i
The Effect of Acid Solubilization Condition on The Yield of
Calcium Extracted from Pacific White Shrimp (
Litopenaeus
vannamei
) Shell
PRACTICAL TRAINING REPORT
This practical training report is submitted for the partial requirement for
Bachelor Degree
By :
Angela Irena Wibawa
12.70.0034
DEPARTMENT OF FOOD TECHNOLOGY
FACULTY OF AGRICULTURAL TECHNOLOGY
SOEGIJAPRANATA CATHOLIC UNIVERSITY
SEMARANG
ii
The Effect of Acid Solubilization Condition on The Yield of
Calcium Extracted from Pacific White Shrimp (
Litopenaeus
vannamei
) Shell
BY:
ANGELA IRENA WIBAWA Student ID : 12.70.0034 Faculty : Agricultural Technology
This practical training report has been approved and supported by examiner in Practical Training Exam on
Departement of Food Technology Faculty of Agricultural Technology Soegijapranata Catholic University
Semarang, July 12th 2015 Technical Advisor I Technical Advisor II
Assoc. Dr. Nattapol Tangsuphoom Dr. Ch.Retnaningsih, MP.
Dean
iii
PREFACE
First of all, the writer wants to say Thank God for His grace and His blessing so this practical training report entitled “The Effect of Acid Solubilization Condition on The Yield of Calcium Extracted from Pacific White Shrimp (Litopenaeus vannamei) Shell could be completed. This practical training report is submitted to fulfill one of the requirements to gain bachelor degree of Agricultural Technology Faculty, Food Technology Department, Soegijapranata Catholic University.
During the making of this report, the writer wants to say thank you to :
1. Assoc. Dr. Nattapol Tangsuphoom as the first advisor who has given the best support and guidance for this practical training, from conducting the experiment until the finishing paper.
2. Dr. Ch.Retnaningsih, MP. as the second advisor who has given the best support and guidance during the internship and the making of this paper.
3. Saranya Dechapinan as the lab partner who helped the experiment and the making of this paper.
4. Assoc. Prof. Dr. Visith Chavasit as Dean of Institute of Nutrition Mahidol University, who has given the opportunity to do the practical training in his faculty. 5. Dr. V. Kristina Ananingsih, ST., MSc. as Dean of Faculty of Agricultural
Technology, Soegijapranata Catholic University, for giving the information and chance to join the practical training abroad.
6. Kartika Puspa Dwiana, S.TP., Practical Training Coordinator of Faculty of Agricultural Technology, Soegijapranata Catholic University, for her understanding and tolerance.
7. My parents, Triwibawa Mulja Widarsa and Liana Mahdalena Imantono as my parents, my little sister, Anita Angelina Wibawa who allowed to get practical training abroad and supported during the practical training.
8. Ivana, Naomi, and Vina, who has giving support, help, and being been best practical training mates during the process.
iv
Finally, the writer wants to apologize if there are accidentally errors during the making of this report and hopes that this report can be useful for the others. The readers also can give suggestions to the writer to improve the content in order to be made as one of the good examples for the next practical training.
