www.elsevier.com / locate / bres
Short communication
An immunogold investigation of the relationship between the amino
acids GABA and glycine and their transporters in terminals in the
guinea-pig anteroventral cochlear nucleus
*
S. Mahendrasingam, C.A. Wallam, C.M. Hackney
MacKay Institute of Communication and Neuroscience, School of Life Sciences, Keele University, Staffordshire ST5 5BG, UK
Accepted 3 October 2000
Abstract
The majority of terminals contacting spherical bushy cell bodies in the guinea-pig anteroventral cochlear nucleus contain GABA, glycine or both (colocalizing). Double labeling with antibodies to each amino acid and the plasma membrane transporter for the other was performed using different sizes of gold particles. The transporter for GABA occurs in the plasma membranes of some terminals containing glycine and vice versa suggesting that colocalizing terminals can retrieve both amino acids. 2000 Elsevier Science B.V. All rights reserved.
Theme: Neurotransmitters, modulators, transporters, and receptors
Topic: Interaction between neurotransmitters
Keywords: Colocalization; GABA; Glycine; GABA transporter (GAT1); Glycine transporter (GLYT2)
Colocalization of GABA and glycine has been reported transporter GAT1, have been investigated using double in both cell bodies and terminals in the mammalian labeling with immunogold, both these transporters having cochlear nuclear complex (for review, see [10]). For been reported previously as occurring in this area or brain example, the spherical bushy cells in the anteroventral regions nearby [4,9,11].
cochlear nucleus (AVCN) that receive the so-called end- For this, pigmented guinea pigs (250–500 g body bulbs of Held, large glutamatergic endings from the weight) were anaesthetized using sodium pentobarbitone cochlear nerve, are also contacted by terminals of non- (Vetalar, 200 mg / kg, I.P) and perfused transcardially with cochlear origin which contain GABA or glycine or both a brief flush of 50 ml of 2% dextran (molecular weight [6,7]. One of the ways in which this colocalization might 60 000–90 000) in 0.1 M sodium phosphate buffer (PB, arise is if the plasma membrane transporters for these pH 7.4), followed immediately by 800 ml of 4% parafor-amino acids occur together in the colocalizing terminals. maldehyde (immunofluorescence), 800 ml of a mixture of This suggestion arises from the fact that in situ hybridiza- 4% paraformaldehyde and 0.05% glutaraldehyde (immuno-tion and immunocytochemical studies have shown a good gold) or 2.5% glutaraldehyde and 1% paraformaldehyde correlation between the presence of the putatively inhib- (ultrastructure only) in the same buffer. The brainstem was itory amino acid neurotransmitters GABA and glycine and then removed, immersed in the same fixative for 2 h and the presence of their specific transporters in the neuronal frontal slices cut on a Vibratome.
membranes [8,12]. To investigate whether this occurs in For immunofluorescent labeling of transporters, 30 mm the AVCN, the reciprocal distributions of GABA and the slices were permeabilised using 0.5% Triton X-100 in glycine transporter GLYT2, and glycine and the GABA phosphate-buffered saline (PBS) for 15 min, preblocked with 1% human serum albumen in PBS (HSA-PBS) containing 0.05% Triton-X 100 for 30 min, then incubated
*Corresponding author. Tel.: 144-1782-583058; fax: 1
44-1782-overnight at 48C in either guinea-pig anti-GLYT2
583055.
E-mail address: [email protected] (C.M. Hackney). [Chemicon, Inc.] (1:5000) or anti-GAT1 antibody
[Chemicon, Inc.] (1:500), followed by the appropriate FITC-conjugated secondary antibody (Sigma,1:20) for 4.5 h.
For ultrastructure alone, 200–300mm slices were post-fixed in 1% osmium tetroxide in 0.1 M phosphate buffer for 1 h, dehydrated via an ethanolic series and propylene oxide and embedded in Durcupan resin (ACM Fluka). For immunogold labeling, slices were cryoprotected by immer-sion in increasing concentrations of glycerol in PB (up to 30%), then plunge frozen and immersed in anhydrous methanol containing 0.5% uranyl acetate at 2858C, brought gradually up to 2458C, rinsed in methanol and infiltrated with increasing concentrations of Lowicryl HM20 resin. This was polymerized with UV light and allowed to warm up to 208C over 33 h. To double label for GABA and GLYT2, ultrathin sections of this tissue were preblocked in 2% HSA in 0.05 M Tris-buffered saline (TBS, pH 7.4) and incubated overnight at 48C in a mixture of polyclonal primary antibodies, rabbit anti-GABA 990 [kindly provided by O.-P. Ottersen and J. Storm-Mathisen, Oslo University] (1:100) and guinea-pig anti-GLYT2 (1:1000), then placed in a mixture of goat anti-rabbit IgG-30 nm gold conjugate (1:20) and goat anti-guinea pig IgG-15 nm gold conjugate (1:20) for 2 h. To double label for glycine and GAT1, a different strategy had to be adopted because both antibodies were raised in the same species. In this case, ultrathin sections were preblocked in 1% HSA-TBS, incubated overnight at 48C in the anti-GAT1 584 [kindly provided by N.C. Danbolt, Oslo University] (1:90), incubated in anti-rabbit IgG-15 nm gold conjugate (1:20) for 2 h, washed in distilled water and air dried. They were then exposed to paraformal-dehyde vapour at 608C for 1 h to denature any remaining primary antibody, washed and preblocked again, then incubated overnight at 48C in rabbit anti-glycine 290 [kindly provided by O.-P. Ottersen and J. Storm-Mathisen, Oslo University] (1:2000) followed by goat anti-rabbit IgG-30 nm gold conjugate (1:10) for 2 h.
