Fuel Cell Technology
Topics
1. A Very Brief History 2. Electrolysis
3. Fuel Cell Basics
- Electrolysis in Reverse - Thermodynamics
- Components
- Putting It Together 4. Types of Fuel Cells
- Alkali
- Molten Carbonate - Phosphoric Acid
- Proton Exchange Membrane - Solid Oxide
5. Benefits
6. Current Initiatives
- Automotive Industry
- Stationary Power Supply Units - Residential Power Units
A Very Brief History
Considered a curiosity in the 1800’s. The first fuel cell was built in 1839 by Sir William Grove, a lawyer and gentleman scientist.
Electrolysis
“What does this have to do with fuel cells?”
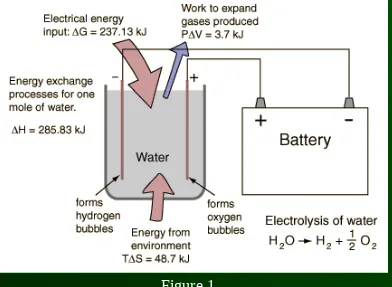
By providing energy from a battery, water (H2O) can be dissociated into the diatomic molecules of hydrogen (H2) and oxygen (O2).
Fuel Cell Basics
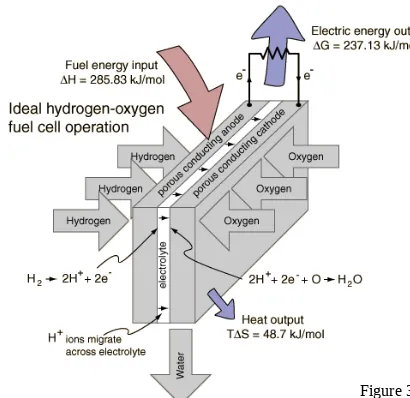
“Put electrolysis in reverse.”
fuel
The familiar process of electrolysis requires work to proceed, if the process is put in reverse, it should be able to do work for us
spontaneously.
The most basic “black box” representation of a fuel cell in action is shown below:
Fuel Cell Basics
Thermodynamics
H2(g) + ½O2(g) H2O(l)
Other gases in the fuel and air inputs (such as N2 and CO2) may be present, but as they are not involved in the electrochemical reaction, they do not need to be considered in the energy calculations.
69.91 J/mol·K
Table 1 Thermodynamic properties at 1Atm and 298K
Enthalpy is defined as the energy of a system plus the work needed to make room for it in an environment with constant pressure.
Fuel Cell Basics
Thermodynamics
Enthalpy of the chemical reaction using Hess’ Law:
ΔH = ΔHreaction = ΣHproducts – ΣHreactants = (1mol)(-285.83 kJ/mol) – (0)
= -285.83 kJ
Entropy of chemical reaction:
ΔS = ΔSreaction = ΣSproducts – ΣSreactants
= [(1mol)(69.91 J/mol·K)] – [(1mol)(130.68 J/mol·K) + (½mol)(205.14 J/mol·K)]
= -163.34 J/K
Heat gained by the system:
ΔQ = TΔS
= (298K)(-163.34 J/K)
Fuel Cell Basics
Thermodynamics
The Gibbs free energy is then calculated by: ΔG = ΔH – TΔS
= (-285.83 kJ) – (-48.7 kJ) = -237 kJ
The external work done on the reaction, assuming reversibility and constant temp. W = ΔG
The work done on the reaction by the environment is:
The heat transferred to the reaction by the environment is: W = ΔG = -237 kJ
ΔQ = TΔS = -48.7 kJ
Fuel Cell Basics
Components
Anode: Where the fuel reacts or "oxidizes", and releases electrons.
Cathode: Where oxygen (usually from the air) "reduction" occurs.
Electrolyte: A chemical compound that conducts ions from one electrode to the other inside a fuel cell.
Catalyst: A substance that causes or speeds a chemical reaction without itself being affected.
Cogeneration: The use of waste heat to generate electricity.