v
1.1.1.2. Institute of Nutrition Mahidol University ... 1
1.1.1.3. Vision ... 2
1.1.1.4. Mission ... 2
1.1.1.5. Faculty Member ... 2
1.1.1.5.1.Administrators ... 2
1.1.2. Purpose of Practical Training ... 3
1.1.3. Time and Place of Practical Training ... 3
2.2.1. Deproteinization of Shrimp Shell ... 6
2.2.2. Calcium Extraction from Shrimp Shell ... 6
2.2.3. Analyses ... 7
2.2.3.1. Ash Content ... 7
2.2.3.2. Calcium Content ... 8
2.2.3.3. Calcium Extraction Yield ... 8
2.2.4. Statistical Analysis ... 8
3. RESULT AND DISCUSSION ... 9
4. CONCLUSION ... 13
5. REFERENCES ... 14
vi
LIST OF TABLES
Table 1. Chemical Composition of The Deproteinized Shrimp Shell ... 10 Table 2. Ash Content of The Remaining Shell from Different
Extraction Conditions ... 10 Table 3. Calcium Content of The Remaining Shell from Different Extraction
Conditions ... 11 Table 4. Calcium Content of The Calcium Solution Obtained from Different
Extractions Conditions ... 12 Table 5. Calcium Extraction Yield Obtained from Different Extraction
vii
LIST OF FIGURES
1
1. INTRODUCTION 1.1.Practical Training 1.1.1. Institution Profile 1.1.1.1. Mahidol University
Mahidol University has its origins in the establishment of Siriraj Hospital in 1888 by His Majesty King Chulalongkorn (Rama V), and the hospital's medical school is the oldest institution of higher learning in Thailand, granting its first medical degree in 1893. Later becoming the University of Medical Sciences in 1943, Mahidol University was renamed with great honor in 1969 by H.M. King Bhumibol Adulyadej , after his Royal Father, H.R.H Prince Mahidol of Songkla , who is widely known as the 'Father of Modern Medicine and Public Health in Thailand'. Mahidol University has since developed into one of the most prestigious universities in Thailand, internationally known and recognized for the high caliber of research and teaching by its faculty, and its outstanding achievements in teaching, research, international academic collaboration and professional services. This diversified institution now offers top quality programs in numerous social and cultural disciplines, including the most doctoral programs of any institution in Thailand, yet has maintained its traditional excellence in medicine and the sciences.
Figure 1. Logo of Mahidol University of Thailand
1.1.1.2. Institute of Nutrition Mahidol University
expertise shown by its faculty in food and nutrition. Since its founding, INMU has fulfilled this mission by conducting research at community and laboratory levels, by providing national and international training and education programs, and by providing technical services in food and nutrition development. The goal has been, and continues to be, the attainment of the highest possible quality of life for individuals, communities, Thai society, and for people living in other countries within and outside the Southeast Asian Region.
1.1.1.3.Vision
Institute of Nutrition Mahidol University strives to become a recognized world leader in food and nutrition by 2015 through innovative research, capacity building and the generation and transfer of advanced knowledge and technologies to effectively address critical food and nutrition challenges at individual, community, national and global levels.
1.1.1.4. Mission
3
1.1.1.5. Faculty Member 1.1.1.5.1. Administrators
The main administrators of the faculty are in follow:
a. M.Sc. and Ph.D. Degree Program in Nutrition: Dr. Gene Charoonruk.
b. M.Sc. Degree Program in Food and Nutritional Toxicology: Asst. Prof. Chaniphun Butryee
c. M.Sc. Degree Program in Food and Nutrition for Development (International program): Asst. Prof. Sitima Jittinandana
d. M.Sc. Degree Program in Nutrition and Dietetics (International program): Asst. Prof. Chanida Pachotikarn
1.1.2. Purpose of Practical Training
a. To give an experience to explore food in the research, so the student can implement the knowledge that they learned in the scientist world or real food industry.
b. To give an opportunity to adapt with new circumstances and neighborhood. c. To broaden the knowledge and experience of international exposure.
1.1.3. Time and Place of Practical Training
The practical training is conducted in the Institute of Nutrition, Mahidol University, Salaya Thailand from 16th January to 26th March 2015.
5
1.2. Research
1.2.1. Background of Research
Calcium is one of the macro mineral which is needed for human body for the skeletal system. Houtkooper (2004) stated that calcium is needed to build and maintain teeth and bones, it helps muscles and nerves to work properly, and also helps to prevent high blood pressure. Inadequate consumption of calcium can cause diseases such as osteoporosis, osteomalacia, and bone fracture. The calcium needed per day for every individual is different and it depends on their ages. Females need more calcium than males since their mass bone more decrease rapidly, especially those who are menopause which reduce the amount of estrogen hormone. Fikawati (2005) also reported that female students need more calcium intake for replenishing than do male students. Thus the risk of osteoporosis is also higher in women than men (Agustin 2009). A study conducted in Bandung City suggested that calcium intake of adolescent students does not meet the requirement yet which is under 75% RDA.