All sections for transmission electron microscopy were stained with uranyl acetate and lead citrate and examined using a JEOL-100CX transmission electron microscope operated at 100 kV.
The animals used for these procedures were maintained and used in accordance with the Principles of Laboratory
Animal Care (NIH publication No. 85-23, revised 1983)
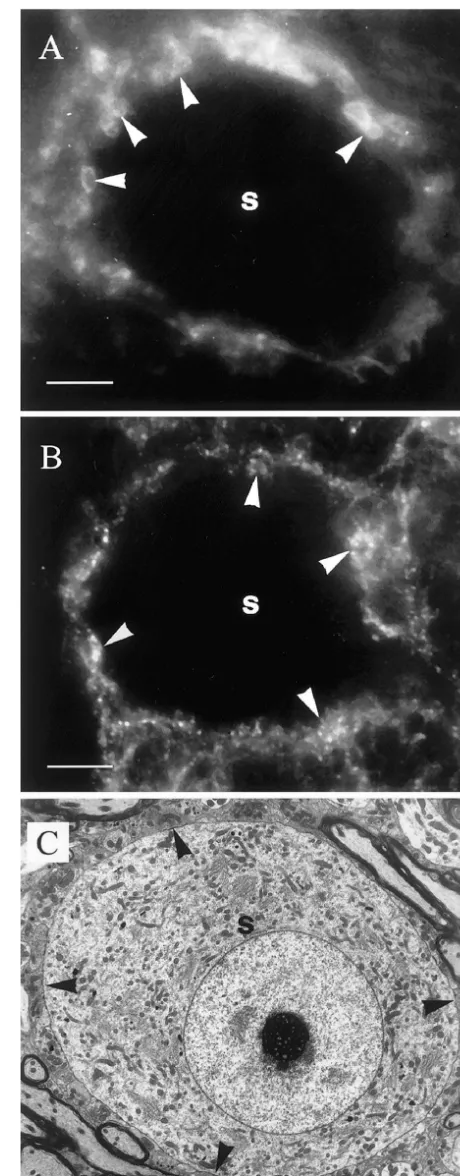
and the Animals (Scientific Procedures) Act, 1986. For both transporters, immunofluorescent labeling was seen around spherical bushy cells (Fig. 1A, B) in similar position to that occupied by the terminals that end on the soma (Fig. 1C). Following immunogold labeling, the large
primary terminals of the cochlear nerve showed little or no Fig. 1. (A, B) Labeling in the AVCN with anti-GLYT2 antibody and anti-GAT1 antibody respectively. In both cases, a spherical bushy cell
labeling for either amino acid or transporter and this was
soma (s) is outlined by labeling, some of which is associated with small
taken to represent a background level. In comparison with
rounded profiles (e.g., arrowheads). (C) Transmission electron micrograph
this, many of the smaller non-primary terminals were showing that the spherical bushy cell soma (s) is contacted by numerous labeled for one or other amino acid and / or transporter with terminals (e.g., arrowheads) in a position corresponding to the fluorescent
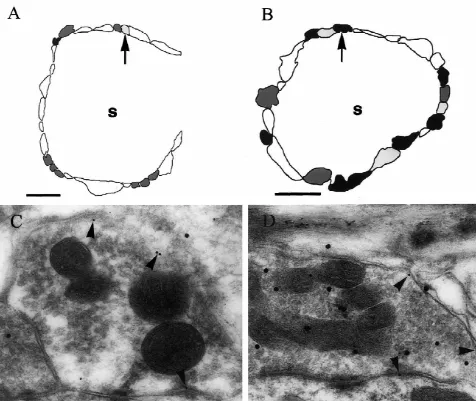
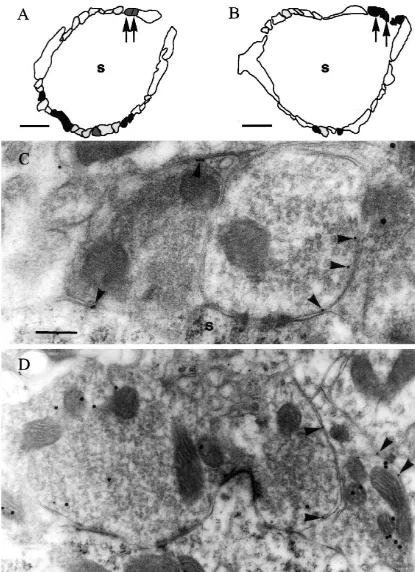
was observed on the plasma membrane of terminals with their plasma membranes but lacking labeling for glycine in no labeling for GABA in their cytoplasm (Fig. 2A, C) but their cytoplasm represent the GABA-only terminals re-also on the membranes of terminals that were labeled for ported previously whilst the ones with labeling for GLYT2 GABA (Fig. 2B, D). Similarly, labeling for GAT1 was but not for GABA are the glycine-only ones [6,7]. It is observed on plasma membranes of terminals with no important to note however that the relatively sparse levels labeling for glycine in their cytoplasm (Fig. 3A, C) but of labeling mean that these terminals could not be iden-also on the membranes of terminals that were labeled for tified with certainty. However, terminals with GLYT2 on glycine (Fig. 3B, D). These different combinations of the plasma membrane and labeling for GABA in their labeling for each transporter and the other amino acid were cytoplasm when that combination of amino acid and observed in terminals adjacent to each other on the same transporter was investigated, seem very likely to be spherical bushy cells (Figs. 2A, B, and 3A, B). Thus, these colocalizing ones. Similarly, those that showed labeling for patterns could not be accounted for by variations in GAT1 on their plasma membranes and labeling for glycine labeling densities between grids. in their cytoplasm when the opposite combination was It is probable that terminals with labeling for GAT1 on tried, are likely to represent this type of terminal too.
