York, 1994, pp. adrenergic and angiotensin type 1 receptors in neurons of
sponta-331–348. neous hypertensive rats, Hypertension 27 (1996) 1277–1283.
[31] E.M. Richards, K. Hermann, C. Summers, M.K. Raisada, M.I. [43] R.S. Weisinger, D.A. Denton, R. Di Nicolantonio, D.K. Hards, M.J. Phyllips, Release of immunoreactive angiotensin II from neuronac¸ McKinley, B. Oldfield, P.G. Osborne, Subfornical organ lesion cultures: adrenergic influences, Am. J. Physiol. 257 (1989) C588– decreases sodium appetite in sodium-depleted rat, Brain Res. 526