Shell of the crustaceans, which is the waste from seafood processing can cause environmental problem because some of flesh left in the shells is the ideal growth media for pathogenic bacteria (Sini et al., 2007). Crustaceans shell consists mainly of chitin, a polymer of N-acetylglucosamine, which naturally exists in complex structure with minerals, especially calcium, and protein. Ravichandran, et al (2009) reported that shrimp shell contains 32.5% protein, 26.6% minerals which mostly is calcium, 9.8% lipid, and 12.3% moisture. Therefore the shell waste is usually utilized as the source for chitin extraction, from which minerals and protein are removed by using strong acid and alkali solutions to solubilize the minerals and protein from the shell. The obtained protein and calcium are frequently discarded as by products.
condition factors such as temperature, time, and pH can influence the yield of the calcium extracted from shrimp shell.
1.2.2.Objectives:
2. RESEARCH METHODOLOGY 2.1. Materials
Frozen shell of Pacific white shrimp (Litopenaeus vannamei) was obtained from a shrimp processing plant of Charoen Pokphand Food (Public) Co., Ltd. Serine endoprotease (Alcalase 2.4L) was donated by Brenntag (Thailand) Co., Ltd. All other chemicals unless otherwise stated were obtained from Sigma Aldrich.
2.2. Methods
2.2.1. Deproteinization of Shrimp Shell
First of all, shrimp shell was thawed and then the thawed shell was mixed with de-ionized water at the weight to volume ratio of 1:10. After that, the enzyme was added into them at the concentration of 0.1% (v/w) of the shell and the mixture was heated in a temperature-controlled water bath for 2 hours at pH 9 and 50oC, which is the optimal condition of the enzyme, to hydrolyze and remove the protein from the shell. After that, the mixture was heated at 90oC for 10 minutes in the water bath to inactivate the enzyme and after that it was put immediately into the ice bath to cool down the room temperature (28°C). The deproteinized shell was separated from protein solution by wire screen and dried in the hot air oven at 50oC for 6 hours. The dried deproteinized shrimp shell was packed and sealed in LDPE bags and kept in the desiccator for further analysis. Chemical composition of the deproteinized shell was analyzed according to AOAC Official Method.
2.2.2. Calcium Extraction from Shrimp Shell
2.2.3. Analyses 2.2.3.1. Ash Content
The ash contents in the shells before and after calcium extraction were analyzed according to AOAC Official Method. The crucible and lid were put in the furnace prior to heating at 550oC for overnight. After that the crucible was cooled in the desiccator for 30 minutes. Then, the crucible and the lid were weighed. One gram of the sample was put in the crucible. After that, the crucible was heated over flow above Bunsen flame with lid half covered until the fume was no longer produced. Then, crucible with lid and sample was put in the furnace and heated at 550oC for overnight. During heating, lid was not covered to prevent loss of fluffy ash but it was put on after the heating was complete, which was noticed as the sample turned to grey. The crucible was then cooled down in the desiccator. The weight of crucible with lid and the remaining ash was recorded and the ash content was calculated based on the following equation:
2.2.3.2. Calcium Content
The calcium contents both in the solution and in the remaining shell from each extraction condition were analyzed by using an atomic absorption spectrometry (AAS) equipped with flame and graphite furnace. The remaining ash from the shell was added 5 ml of 4 N nitric acid solution and diluted with distilled water to obtain the ash solution of the final volume of 50 ml. For calcium content analysis by AAS, aliquots (125 µl) of the ash solution from the remaining shell and the calcium solution were added with 2.5 ml of 4 N nitric acid solution and 5 ml of 5% lanthanum oxide and diluted with distilled water to the final volume of 25 ml prior to AAS analysis.
2.2.3.3. Calcium Extraction Yield
9
2.2.4. Statistical Analysis
3. RESULTS AND DISCUSSION
Hartini et al. (2003) stated that the source of calcium is widely-available in broccoli, cabbage, legumes, milk, dairy products and also fortified products such as soy milk. Houtkooper (2004) also added that the calcium needed for individual ages between 9-18 years old is 1300 miligram each day. The adults between 19-50 years old need 1000 miligram calcium per day and for people ≥ 50 years old need 1200 miligram calcium per day. Fikawati (2005) reported that 76.2% from 1254 students consume the calcium less than 75% of RDA and the RDA for Indonesian is 1200 miligram/ day for the teenagers.