Harnessing otherwise wasted heat boosts the efficiency of power-generating systems.
Reformer: A device that extracts pure hydrogen from hydrocarbons.
Direct Fuel Cell: A type of fuel cell in which a hydrocarbon fuel is fed directly to the fuel cell stack, without requiring an external
Fuel Cell Basics
Putting it together.
Types of Fuel Cells
The five most common types: Alkali
Molten Carbonate Phosphoric Acid
Alkali Fuel Cell
compressed hydrogen and oxygen fuel
potassium hydroxide (KOH) electrolyte
~70% efficiency
150˚C - 200˚C operating temp. 300W to 5kW output
requires pure hydrogen fuel and platinum catylist → ($$) liquid filled container → corrosive leaks
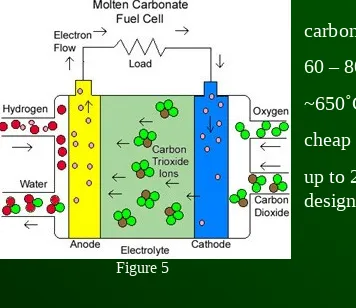
Molten Carbonate Fuel Cell (MCFC)
carbonate salt electrolyte
60 – 80% efficiency
~650˚C operating temp.
cheap nickel electrode catylist
up to 2 MW constructed, up to 100 MW designs exist
Figure 5
The operating temperature is too hot for many applications.
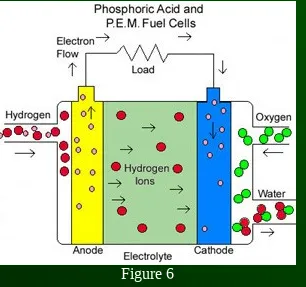
Phosphoric Acid Fuel Cell (PAFC)
phosphoric acid electrolyte
40 – 80% efficiency
150˚C - 200˚C operating temp
11 MW units have been tested
sulphur free gasoline can be used as a fuel
Figure 6
The electrolyte is very corrosive
Proton Exchange Membrane (PEM)
thin permeable polymer sheet electrolyte
40 – 50% efficiency
50 – 250 kW
80˚C operating temperature
electrolyte will not leak or crack
temperature good for home or vehicle use
platinum catalyst on both sides of membrane → $$
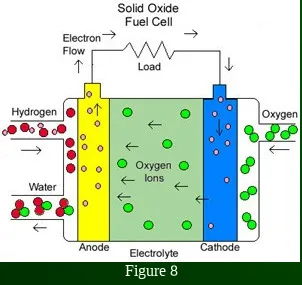
Solid Oxide Fuel Cell (SOFC)
hard ceramic oxide electrolyte
~60% efficient
~1000˚C operating temperature
cells output up to 100 kW
high temp / catalyst can extract the hydrogen from the fuel at the electrode
high temp allows for power generation using the heat, but limits use
SOFC units are very large
solid electrolyte won’t leak, but can crack
Benefits
Efficient: in theory and in practice
Portable: modular units
Reliable: few moving parts to wear out or break
Fuel Flexible: With the a fuel reformer fuels such as natural gas, ethanol, methanol, propane, gasoline, diesel, landfill gas,
wastewater treatment digester gas, or even ammonia can
be used
Environmental: produces heat and water (less than combustion in both cases) near zero emission of CO and NOx
Current Initiatives
Automotive Industry
Most of the major auto manufacturers have fuel cell vehicle (FCV) projects
currently under way, which involve all sorts of fuel cells and hybrid combinations of conventional combustion, fuel reformers and battery power.
Considered to be the first gasoline powered fuel cell vehicle is the H20 by GM:
GMC S-10 (2001)
fuel cell battery hybrid low sulfur gasoline fuel 25 kW PEM
40 mpg
112 km/h top speed
Fords Adavanced Focus FCV (2002) fuel cell battery hybrid
85 kW PEM
~50 mpg (equivalent)
4 kg of compressed H2 @ 5000 psi
Approximately 40 fleet vehicles are planned as a market introduction for Germany, Vancouver and California for 2004.
