Summary A mixture of tritiated and deuterated gibberellin A4 (GA4) was injected into the xylem of Norway spruce (Picea
abies (L.) Karst) propagules, below an elongating shoot, or
applied directly on the needles of an elongating shoot. The distribution of [2H
2]GA4 and [3H]GA4 in the needles, stems and buds was determined after 4, 12 and 24 h. After 4 h, most of the xylem-injected GA4 was found in the needles, whereas after 24 h, most of the GA4 was found in the stem, with a small portion in the lateral buds. Of the GA4 applied to the needles, 51% of the radioactivity recovered after 24 h was found in the stem and 2% in the lateral buds. Mixtures of tritiated and deuterated GA4 and GA9 were injected into elongating shoots of one abundant-flowering family and one limited-flowering family, grown either under conditions inductive for flowering (hot and dry, HD) or under noninductive conditions for flow-ering (cool and wet, CW). Shoots of both CW-and HD-treated propagules converted [2H
2]GA9 to [2H2]GA51, [2H2]GA4, [2H
2]GA34 and [2H2]GA1, whereas [2H2]GA4 was converted to [2H
2]GA34, [2H2]GA1 and [2H2]GA8. In shoots of both CW-treated clones, the main metabolite of [3H]GA
9 was in the GA51 region. The HD-treated propagules converted more [3H]GA
9 to putative GA4 than the CW-treated propagules. The main meta-bolite of [3H]GA
4 was in the GA34 region. Radioactive meta-bolites were also found in the GA1 and GA8 regions.
Keywords: cone bud production, deuterium, flowering, gas chromatography--mass spectrometry, high-performance liquid chromatography, metabolism, transport, tritium.
Introduction
The growth pattern of mature temperate-zone conifers is fixed or determined; that is, the initiation of a bud and cell division and differentiation therein, are separated in time, usually by a winter season, from cell elongation (see Cannell et al. 1976). The initiation of new buds starts during elongation of the preformed shoot. In the Pinaceae family, the formation of cone buds or strobili can be induced by specific gibberellins (GAs) (Pharis et al. 1986), mainly the less polar GA4 and GA7, in combination with stress treatments, such as controlled drought
and heat (Philipson 1983, Bonnet-Masimbert and Zaerr 1987, Pharis et al. 1987).
Both endogenous and exogenously applied GAs are con-tinuously transported and metabolized in plant cells. Because these processes affect the availability of a specific GA in a particular compartment per unit time, they influence or deter-mine the eventual differentiation of the bud primordia. It is therefore important to determine where the GAs are synthe-sized, how they are transported and how physiologically active GAs and their immediate precursors are metabolized.
The major GAs of Norway spruce (Picea abies (L.) Karst.) are GA9, GA4, GA1 and GA3 (Odén et al. 1982, 1987). Meta-bolic studies of GAs in Picea sitchensis (Bong.) Carr. have
shown that [2H
2]GA9 is converted to [2H2]GA4 and that [2H
2]GA4 is converted to [2H2]GA1 and [2H2]GA34 (Moritz et al. 1989). In P. abies, [2H
2]GA9 is converted to [2H2]GA4, [2H
2]GA51 and a deuterated cellulase-hydrolyzable GA9 -con-jugate (Mortiz and Odén 1990).
We investigated the transport of exogenously applied deu-terated and tritiated GA4 and the metabolism of exogenously applied deuterated and tritiated GA9 and GA4 in shoots of abundant- and limited-flowering clones of P. abies grown under conditions favorable for flowering (hot and dry) and under conditions unfavorable for flowering (cool and wet).
Materials and methods
Plant material
Five-year-old, container-grown P. abies grafted propagules, about 1.5 m high, from about 60-year-old ortets, were used in the experiments. One clone (G2024, Holkastorp, latitude 57°05′, longitude 14°47′, altitude 260 m a.s.l.) was categorized as abundant flowering. Another clone (F2100, Markarmåla, latitude 57°56′, longitude 14°58′, altitude 275 m a.s.l.) was categorized as limited flowering. On May 10, 1991, half of the trees (16 ramets) were placed in a greenhouse providing a computer-controlled day/night temperature of 30/25 °C. Drought stress was monitored by measuring needle water potential (Ψ) with a Scholander pressure chamber.