Extracted Calcium from the shrimp shell can be utilized for the fortification because it is cheap but there are several factors that can influence the percent yield of shrimp shell such as pH, acid, temperature and time and this study used two parameters to find the suitable condition for the % extraction yield. Before it obtained % extraction yield, it used deproteinization to remove the protein and get the calcium from the Pacific White shrimp shell. Then, calcium extracted with HCl to get the mixture of calcium with acid from the shrimp shell. Percon et al (2003) reported that HCl didn’t give the good effect to the molecular weight in the demineralization of chitin but Poeloengasihhernawan (2009) stated it has high ability to remove ash and protein than acetic acid. After that, AAS was used to analyze the extracted calcium.
The result of chemical compostion of the deproteinized shrimp shell is shown in the table 1.
Table 1. Chemical Composition of The Deproteinized Shrimp Shell
Composition Content (/100 g)*
Wet weight basis Dry weight basis
Moisture 9.96 g 90.05 g
Protein (N x 6.25) 41.79 g 46.41 g
Ash 25.48 g 28.30 g
Calcium 8365.805 mg 9290.280 mg
Dietary fiber 30.38 g 33.74 g
11
From the data above, it showed that the calcium is the highest composition from the deproteinized shrimp shell based on the wet weight basis which is 8365.805 mg/100 g and the dry weight basis which is 9290.280 mg/100 g. On the previous study by Ravichandran (2009), it mentioned that the calcium content in the shrimp shell in Indian White Shrimp (Penaeus indicus) is higher than its flesh so shrimp shell is the good source of calcium. It is also supported by Okoye (2005) that shrimp waste is good source for calcium but the bioavailability for the protein is low for the broiler chicken.
The result of ash content of the remaining shell from different extraction conditions is shown in the table 2.
Table 2. Ash Content of The Remaining Shell from Different Extraction Conditions
*Mean ± standard deviation of triplicate samples
From the table 2, it showed that the higher temperature, the lower ash content and the longer time, the lower ash content too but the data in the temperature 28oC, 4 hours showed that the longer time, the higher ash content. Poeloengasihhernawan (2009) stated that the ash of the shrimp shell are higher than those of heads because of the different chemical composition of the raw material but preconditioned shell has lower ash content than head and after the demineralization process, shell has higher ash content than head. Fall (2012) reported that the highest ash was obtained from 100% shrimp shell meal for hybrid tilapia and it could substitute the soybean meal up to 60% for hybrid tilapia’s meal. Trung (2012) also reported that The purified chitosan showed the higher quality than chitosan especially lower ash and protein content. Then shrimp shell powder also has the higher ash than raw chitin (Khorrami, 2012).
Temperature(oC) Ash content (mg/100g)*
1 hour 2 hours 4 hours
28 3.90 ± 0.24 3.81 ± 0.20 5.00 ± 0.35
50 3.00 ± 0.34 3.12 ± 0.09 2.94 ± 0.21
The result of calcium content of the remaining shell from different extraction conditions is shown in table 3.
Table 3. Calcium Content of The Remaining Shell from Different Extraction Conditions
*Mean ± standard deviation of triplicate samples
From the data above, it showed that the higher temperature, the lower calcium content from the remaining shell and the longer time, the lower calcium content too but the calcium content increased slightly at 4 hours for each temperature.
The result of calcium content of the calcium solution obtained from different extractions conditions is shown in the table 4.
Table 4. Calcium Content of The Calcium Solution Obtained from Different Extraction Conditions
*Mean ± standard deviation of triplicate samples
Based on the data above, it showed that the higher temperature, the higher calcium content of the calcium solution and the longer time, the higher calcium content of the solution too.