Current Initiatives
Automotive Industry
Figure 10
Daimler-Chrysler NECAR 5 (introduced in 2000)
85 kW PEM fuel cell
methanol fuel
reformer required
150 km/h top speed
version 5.2 of this model completed a California to Washington DC drive awarded road permit for Japanese roads
Current Initiatives
Automotive Industry
Mitsubishi Grandis FCV minivan
fuel cell / battery hybrid
68 kW PEM
compressed hydrogen fuel
140 km/h top speed
Plans are to launch as a production vehicle for Europe in 2004.
Current Initiatives
Automotive Industry
Current Initiatives
Stationary Power Supply Units
A fuel cell installed at McDonald’s restaurant, Long Island Power Authority to install 45 more fuel cells across Long Island, including homes.(2) Feb 26, 2003
More than 2500 stationary fuel cell systems have been installed all over the world - in hospitals, nursing homes, hotels, office buildings, schools, utility power plants, and an airport terminal, providing primary power or backup. In large-scale building systems, fuel cells can reduce facility energy service costs by 20% to 40% over conventional energy service.
Current Initiatives
Residential Power Units
There are few residential fuel cell power units on the market but many designs are undergoing testing and should be available within the next few years. The major technical difficulty in producing residential fuel cells is that they must be safe to install in a home, and be easily maintained by the average homeowner.
Residential fuel cells are typically the size of a large deep freezer or furnace, such as the Plug Power 7000 unit shown here, and cost $5000 - $10 000.
If a power company was to install a residential fuel cell power unit in a home, it would have to charge the homeowner at least 40 ¢/kWh to be economically
profitable.(3) They will have to remain a backup power supply for the near future.
Future
“...projections made by car companies themselves and energy and automotive experts concur that around 2010, and perhaps earlier, car manufacturers will have mass production capabilities for fuel cell vehicles, signifying the time they would be economically available to the average consumer.” Auto Companies on Fuel Cells, Brian Walsh and Peter Moores, posted on www.fuelcells.org
Technical and engineering innovations are continually lowering the capital cost of a fuel cell unit as well as the operating costs, but it is expected that mass
production will be of the greatest impact to affordability.
A commercially available fuel cell power plant would cost about $3000/kW, but would have to drop below $1500/kW to achieve widespread market penetration.
Future
internal combustion obsolete?
solve pollution problems?
common in homes?
better designs?
higher efficiencies?
cheaper electricity?
reduced petroleum dependency?
References
(1) FAQ section, fuelcells.org
(2) Long Island Power Authority press release: Plug Power Fuel Cell Installed at McDonald’s Restaurant, LIPA to Install 45 More Fuel Cells Across Long Island, Including Homes,
http://www.lipower.org/newscenter/pr/2003/feb26.fuelcell.html
(3) Proceedings of the 2000 DOE Hydrogen Program Review: Analysis of Residential Fuel Cell Systems & PNGV Fuel Cell Vehicles, http://www.eere.energy.gov/hydrogenandfuelcells/pdfs/28890mm.pdf
Figures
1, 3 http://hyperphysics.phy-astr.gsu.edu/hbase/thermo/electrol.html 4 – 8 http://fuelcells.si.edu/basics.htm
10 http://www.moteurnature.com/zvisu/2003/focus_fcv/focus_fcv.jpg
11 http://www.granitestatecleancities.org/images/Hydrogen_Fuel_Cell_Engine.jpg 12 http://www.in.gr/auto/parousiaseis/foto_big/Necar07_2883.jpg
13 http://www3.caradisiac.com/media/images/le_mag/mag138/oeil_mitsubishi_grandis_big.jpg 14 http://www.lipower.org/newscenter/pr/2003/feb26.fuelcell.html
15 http://americanhistory.si.edu/csr/fuelcells/images/plugpwr1.jpg
Table 1 http://hyperphysics.phy-astr.gsu.edu/hbase/tables/therprop.html#c1
Fuel cell data from: Types of Fuel Cells, fuelcells.org