33 Ohkawa, H. et al. (1998) Molecular mechanisms of herbicide resistance. Rev. Toxicol. 2, 245–252
34 Inui, H. et al. (1999) Herbicide metabolism and cross-tolerance in transgenic potato plants expressing human CYP1A1. Pestic. Biochem. Physiol. 64, 33–46 35 Lacour, T. and Ohkawa, H. (1999) Engineering and biochemical
characterization of the rat microsomal cytochrome P450 to ferredoxin-NADP(1) reductase from plant chloroplasts. Biochim. Biophys. Acta 1433, 87–102
36 Pierrel, M.A. et al. (1994) Catalytic properties of the plant cytochrome P450 CYP73 expressed in yeast. Substrate specificity of a cinnamate hydroxylase. Eur. J. Biochem. 224, 835–844
37 Cabello-Hurtado, F. et al. (1998) Cloning, expression in yeast and functional characterization of CYP81B1, a plant P450 which catalyses in-chain hydroxylation of fatty acids. J. Biol. Chem. 273, 7260–7267
38 Robineau, T. et al. (1998) The chemically inducible plant cytochrome P450 CYP76B1 actively metabolizes phenylurea and other xenobiotics. Plant Physiol. 118, 1049–1056
39 Siminsky, B. et al. (1999) Expression of a soybean cytochrome P450 monooxygenase cDNA in yeast and tobacco enhances the metabolism of phenylurea herbicides. Proc. Natl. Acad. Sci. U. S. A. 96, 1750–1755
R
oots, rhizomes and other plant organs in the soil usually obtain O2 for respiration directly from their immediateenvironment – the soil gaseous atmosphere. But when the soil becomes excessively wet, transfer of O2 from the air into the
soil is effectively blocked because the larger soil pores, which are usually air-filled, are water-filled instead. Without replenishment from the air, any dissolved O2remaining in the soil is quickly
con-sumed by microorganisms and plants, and the soil is then no longer able to supply O2. Aerenchyma – tissue comprising a high
proportion of gas-filled spaces or lacunae – provides the plant with an alternative strategy for obtaining O2. The interconnected
lacunae, extending from below the ground up into the stems and leaves, make up an internal aeration system, enabling parts of the plant to survive or grow for a time in environments that are O2-deficient or even completely devoid of O2.
Spaces form within aerenchymatous organs, either by cell separation at the middle lamella during development (shizogeny), or by cell death and dissolution (lysigeny). Sometimes, as in Sagittaria lancifolia, both processes occur, with lysigenous aerenchyma in the roots and shizogenous aerenchyma in the leaf petiole1. In lysigenous aerenchyma formation in the root
cortex of maize, rice and Sagittaria, cell death is first detected at a distance of a cm or less from the root apical meristem, in the zone where cell elongation has just been completed2–4
. The space created by the death of cells becomes increasingly promi-nent in older zones behind the tip. The system of gas-filled lacunae therefore develops acropetally, extending into the root towards the tip, simultaneous with the root’s extension into the soil.
Aerenchyma function
Lysigenous aerenchyma provides not only an internal pathway for O2transfer, but also simultaneously reduces the number of O2
-consuming cells, a feature that might assist in low O2
environ-ments. It has been suggested that aerenchymatous organs are often water- or fluid-filled5
, a feature that would prevent them from functioning in O2transfer. However, evidence from the
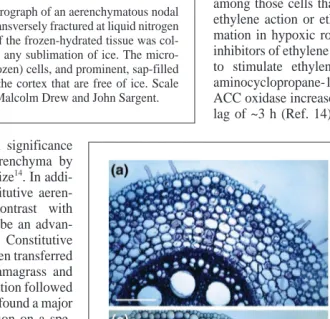
measure-ment of gas-filled porosity, microscopic observation (Fig. 1) and O2transport
6is overwhelming – the lacunae are indeed usually
gas-filled and therefore are able to transfer gases by convection and diffusion.