Temperature(oC) Calcium content (mg/100g)*
1 hour 2 hours 4 hours
28 1052.92 ± 89.03 1001.29 ± 47.89 1010.66 ± 188.64 50 971.05 ± 112.71 941.47 ± 76.15 955.44 ± 149.02 70 954.58 ± 183.90 769.50 ± 51.60 803.10 ± 33.00
Temperature(oC) Calcium content (mg/100g)
1 hour 2 hours 4 hours
13
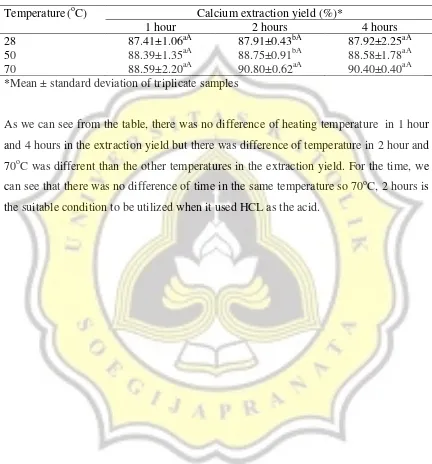
The result of calcium extraction yield obtained from different extraction conditions is shown in the table 5.
Table 5. Calcium Extraction Yield Obtained from Different Extraction Conditions
*Mean ± standard deviation of triplicate samples
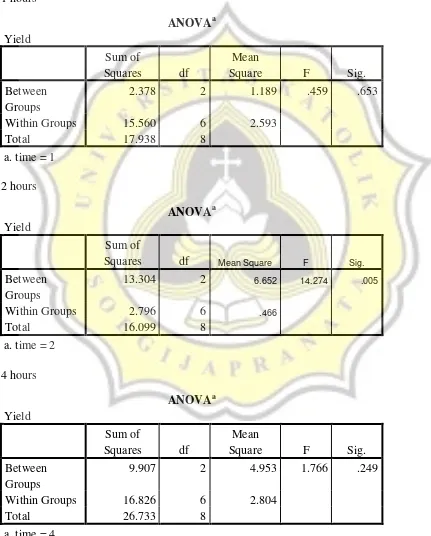
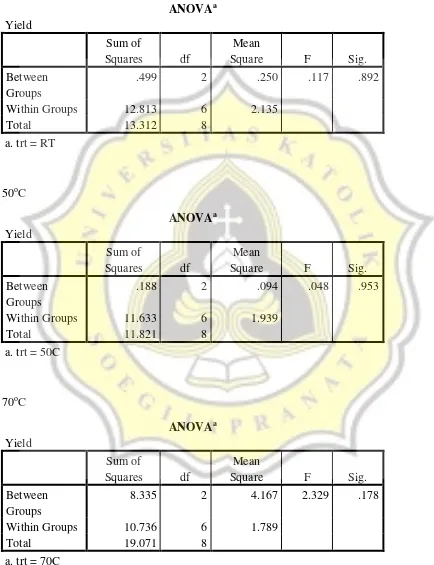
As we can see from the table, there was no difference of heating temperature in 1 hour and 4 hours in the extraction yield but there was difference of temperature in 2 hour and 70oC was different than the other temperatures in the extraction yield. For the time, we can see that there was no difference of time in the same temperature so 70oC, 2 hours is the suitable condition to be utilized when it used HCL as the acid.
Temperature(oC) Calcium extraction yield (%)*
1 hour 2 hours 4 hours
28 87.41±1.06aA 87.91±0.43bA 87.92±2.25aA
50 88.39±1.35aA 88.75±0.91bA 88.58±1.78aA
14
4. CONCLUSION
15
5. REFERENCES
Agustin, R. The Relationship between Status Nutrition, Lifestyle, and Habits of Calcium and Vitamin D with Genesis Osteoporosis and Osteoponia on Citizens ≥ 45 years in Taman Wisma Asri North Bekasi. FKMUI; 2009.
AOAC. 1990. Official methods of analysis of the Association of Official Analytical Chemists. 15th edition. Washington, DC, Association of Official Analytical Chemists.
Fall, Jean, Yi-Theng Tseng, Diegane Ndong, Shin-Shyn Sheen. The Effects of Replacement of Soybean Meal by Shrimp Shell Meal on the Growth of Hybrid Tilapia (Oreochromis niloticus x Oreochromis aureus) Reared Under Brackish Water. International Journal of Fisheries and Aquaculture Vol.4 (5), pp. 85-91, April 2012 . Available online a
Fikawati, S., Syafiq, A., Puspasari, P. The Factors Associated with Calcium Intake in Young in Bandung. Vol.24 No.1. Universa Medicina; 2005.