Drought-Transport and metabolism of gibberellins in relation to flower bud
differentiation in Norway spruce (
Picea abies
)
PER CHRISTER ODÉN,
1QING WANG,
2KARL-ANDERS HÖGBERG
3and MARTIN
WERNER
31 Department of Silviculture, Swedish University of Agricultural Sciences, S-901 83 Umeå, Sweden
2 Department of Forest Genetics and Plant Physiology, Swedish University of Agricultural Sciences, S-901 83 Umeå, Sweden
3 Forest Research Institute, Ekebo, S-268 00 Svalöv, Sweden
Received February 22, 1994
treated propagules were watered when the mean value of needle Ψ reached −2 MPa (HD-treatment). The remaining trees were placed outside and watered daily (CW-treatment).
Application and injection of labeled GAs
The tritiated GAs were [2,3-3H]GA
9, 1.7 PBq mol−1 (Dr. Alan Crozier, Glasgow University, Glasgow, U.K.) and [1,2-3H]GA
4, 1.4 PBq mol−1 (Amersham, U.K.). The tritiated GAs were purified by reverse-phase high-performance liquid chro-matography (HPLC) before use. The deuterated GAs were [17,17-2H]GA
9 and [17,17-2H]GA4 (Prof. Lewis N. Mander, Research School of Chemistry, Australian National University, Canberra, Australia).
Transport experiment Ten µl of a solution of 17 kBq [3H]GA 4 plus 1 µg [2H
2]GA4 (about 3 nmol) per µl ethanol was either injected into the pith--xylem below the expanding terminal shoot on the second whorl of branches, or evenly applied with a microsyringe to the needles on a similar expanding shoot. Three shoots were treated on each of two grafted propagules of each HD-treated clone per mode of application (shoot injec-tion or leaf applicainjec-tion). One shoot per propagule was har-vested after 4, 12 and 24 h, separated into needles, stem and lateral buds, and each part frozen in liquid N2 and stored at
−80 °C until analyzed. Applications were made when the shoots had elongated to 99% of their total length, i.e., slightly before the onset of bud initiation.
Metabolism experiment Solutions of 17 kBq [3H]GA 9 plus 1 µg [2H
2]GA9 (about 3 nmol) per µl ethanol and 17 kBq [3H]GA
4 plus 1 µg [2H2]GA4 were used in the metabolism experiments. Two liters of each GA solution was injected into the pith--xylem immediately below two elongating terminal shoots on the second whorl of branches on two grafted propagules of the abundant-flowering clone and two grafted propagules of the limited-flowering clone. The shoots were harvested after 24 h, frozen in liquid N2 and stored at −80 °C until analyzed. Applications were made when the shoots had elongated to about 99% of their final length.
Extraction and purification
The shoots from the transport experiment were divided into needles, stem and lateral buds, whereas shoots from the meta-bolism experiment were frozen intact. The material from the two replicate propagules was combined and homogenized in 100% methanol (MeOH) at 4 °C, 20 ml per g fresh weight (gFW), containing 0.02% (w/v) dithiocarbamic acid as antioxi-dant. After extraction for 2 h at 4 °C, the MeOH was filtered off and the tissue debris washed with another 50 ml of MeOH. The pooled filtrates were evaporated to water phase under reduced pressure at 35 °C. The aqueous residue was adjusted to about 5 ml with 0.1 M sodium phosphate buffer, pH 8.0, and applied to a 150 × 10 mm i.d. column packed with poly-N -vinylpolypyrrolidone (PVPP). The column was eluted with 100 ml of 0.1 M sodium phosphate buffer, pH 8.0. The buffer phase was adjusted to pH 3.0 and extracted with 3 × 50 ml of ethylacetate (EtOAc). The water was removed from the com-bined EtOAc-phase by freezing and filtering, and the EtOAc
was finally evaporated to dryness under reduced pressure at 35 °C. The samples were further purified by HPLC.
High-performance liquid chromatography
The HPLC system consisted of two LKB 2150 pumps con-nected to the column by a Rheodyne injector. The pumps were controlled by an LKB 2152 gradient controller. All samples were first purified by reverse-phase HPLC on a column, 200 × 4.6 mm i.d., packed with 5 µm Nucleosil C18. The mobile phase was a linear gradient from 100% water with 1% acetic acid to 100% MeOH with 1% acetic acid. The gradient sweep time was 60 min, and the flow rate was 1 ml min−1. Sixty 1-ml fractions were collected, and radioactivity in 0.1 ml of each fraction was determined by liquid scintillation spectrometry.