Aerenchyma formation is inducible by flooding in maize7,8
, in the coastal grass Spartina patens9 and in many other native
species, both monocot and dicot, that occupy wetland habitats10
. By contrast, in the maize relatives Tripsacum dactyloides (eastern gamagrass) and Zea luxurians (teosinte)11
, as well as in wetland species, such as rice3,12and S. lancifolia1,4, aerenchyma forms
con-stitutively, apparently without any requirement for an external stimulus. Eastern gamagrass possesses notable tolerance to drought and flooding, which is thought to be because of its deep rooting pattern and constitutive aerenchyma13. The roots have
been found to exceed 1.8 m in depth, penetrating a resistant clay pan at or below 0.90 m, apparently when the soil was wet and mechanically least resistant. Therefore aerenchyma was likely to have assisted oxygenation of the root at that time. In general, soil compaction can lower the oxygen concentration of the soil (fewer large, gas-filled pores for gas exchange with the atmosphere). Therefore, aerenchyma could be potentially beneficial for growth or function of roots in a densely packed soil layer. These considerations
Programmed cell death and
aerenchyma formation in roots
Malcolm C. Drew, Chuan-Jiu He and Page W. Morgan
Lysigenous aerenchyma contributes to the ability of plants to tolerate low-oxygen soil environments, by providing an internal aeration system for the transfer of oxygen from the shoot. However, aerenchyma formation requires the death of cells in the root cortex. In maize, hypoxia stimulates ethylene production, which in turn activates a signal transduction pathway involving phosphoinositides and Ca21. Death occurs in a predictable pattern, is
regulated by a hormone (ethylene) and provides an example of programmed cell death.
Danièle Werck-Reichhart*, Alain Hehn and Luc Didierjean are at the Dept of Cellular and Molecular Enzymology, Institute of Plant Molecular Biology, Centre National de la Recherche Scientifique UPR 406, 28 rue Goethe, 67083 Strasbourg Cedex, France.
*Author for correspondence (tel 133 3 88 35 83 32;
might explain the functional significance of the induction of root aerenchyma by mechanical impedance in maize14. In
addi-tion, they suggest that constitutive aeren-chyma development, in contrast with induction after stress, might be an advan-tage in some environments. Constitutive aerenchyma formation has been transferred successfully from eastern gamagrass and teosinte to maize by hybridization followed by backcrossing11. This study found a major
gene for aerenchyma formation on a spe-cific chromosome arm in eastern gama-grass. It will be interesting to see whether improved resistance to flooding is associ-ated with the constitutive formation of aerenchyma in these lines. In crops such as soybean15
, and in Ranunculus species grow-ing in river floodplains16, aerenchyma
formation is associated with flooding toler-ance. However, formation of a prominent aerenchyma by shizogeny or lysigeny is not an invariable requirement for the ad-equate internal transfer of O2. In Carex
pseudocyperus17, the cortical cells remain
intact, and fine intercellular spaces extend throughout the root and into the apical zone, the average porosity of 20% provid-ing sufficient O2for the species to tolerate
anaerobic environments.
Induction of lysigenous aerenchyma Hypoxia under laboratory conditions readily induces aerenchyma formation in the roots of maize (Fig. 2), and hypoxia probably accounts for the induction of aerenchyma in roots in flooded soils. Hypoxia might be a much more common occurrence within roots than has been supposed. Even in well-oxygenated surroundings, the rate of O2
consumption during respiration, especially
at warm temperatures, can outpace the rate of supply of O2to the
respiring cells. Microsensors for O2 have shown steep
concen-tration gradients across roots, from 20.6% O2(the concentration
in air) at the epidermal surface of a maize root at 298C, to less than half that in the center of the stele near the root apex18
. Non-aerenchymatous maize roots in a low-O2environment and
receiv-ing O2 principally from the shoot via intercellular spaces, had
steep longitudinal as well as radial gradients, with only 2.5% O2in
the cortex and 1.0% in the stele at 70 mm below the stem base19
. Thus, conditions conducive to aerenchyma formation might be common.