Hartini TNS, Winkvist A, Lindholm L, Stenlund H, Persson V, Nurdiati DS, and Surjono A & Hakimi M. Nutrient intake and iron status of urban poor and rural poor without access to rice fields are affected by the emerging economic crisis: the case of pregnant Indonesian women. Eur J Clin Nutr. 2003 May; 57(5) : 654. Available
fro
Houtkooper, L., Farrell, V. A. Calcium supplement guidelines. Arizona Cooperative Extension ; 2004.
Khorrami, M, G. D. Najafpour, H. Younesi, M. N. Hosseinpour. Production of Chitin and Chitosan from Shrimp Shell in BatchCulture of Lactobacillus plantarum. 2012. Chem. Biochem. Eng. Q. 26 (3) 217–223.
Okoye, F.C., G.S. Ojewola and K. Njoku-Onu. Evaluation of Shrimp Waste Meal as A Probable Animal Protein Source for Broiler Chicken. 2005. Int. J. Poult Sci., pp: 458-461.
Poeloengasihhernawan, Crescentiana D., Satriyo K.Wahono Suharto, M. Kismurtono. Optimization of Chitin Production from Penaeus monodon Shells at Ambient Temperature. Surabaya; 22 Dec. 2009, ISSN 2086-1931.
Ravichandran, S., Rameshkumar, G. Prince, A. R. Biochemical composition of shell and flesh of the Indian white shrimp (Penaeus indicus). 2009.American-Eurasian Journal of Scientific Research 4 (3), 191-194. Available
Sini, T. K., Santhosh, S., Mathew, P. T. Study on the production of chitin and chitosan from shrimp shell by using Bacillus subtilis fermentation. Carbohydrate Research. 2007
Nov 26; 342(16): 2423. Available from
Trung, Trang Si, Pham Thi Dan Phuong. Bioactive Compounds from By-Products of Shrimp Processing Industry in Vietnam. Journal of Food and Drug Analysis, Vol. 20,
17
Appendix A: Table of Result
Table 1. Result of ANOVA for Different Time
Table 2. Result of ANOVA for Different Temperature
Room Temperature (28oC)
19
Table 3. Result of Post Hoc Test for Different Time
1 hour
Means for groups in homogeneous subsets are displayed.
a. Uses Harmonic Mean Sample Size = 3.000.
Means for groups in homogeneous subsets are displayed.
a. Uses Harmonic Mean Sample Size = 3.000.
4 hours
Means for groups in homogeneous subsets are displayed.
a. Uses Harmonic Mean Sample Size = 3.000.
b. time = 4
Table 4. Result of Post Hoc Test for Different Temperature
Room Temperature (28oC)
yieldb
Means for groups in homogeneous subsets are displayed.
a. Uses Harmonic Mean Sample Size = 3.000.
21
Means for groups in homogeneous subsets are displayed.
a. Uses Harmonic Mean Sample Size = 3.000.
Means for groups in homogeneous subsets are displayed.
a. Uses Harmonic Mean Sample Size = 3.000.
Table 5. Result of % Calcium Extraction Yield
Condition time rep Wet dried sample
23
Appendix B: Documentation
Figure 1. Raw Shrimp Shell
Figure 2. Dried Shrimp Shell
Figure 3. Calcium Extraction
24
Appendix : Result of Atomic Absorption
25
The Result of Atomic Absorption Spectrocopy from
Calcium content in HCL Solution
Concentration Intensity
70 1 1 0.0625 25 0.102 4.23679 1695 169
70 1 1 0.0625 25 0.148 6.18182 2473 247
70 2 1 0.125 25 0.277 11.63636 2327 233
70 2 1 0.125 25 0.256 10.74841 2150 215
70 4 1 0.0625 25 0.147 6.13953 2456 246
70 4 1 0.0625 25 0.128 5.33615 2134 213
70 2 2 0.0625 25 0.136 5.67442 2270 227
70 2 2 0.0625 25 0.14 5.84355 2337 234
70 4 2 0.0625 25 0.14 5.84355 2337 234
70 4 2 0.0625 25 0.143 5.97040 2388 239
70 1 3 0.125 25 0.246 10.32558 2065 207