Radioactivity of the fractions from the transport experiment corresponding to the elution volume of GA4 was determined by liquid scintillation spectrometry, and the content of [2H
2]GA4 of the same fractions was determined by gas chro-matography--mass spectrometry (GC--MS).
The radioactive fractions from the metabolism experiment were pooled and further purified by normal-phase HPLC using a column, 200 × 4.6 mm i.d., packed with 5 µm Nucleosil NO2. The mobile phase was a linear gradient from 75% n-heptane,
half saturated with 0.5 M formic acid in water, and 25% EtOAc/water/formic acid (98.5/1/0.5, v/v) to 100% EtOAc/ water/formic acid (98.5/1/0.5, v/v). The gradient sweep time was 60 min, and the flow rate was 2 ml min−1. Sixty 2-ml fractions were collected, and radioactivity in 0.2 ml of each fraction was determined by liquid scintillation spectrometry. Fractions belonging to each peak were combined and analyzed by GC--MS.
Identification and quantitation of metabolites by GC--MS
Radioactive fractions were methylated with ethereal dia-zomethane and, after evaporation, were trimethylsilylated by adding 50 µl of N-methyl-N-trimethylsilytrifluoroacetamid.
The mixtures were heated to 80 °C for 20 min and, after evaporation, dissolved in n-heptane. The samples were then injected splitless into a Hewlett Packard 5890 GC equipped with a fused silica glass capillary column, SE-30 chemical bonded phase 0.25 µm, 25 m long, i.d. 0.25 mm (Quadrex). The injector temperature was 230 °C, and the column tempera-ture was 80 °C for the first minute. Subsequently the column temperature was increased by 20 °C min−1 to 200 °C, and then by 4 °C min−1 to 250 °C. The column effluent was led into the ion source of a Hewlett Packard 5970 mass selective detector with an interface temperature of 275 °C. The electron energy was 70 eV. The electron impact spectra were recorded and processed by a Hewlett Packard 9133 data system. The amounts of [2H
2]GA4 transported in the plants were monitored by selected ion monitoring (SIM) at m/z 420 and 286. The metabolites of exogenously applied [2H
2]GA9 and [2H2]GA4 were identified by full-scan GC--MS, scanning between m/z 80 and 650. Each sample was also co-injected with a series of
nondeuterated standards supplied by Prof. Lewis N. Mander, Canberra, Australia, and Dr. Peter Hedden, Bristol, U.K.
Results
Transport of tritiated and deuterated GA4
When tritiated plus deuterated GA4 was injected into the pith--xylem immediately below elongating shoots of Norway spruce, the radioactivity in the GA4 fraction and in [2H2]GA4 was transported to the stem and needles above the application point (Table 1). The amount of radioactivity decreased with time in the needles, but increased in the stem and lateral buds. After 24 h, 18% of the total recovered radioactivity in the GA4 fraction was detected in the needles, 77% in the stem and 5% in the lateral buds. The concentrations of [2H
2]GA4 in stem and bud tissues on a fresh weight basis after 24 h were 1.8 and 1.4 µg gFW−1, respectively. The concentration of [2H2]GA4 in the needles decreased from 0.6 µg gFW−1 after 4 h to 0.2 µg gFW−1 after 24 h.
When the tritiated plus deuterated GA4 solution was applied directly to the needles of the elongating shoot, the radioactivity in the GA4 fraction and in [2H2]GA4 decreased in the needles over the 24-h period. During the same time, the corresponding GAs increased in all bud and stem samples except the 24-h stem sample (Table 1). Injection of GA4 into the pith--xylem resulted in higher concentrations of [2H
2]GA4 in the stem and buds than direct application of GA4 to the needles. It is possible that the addition of a surfactant would have enhanced the uptake of GA4 solution from the needle surface.
The amount of radioactivity in the fraction corresponding in retention time with the standard GA4 fraction and the concen-tration of [2H
2]GA4 were not corrected for metabolism or other losses during the extraction and purification procedures; how-ever, we estimate that, in the 24-h samples, these losses were from 50 to 75%.