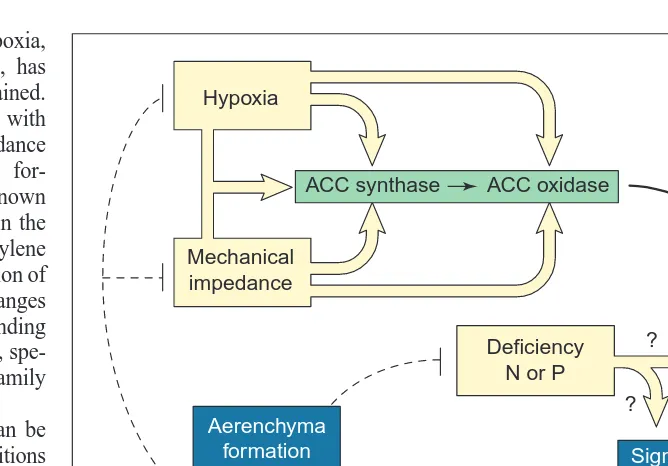
The involvement of ethylene in aerenchyma formation is well documented for maize6
; under well-oxygenated (normoxic) con-ditions, low concentrations of ethylene induce cell death precisely among those cells that would succumb to hypoxia. Inhibitors of ethylene action or ethylene biosynthesis block aerenchyma for-mation in hypoxic roots, whereas in ethylene-treated roots only inhibitors of ethylene action are effective. The effect of hypoxia is to stimulate ethylene biosynthesis, and the activities of 1-aminocyclopropane-1-carboxylic acid (ACC) synthase and of ACC oxidase increase in extracts from hypoxic roots following a lag of ~3 h (Ref. 14). Mechanical impedance also increases the
Fig. 2. Transverse section of maize nodal roots in a four-day-old zone. (a) Well-oxygenated
(nor-moxic) control root, lacking aerenchyma. Note the radial packing of cells in the inner cortex and hexagonal packing in the outer cortex. (b) Hypoxic root, with lysigenous aerenchyma in the mid cortex. (c) Well-oxygenated root treated for four days with okadaic acid, an inhibitor of protein phosphatases. Note that aerenchyma is beginning to form. (d) Hypoxic root treated for four days with EGTA [ethylene glycol-bis (b-aminoethyl ether) N,N,N9,N9-tetraacetic acid] to complex Ca21, thereby eliminating aerenchyma formation. (e) Hypoxic root stained with neutral red with
color showing pH ,7.0. Cell acidification can be seen in the mid cortex, which later dies and degrades. (f) Hypoxic root showing uptake of Evans blue by cells in the mid cortex, indicating a loss of ability to exclude this molecule at an early stage in cell degeneration. Scale bars = 0.25 mm.
Fig. 1. Scanning electron micrograph of an aerenchymatous nodal
activities of these enzymes, and hypoxia, together with mechanical impedance, has a synergistic effect that is unexplained. However, the burst of extra ethylene with hypoxia or with mechanical impedance probably accounts for aerenchyma for-mation. In maize roots, it is not known whether the relatively rapid increase in the activity of enzymes involved in ethylene biosynthesis is as a result of the activation of pre-existing enzymes, or if there are changes in the transcript level of the corresponding genes. In roots of tomato20,21
and rice22
, spe-cific genes in the ACC synthase gene family are induced by O2deficiency.
In maize, aerenchyma formation can be induced under well-oxygenated conditions in nutrient solution by the transient shortage of either a N or a P source (Fig. 3). This is ethylene-dependent, and does not require the augmentation of ethylene production, but rather an increase in sensitivity to ethylene23.
There is uncertainty whether induction of cell death in aerenchyma formation is related to root cortical death (RCD), a form of non-pathogenic death that begins in the cortex and spreads towards the endodermis24
. RCD has been detected in roots of many species in the grass family, including wheat, barley and maize. It is characterized by a loss of nuclear
staining with acridine orange, a symptom that coincides with the loss of both membrane permeability and esterase activity, with fluores-cein diacetate as a substrate. However, RCD has not been examined in relation to its inducibility by ethylene.
Although aerenchyma formation in rice is thought to be consti-tutive12, in some varieties additional cell death occurs in response
to ethylene or hypoxia3,25
. Rice and other wetland species have several anatomical adaptations that help minimize loss of O2to the
surrounding soil, thereby conserving more for respiration by the apical meristem. These structures include a suberized hypoder-mis, and a layer of lignified cells immediately interior to the hypo-dermis, both of which are only slightly gas
permeable. Apart from restricting the loss of O2, these cell layers are likely to hold
in ethylene as well, even if it is slowly synthesized. The question remains whether the constitutive formation of aerenchyma in wetland species is really a response to entrapped ethylene.