[2] P.M. Burger, J. Hell, E. Mehl, C. Krasel, F. Lottspeich, R. Jahn,
Similar observations have been made in the rat nucleus
GABA and glycine in synaptic vesicles: storage and transport
tractus solitarii [1] suggesting that these patterns are found
characteristics, Neuron 7 (1991) 287–293.
in other brain regions too. [3] H. Christensen, E.M. Fyske, F. Fonnum, Uptake of glycine into It is generally believed that the uptake mechanism in synaptic vesicles isolated from rat spinal cord, J. Neurochem. 54
synaptic vesicles does not distinguish between GABA and (1990) 1142–1147.
[4] E. Friauf, C. Aragon, S. Lohrke, B. Westenfelder, F. Zafra,
De-glycine [2,3,5]. Thus it seems likely that both are available
velopmental expression of the glycine transporter GLYT2 in the
for release by colocalizing terminals. The results presented
auditory system of rats suggests involvement in synapse maturation,
here additionally suggest that colocalizing terminals are J. Comp. Neurol. 412 (1999) 17–37.
capable of taking up both GABA and glycine when [5] P. Jonas, J. Bischofberger, J. Sandkuhler, Corelease of two fast
released. The intriguing question that remains now is what neurotransmitters at a central synapse, Science 281 (1998) 419–424. [6] J. Kolston, K.K. Osen, C.M. Hackney, O.-P. Ottersen, J.
Storm-the post-synaptic effects of such co-release might be and in
Mathisen, An atlas of glycine-like and GABA-like immunoreactivity
the case of the auditory brainstem at least, why this
and colocalization in the cochlear nuclear complex of guinea pig,
particular pathway requires these types of inputs alongside Anat. Embryol. 186 (1992) 443–465.
single GABAergic and glycinergic ones. [7] S. Mahendrasingam, C. Wallam, C.M. Hackney, Distribution of immunogold labelling for GABA and glycine in nerve terminals on spherical bushy cells in the guinea-pig anteroventral cochlear nucleus, Brit. J. Audiol. 32 (1998) 78–79.
Acknowledgements
[8] A. Minelli, N.C. Brecha, C. Karschin, S. DeBiasi, F. Conti, GAT-1, a high-affinity plasma membrane transporter, is localized to neurons
Supported by the Wellcome Trust, an MRC Studentship and astroglia in the cerebral cortex, J. Neurosci. 15 (1995) 7734–
and Defeating Deafness: The Hearing Research Trust. 7746.
[9] R. Radian, O.-P. Ottersen, J. Storm-Mathisen, M. Castel, B.I.
Thanks are also due to N.C. Danbolt, O.-P. Ottersen and J.
Kanner, Immunocytochemical localization of the GABA transporter
Storm-Mathisen, Department of Preclinical Medicine, Oslo
in rat brain, J. Neurosci. 10 (1990) 1319–1330.
University for providing antibodies, to Mrs. J. Kott for [10] R.J. Wenthold, Neurotransmitters of brainstem auditory nuclei, in: technical assistance and to Dr. D.N. Furness for help in R.A. Altschuler, R.P. Bobbin, B.M. Clopton, D.W. Hoffman (Eds.),
producing the plates. Neurobiology of Hearing: The Central Auditory System, Raven Press Ltd, New York, 1991, pp. 121–139.
[11] X.-X. Yan, C.E. Ribak, Increased expression of GABA transporters, GAT-1 and GAT-3, in the deafferented superior colliculus of the rat,
References Brain Res. 783 (1998) 63–76.