Metabolism of [3H]GA9 + [2H2]GA9 and [3H]GA4 +
[2H2]GA4 in shoots of CW-and HD-treated propagules
Shoots that had elongated to about 99% of their final length converted tritiated and deuterated GA9 and GA4 to several
metabolites. Some of these were identified by GC--MS and others were traced by radioactivity. Four radioactivity profiles after normal-phase HPLC of samples from shoots containing [3H]GA
9-and [3H]GA4-metabolites are shown in Figures 1 and 2. Shoots of both CW-and HD-treated propagules of the abun-dant-flowering clone converted [2H
2]GA9 to [2H2]GA51, [2H
2]GA4, [2H2]GA34 and [2H2]GA1. The [2H2]GA34 compound was traced by its deuterium because both tritium ions were lost during 2β- and 3β-hydroxylations. Metabolites were identified by comparing the relative intensities of several ions and
reten-Table 1. Recovery of tritiated and deuterated GA4 in needles, stem and lateral buds of Norway spruce shoots after xylem injection or needle application of 170 kBq [3H]GA4 and 10 µg [2H2]GA4. Assessments were made on the HPLC fraction eluting at the retention time of GA4. Results are presented as percent of total radioactivity recovered and as µg per gFW.
Treatment Needles Stem Lateral buds
[3H]GA4 [2H2]GA41 [3H]GA4 [2H2]GA41 [3H]GA4 [2H2]GA41 % of total µg gFW−1 % of total µg gFW−1 % of total µg gFW−1
Xylem injection after 4h 57 0.6 42 0.8 1 0.2
Xylem injection after 12 h 32 0.3 66 1.3 2 0.4
Xylem injection after 24h 18 0.2 77 1.8 5 1.4
Needle application after 4 h 85 1.6 15 0.2 0 0
Needle application after 12 h 55 1.2 44 1.5 1 0.4
Needle application after 24 h 47 0.9 51 1.1 2 0.8
1 Quantitations based on m/z peak areas relative to co-injected GA
4 standards.
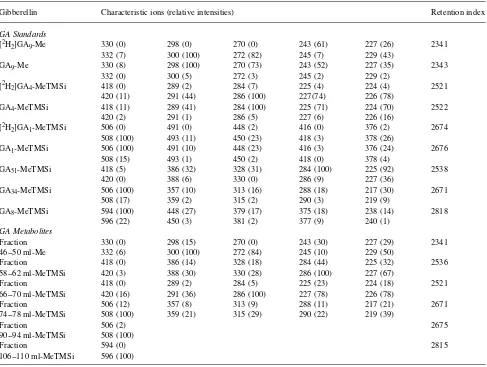
tion characteristics of compounds in the sample fractions with known GA standards (Table 2). In shoots of both CW-and HD-treated propagules, [2H
2]GA4 was converted to [2H2]GA34, [2H
2]GA1 and [2H2]GA8 (Table 2).
We attempted to quantify the rates of conversion by calcu-lating the amount of radioactivity in each of the identified GA regions and relating this amount to the total amount of radio-activity recovered after normal-phase HPLC (Table 3). The main metabolite of [3H]GA
9 in shoots of both CW-treated clones was in the GA51 region, but radioactivity was also detected in the GA4 and GA1 regions. The HD-treated propa-gules of both clones converted [3H]GA
9 to radioactive metabo-lites in the same manner; however, the amount of putative [3H]GA
4 was higher in the shoots of HD-treated propagules than in the shoots of CW-treated propagules.
The main metabolite of [3H]GA
4 was usually in the GA34 region. Radioactive metabolites were also found in the GA8 and GA1 regions, and there was slightly more putative [3H]GA1 in the CW-treated propagules than in the HD-treated propagules. Total metabolism was slower in shoots of CW-treated propagules than in shoots of HD-CW-treated propagules, as indicated by the higher proportion of radioactivity in the region of the [3H]GA
9 or [3H]GA4 precursor. Another indication of higher metabolic rates in shoots of HD-treated propagules than in shoots of CW-treated propagules was the slightly higher proportion of unknown metabolites in the HD-treated propagules.
Unknown amounts of both radiolabeled and deuterium-la-beled metabolites were discarded during the purification be-cause only the acidic EtOAc fractions were analyzed.