Ethylene signal transduction pathway Induction of cell death in the root cortex of maize has been examined in relation to the possible pathway downstream of ethylene26.
A variety of agonists and antagonists of par-ticular steps in signal transduction have been used in this pharmacological approach. In well-oxygenated roots, cell death can be ini-tiated by GTP-g-S, which activates hetero-trimeric G proteins. Death was also induced by okadaic acid (Fig. 2), which inhibits pro-tein phosphatases, thereby tending to main-tain proteins in a phosphorylated state. The importance of protein phosphorylation has
been confirmed by exposing roots to K252a, an inhibitor of protein kinases (especially of protein kinase C), that blocks cell death in hypoxic roots. Neomycin is also effective in preventing death dur-ing root growth under hypoxia by interferdur-ing in phosphoinositide metabolism and both EGTA [ethylene glycol-bis (b-aminoethyl ether) N,N,N9,N9-tetraacetic acid] and ruthenium red prevent death by lowering the Ca21
concentration in the cytosol (Fig. 2). By con-trast, reagents that tend to raise cytosolic Ca21
, such as thapsigar-gin or caffeine, promoted death in well-oxygenated roots. Based on these observations, it is possible to tentatively construct a signal transduction pathway for ethylene signaling in cortical cell death Fig. 3. Model of induction of programmed cell death (PCD) in cortical cells of maize roots
by environmental factors. PCD is viewed here as part of a feedback loop to acclimate roots to sub-optimal soil conditions. For hypoxia and mechanical impedance, the supply of oxy-gen allowed by aerenchyma helps sustain aerobic metabolism and growth. For nutrient de-ficiency, cell death and lysis might provide essential inorganic nutrients and metabolites to maintain meristematic cells.
T
rends in Plant Science
Hypoxia
ACC synthase ACC oxidase
Ethylene
Receptor
Signaling cascade
Cell death
Mechanical impedance
Deficiency N or P
?
?
Aerenchyma formation
Fig. 4. Proposed ethylene signal transduction pathway initiating cell death in cortical cells in
maize roots.
T
rends in Plant Science
Ethylene
G protein Phosphatidyl inositol 4,5 bisphosphate
Phospholipase C
Diacylglycerol Inositol 1,4,5 trisphosphate
Protein kinase C Cytosolic Ca2+
Protein kinase (Ca2+- or Ca-CaM-dependent)
(Fig. 4). As evidence that the reagents interfered with the ethylene signal transduction pathway, rather than with a hypoxia pathway per se, it was checked that the treatments that blocked cell death in hypoxic roots were also effective in well-oxygenated roots exposed to ethylene (C-J. He and M.C. Drew, unpublished). None of the reagents inhibited ethylene production in hypoxic roots26.
The possibility that Ca21
might be involved in cell signaling under low O2conditions was established in cell cultures of maize
27.
Anoxia quickly raised cytosolic Ca21
and induced alcohol dehy-drogenase (ADH) activity. These responses could be simulated in well-oxygenated cells by manipulating cytosolic Ca21
using ruthen-ium red or caffeine. However, the relationship between anoxia and Ca21
is probably independent of ethylene because ethylene synthe-sis is blocked in the absence of O2 (anoxia) and, in addition, anoxic
cells fail to respond to ethylene6
. It is not known whether ethylene and anoxia share part of the same signal transduction pathway, other than at the level of Ca21
. Equally uncertain is the extent to which ethylene signal transduction in different species follows the same pathway. In Arabidopsis, molecular genetic studies have pro-vided a tentative sequence in which putative ethylene-receptors, and downstream, several classes of protein kinases, have a defined role28,29– comparable information is not available for other species.