Discussion
When labeled GA4 was injected into the pith--xylem below the elongating shoot, it was transported in the transpiration stream upward to the needles. From the needles, transport was di-rected toward the adjacent stem and lateral buds, as indicated by the finding that needle-applied labeled GA4 was rapidly transported to the adjacent stem and lateral buds. The absolute amounts of labeled GA4 in the various tissues may also reflect differences in metabolic rates. After 24 h, more than 50% of the labeled GA4 was metabolized to other compounds. Unfor-tunately, a suitable internal standard (e.g., [2H
5]GA4) for cor-rection of losses during the extraction and purification procedure was not available.
The most effective GAs for promoting flowering and shoot elongation in conifers belonging to the Pinaceae are GA4 and GA7. An additional OH-group in position 13, as in GA1 and GA3, blocks florigenic activity. Compound GA9, which lacks the 13-OH group, is probably active because of its enzymatic conversion to GA4, which catalyzed by a 3β-hydroxylase.
Compound [2H
2]GA9 was converted to [2H2]GA51, [2H
2]GA4, [2H2]GA34 and [2H2]GA1 (Table 2). Part of this metabolic pathway has been established for P. abies (Moritz
and Odén 1990). The 2β-hydroxylase catalyzed formation of GA51 and GA34 are catabolic steps, yielding physiologically inactive compounds (Graebe 1987). The formation of [3H]GA
51 from [2,3-3H]GA34 resulted in loss of half the trit-ium, and the formation of [3H]GA
34 resulted in a complete loss of tritium. It was therefore not possible to detect GA34 by determining the radioactivity (Figure 1), but it was detected by GC--MS of [2H
2]GA34. The loss of [3H] from [2,3-3H]GA9 confirmed that the stereochemistry is β and that there is little or no nonspecific labeling with [3H] elsewhere in the molecule.
Compound [2H
2]GA4 was converted to [2H2]GA34, [2H2]GA1 and [2H
2]GA8 (Table 2). The formation of the physiologically inactive GA34 involves a 2β-hydroxylation of GA4, the forma-tion of GA1 involves a C-13-hydroxylation, and a further 2β-hydroxylation of GA1 yields the inactive GA8. Neither [2H
2]GA7 nor [2H2]GA3 was detected by GC--MS. Analogous to the loss of tritium from [2,3-3H]GA
9 during metabolism, half of the tritium was lost when [2,3-3H]GA
4 was converted to [3H]GA
34 and [3H]GA8.
An attempt to quantify the rates of metabolism of [3H]GA 9 and [3H]GA
4 in an abundant- and a limited-flowering clone each subjected to favorable (HD) and unfavorable (CW) flow-ering conditions resulted in some consistent differences; how-ever, the quantitations are based on radioactivity of unknown compounds in fractions corresponding to the elution volumes of various GAs. In shoots of HD-treated propagules, [3H]GA
9 was 3β-hydroxylated to a greater extent than [3H]GA
4. Con-versely, in shoots of CW-treated propagules, [3H]GA
9 was 2β-hydroxylated to [3H]GA
51 to a greater extent than in HD-treated propagules. The amount of [3H]GA
1, the 13-hydroxy-Figure 2. Radioactivity profile after normal-phase HPLC of [3H]GA4
lation product of [3H]GA
4, was approximately the same in both HD-and CW-treated propagules. However, when related to the total amount of [3H]GA
4, it was more rapidly 13-hydroxylated
to [3H]GA
1 in the CW-treated plants than in the HD-treated plants. The CW-treated propagules also 2β-hydroxylated more [3H]GA
4 to [3H]GA34 than the HD-treated propagules. The Table 2. GC--MS Analyses, full-scan mode, scanning between m/z 80 to 650, of deuterated and nondeuterated GA standards and of metabolites in Norway spruce after injections of [2H2]GA9 and [2H2]GA4.