Part of the proposed scheme (Fig. 4) for the initiation of cell death in the ethylene signal transduction pathway of maize roots has been tested. Changes in phopholipase activity and in inositol 1,4,5 trisphosphate (IP3) in maize roots following either the
im-position of hypoxia or of exogenous ethylene have been examined (C-J. He, P.W. Morgan and M.C. Drew, unpublished). Phospholi-pase C uses plasma membrane-bound phosphatidyl inositol bis-phosphate as a substrate, releasing IP3 to the cytosol, where it
gates Ca21
channels at the endoplasmic reticulum or tonoplast, thereby raising cytosolic Ca21
. Measurements were made of the apical 5 mm zone, which was well ahead of the first signs of cell death or lysis detected under the light microscope at .10 mm from the apical tip. Phospholipase C activity in the plasma mem-brane fraction rose sharply with hypoxia or ethylene, with an approximate doubling of activity in 4 h (C-J. He, P.W. Morgan and M.C. Drew, unpublished). IP3 also showed a corresponding
rise, which is consistent with the higher level of enzyme activity and with the proposed rise in cytosolic Ca21
in response to IP3.
These results are compatible with a role for a phosphoinositide signal transduction pathway in cell death in maize roots (Fig. 4).
Programmed cell death
Programmed cell death (PCD) can be defined as death resulting from the activation of a specific biochemical pathway that is under the genetic control of the cell, and therefore it amounts to cellular suicide30,31. Apoptosis is a particular form of PCD, which
is defined in animal cells by a combination of changes. These include:
• Nuclear condensation. • Activation of Ca21
-dependent endonucleases with DNA frag-mentation into characteristic, internucleosomal lengths of 180–200 bp or multiples thereof.
• Nuclear and plasma membrane blebbing with expulsion of apoptotic bodies.
By contrast, necrosis refers to ‘accidental’ cell death in response to an injurious environmental factor, such as heat stress or a toxin; it is not normally associated with development, and it does not require the activation of signal transduction pathways. Necrosis (also known as oncosis) is characterized typically by a loss of plasma membrane integrity, sometimes with cellular swelling, followed by organelle degeneration, with DNA degradation tak-ing place relatively late in the process.
Cell death during lysigenous aerenchyma formation occurs in a precise, predictable pattern during differentiation, and in some species is induced by ethylene, supporting the PCD idea, but information on nDNA degradation in this context has not been published. Because cell death is not synchronous in the root cor-tex and occurs among a population of normal cells, DNA ladder-ing might not give unambiguous answers. An alternative approach would be to detect DNA fragmentation in individual cells in situ, using the TUNEL reaction to label the exposed 39ends of the DNA, a method that has already been used with roots to demon-strate PCD in cap cells32
. In S. lancifolia, electron microscopy reveals nuclear condensation and nuclear blebbing in lysigenous aerenchyma suggesting that PCD is involved4
. In rice and maize3
, early signs of cell degeneration are staining with neutral red and permeation of Evans blue – a dye that is normally excluded by the plasma membrane of healthy cells (Fig. 2).
It has been suggested that cell death in rice roots, which always begins among cells in the mid-cortex (as it does in maize) takes place by PCD, and that the subsequent death of cells, which spreads out to surrounding cortical cells, is by necrosis3. It is
thought that a factor diffuses symplastically from the initiating cells to the remainder of the cortex in a radial direction, which is the direction that most plasmodesmatal connections occur in roots, but what that factor might be is obscure. The pattern of cell death and lysis in maize aerenchyma formation begins in the mid-cortex but unlike rice, spreads tangentially as well as radially. In Carex species, as well as in Eriophorum angustifolium and Lysimachia nummularia, cell death spreads only in a tangential direction10. Cell
packing also has a marked influence on whether shizogenous or lysigenous aerenchyma forms or not. Aerenchyma develops pref-erentially where the pre-aerenchyma packing of cells in the cortex is radial in transverse section, which is the pattern found in many wetland species10
. By contrast, aerenchyma rarely occurs where there is non-radial, hexagonal packing, which is chiefly found in non-wetland species, or in the outer cortical layers that do not par-ticipate in aerenchyma formation in typical wetland species. These differences in the pattern of aerenchyma formation are difficult to explain in terms of diffusion of a necrotic factor.