Gibberellin Characteristic ions (relative intensities) Retention index
GA Standards
[2H2]GA9-Me 330 (0) 298 (0) 270 (0) 243 (61) 227 (26) 2341
332 (7) 300 (100) 272 (82) 245 (7) 229 (43)
GA9-Me 330 (8) 298 (100) 270 (73) 243 (52) 227 (35) 2343
332 (0) 300 (5) 272 (3) 245 (2) 229 (2)
[2H2]GA4-MeTMSi 418 (0) 289 (2) 284 (7) 225 (4) 224 (4) 2521
420 (11) 291 (44) 286 (100) 227(74) 226 (78)
GA4-MeTMSi 418 (11) 289 (41) 284 (100) 225 (71) 224 (70) 2522
420 (2) 291 (1) 286 (5) 227 (6) 226 (16)
[2H2]GA1-MeTMSi 506 (0) 491 (0) 448 (2) 416 (0) 376 (2) 2674
508 (100) 493 (11) 450 (23) 418 (3) 378 (26)
GA1-MeTMSi 506 (100) 491 (10) 448 (23) 416 (3) 376 (24) 2676
508 (15) 493 (1) 450 (2) 418 (0) 378 (4)
GA51-MeTMSi 418 (5) 386 (32) 328 (31) 284 (100) 225 (92) 2538
420 (0) 388 (6) 330 (0) 286 (9) 227 (36)
GA34-MeTMSi 506 (100) 357 (10) 313 (16) 288 (18) 217 (30) 2671
508 (17) 359 (2) 315 (2) 290 (3) 219 (9)
GA8-MeTMSi 594 (100) 448 (27) 379 (17) 375 (18) 238 (14) 2818
596 (22) 450 (3) 381 (2) 377 (9) 240 (1)
GA Metabolites
Fraction 330 (0) 298 (15) 270 (0) 243 (30) 227 (29) 2341
46--50 ml-Me 332 (6) 300 (100) 272 (84) 245 (10) 229 (50)
Fraction 418 (0) 386 (14) 328 (18) 284 (44) 225 (32) 2536
58--62 ml-MeTMSi 420 (3) 388 (30) 330 (28) 286 (100) 227 (67)
Fraction 418 (0) 289 (2) 284 (5) 225 (23) 224 (18) 2521
66--70 ml-MeTMSi 420 (16) 291 (36) 286 (100) 227 (78) 226 (78)
Fraction 506 (12) 357 (8) 313 (9) 288 (11) 217 (21) 2671
74--78 ml-MeTMSi 508 (100) 359 (21) 315 (29) 290 (22) 219 (39)
Fraction 506 (2) 2675
90--94 ml-MeTMSi 508 (100)
Fraction 594 (0) 2815
106--110 ml-MeTMSi 596 (100)
Table 3. Quantitative metabolism of [3H]GA9 and [3H]GA4 in shoots from two clones of Norway spruce that had reached 99% of total shoot elongation. Clones were grown under either cool and wet (CW) or hot and dry (HD) conditions, and each of two shoots per grafted propagule were injected in the xylem with either 34 kBq of [3H]GA
9 or 34 Bq of [3H]GA4. Results are expressed as % of total recovered radioactivity.
Precursor Metabolite Abundant-flowering clone (G2024) Limited-flowering clone (F2100)
CW (%) HD (%) CW (%) HD (%)
GA9 GA9 57.1 51.3 55.9 50.3
GA9 GA511 9.9 7.1 11.3 9.7
GA9 GA41 6.7 15.2 6.2 10.5
GA9 GA11 2.3 2.1 3.0 3.2
Known metabolites 76.0 75.7 76.6 73.7
GA4 GA4 54.3 50.2 55.2 48.8
GA4 GA341 14.2 13.3 11.5 7.7
GA4 GA1 8.1 5.7 10.2 9.6
GA4 GA81 2.8 2.1 3.5 1.8
Known metabolites 79.4 71.3 80.4 67.9
amount of radioactivity in the [3H]GA
1 region was thus slightly higher in CD-treated shoots than in HD-treated shoots.
The HD-treated shoots metabolized GA9 and GA4 more rapidly than the CW-treated shoots; however, the CW-treated shoots had a more efficient 2β-hydroxylase system, yielding higher amounts of inactive GA51 and GA34 than the HD-treated shoots. Cultural treatments or environmental stresses known to increase flowering in Pinaceae conifers may thus act by alter-ing the quantities and metabolic turnover of GA4 (Pharis and Ross 1986, Pharis et al. 1987) and possibly GA7 (Pharis et al. 1987). The hypothesis that imposed stress decreases shoot elongation, a strong sink for GAs, resulting in an accumulation of less polar GAs (GA4, GA7 and GA9), thereby stimulating flowering, was first questioned by Ross (1991a), who found
that enhanced flowering of root-pruned Picea glauca
(Moench) Voss propagules was not correlated with decreased shoot elongation. Based on our findings, we conclude that the availability of active GAs is deliberately regulated in the spe-cific organ compartments of the shoot, and that their metabo-lism is directly influenced by various factors including root activity, stomatal turgor (e.g., tissue Ψ) and temperature (Moritz and Odén 1990, Ross 1991b).
References
Bonnet-Masimbert, M. and J.B. Zaerr. 1987. The role of plant growth regulators in promotion of flowering. Plant Growth Regul. 6:13--35. Cannell, M.G.R., S. Thompson and R. Lines. 1976. An analysis of inherent differences in shoot growth within some north temperate conifers. In Tree Physiology and Yield Improvement. Eds. M.G.R.
Cannell and F.T. Last. Academic Press, London, pp 173--205. Gaskin, P., J. MacMillan, R.D. Firn and R.D. Pryce. 1971. ‘‘Parafilm:’’
a convenient source of n-alkane standards for determination of gas chromatographic retention indices. Phytochemistry 10:1155--1157. Graebe, J.E. 1987. Gibberellin biosynthesis and control. Annu. Rev.
Plant Physiol. 38:419--465.
Kovats, E. 1958. Gas-chromatographische Charakterisierung organis-cher Verbindungen. Teil 1: Retentions indices aliphatisorganis-cher halo-genide, alkohole, aldehyde und ketone. Helv. Chim. Acta 41:1915--1932.
Moritz, T. and P.C. Odén. 1990. Metabolism of tritiated and deuterated gibberellin A9 in Norway spruce (Picea abies) shoots during the period of flower bud differentiation. Physiol. Plant. 79:242--249. Moritz, T., J.J. Philipson and P.C. Odén. 1989. Metabolism of tritiated
and deuterated gibberellins in Sitka spruce (Picea sitchensis) during
the period of flower bud differentiation. Physiol. Plant. 77:39--45. Odén, P.C., B. Andersson and R. Gref. 1982. Identification of
gibberel-lin A9 in extracts of Norway spruce (Picea abies (L.) Karst.) by
combined gas chromatography--mass spectrometry. J. Chromatogr. 247:133--140.
Odén, P.C., L. Schwenen and J.E. Graebe. 1987. Identification of gibberellins in Norway spruce (Picea abies (L.) Karst.) by
com-bined gas chromatography--mass spectrometry. Plant Physiol. 84:516--519.
Pharis, R.P. and S.D. Ross. 1986. Hormonal promotion of flowering in Pinaceae family conifers. In Handbook of Flowering, Vol. 5. Ed. A. Halevy. CRC Press, Boca Raton, FL, pp 269--286.
Pharis, R.P., D. Tomchuk, F.D. Beall, R.M. Rauter and G. Kiss. 1986. Promotion of flowering in white spruce (Picea glauca) by
gibberel-lin A4/7, auxin (naphthaleneacetic acid), and the adjunct cultural treatments of girdling and Ca(NO3)2 fertilization. Can. J. For. Res. 16:340--345.
Pharis, R.P., J.E. Webber and S.D. Ross. 1987. The promotion of flowering in forest trees by gibberellin A4/7 and cultural treatments: a review of the possible mechanisms. For. Ecol. Manage. 19:65--84. Philipson, J.J. 1983. The role of gibberellin A4/7, heat and drought in the induction of flowering in Sitka spruce. J. Exp. Bot. 34:291--301. Ross, S.D. 1991a. Effect of heat sums and of heat applied separately to shoots and roots on flowering in potted Picea glauca grafts. Can. J. For. Res. 21:672--679.
![Table 1. Recovery of tritiated and deuterated GA4application of 170 kBq [are presented as percent of total radioactivity recovered and as in needles, stem and lateral buds of Norway spruce shoots after xylem injection or needle3H]GA4 and 10 µg [2H2]GA4](https://thumb-ap.123doks.com/thumbv2/123dok/1015368.923752/3.612.321.485.213.487/recovery-tritiated-deuterated-application-presented-radioactivity-recovered-injection.webp)
![Figure 2. Radioactivity profile after normal-phase HPLC of [3H]GA4-metabolites in shoots of Norway spruce propagules of an abundant-flowering clone (G2024)](https://thumb-ap.123doks.com/thumbv2/123dok/1015368.923752/4.612.56.220.72.343/figure-radioactivity-profile-metabolites-norway-propagules-abundant-flowering.webp)