One of the earliest signs of cell death in maize roots is the rupture of the tonoplast2. The release of hydrolytic enzymes sequestered in
the vacuole and the sudden cytoplasmic acidosis caused by the liber-ation of the acidic vacuolar sap might be sufficient to kill the ethyl-ene-responsive cell. In rice roots also, cells in the mid-cortex that are the first to die, are also the first to show signs of acidosis as detected by neutral-red staining3
. There is an obvious similarity here with the death of xylem cells (tracheary elements, TE), which die at the com-pletion of their differentiation. In Zinnia, in cell culture, induction of cell death during TE formation is associated with a serine protease that appears to activate Ca21
influx into the cytosol, the Ca21
then triggering nDNA fragmentation as part of the PCD process33.
Rup-ture of the tonoplast is also an early event in TE death, therefore the parallel to cell death in the root cortex is striking.
Death of root cortical cells is followed by degradation and total lysis of the cytoplasm, with only a few remnants of the cell wall remaining2
. This has to be accomplished by a whole suite of enzymes, but this aspect has received little attention. One enzyme that is closely involved is cellulase (b1,4-endoglucanase), which is always tightly coupled to cell death; promotors of cell death (ethylene, Ca21
, okadaic acid) increase cellulase activity, whereas inhibitors of cell death block any rise in its activity26. It appears as if
(and indeed many other enzymes) must also be involved in the degradation process, and it is important to distinguish those that are involved in degradation from those that are involved in the signaling of cell death.
An intriguing question concerns the highly localized pattern of cell death in the root cortex. In maize, the epidermis, hypodermis, endodermis and stele are unaffected. Even in the cortex, some cells remain intact and healthy, forming radial bridges immediately adjacent to lysing cells. If lytic enzymes are freely released from dying cells, what limits their spread? The same question arises in the highly localized lysis of the primary cell wall and cytoplasmic contents of TEs in the vicinity of healthy parenchymatous cells33.
Conclusions
Aerenchyma formation by cell death and lysigeny provides an ideal model system for examining the initiation of PCD by envi-ronmental factors or by the phytohormone, ethylene. Information is accumulating on the signal transduction pathway, only parts of which may have features in common with that in Arabidopsis. A rise in cytosolic Ca21
and rupture of the tonoplast are early events common to death of TEs and root cortical cells, and it will be interesting to explore the similarities between these two systems further. If it is shown conclusively that PCD is the mechanism involved in lysigenous aerenchyma formation, it will be of inter-est to determine the extent to which the process resembles that of apoptosis in animal cells.
Acknowledgements
Research on aerenchyma formation in M.C.D.’s and P.M.W.’s laboratories is supported by a competitive grant from the US Dept of Agriculture, National Research Initiative program.
References
1 Shussler, E. and Longstreth, D.J. (1996) Aerenchyma develops by cell lysis in roots and cell separation in leaf petioles in Sagittaria lancifolia
(Alismataceae). Am. J. Bot. 83, 1266–1273
2 Campbell, R. and Drew, M.C. (1983) Electron microscopy of gas space (aerenchyma) formation in adventitious roots of Zea mays L. subjected to oxygen shortage. Planta 157, 350–357
3 Kawai, M. et al. (1998) Cellular dissection of the degradation pattern of cortical cell death during aerenchyma formation of rice roots. Planta 204, 277–287 4 Shussler, E.E. and Longstreth, D.J. Changes in cell structure during the
formation of root aerenchyma in Sagittaria lancifolia (Alismataceae). Am. J. Bot. (in press)
5 Canny, M.J. (1995) Apoplastic water and solute movement: new rules for an old space. Annu. Rev. Plant Physiol. Plant Mol. Biol. 46, 215–236 6 Drew, M.C. (1997) Oxygen deficiency and root metabolism: injury and
acclimation under hypoxia and anoxia. Annu. Rev. Plant Physiol. Plant Mol. Biol. 48, 223–250
7 Drew, M.C. et al. (1989) Decreased ethylene biosynthesis, and induction of aerenchyma, by nitrogen- or phosphate-starvation in adventitious roots of Zea mays L. Plant Physiol. 91, 266–271
8 He, C.J. et al. (1994) Induction of enzymes associated with lysigenous aerenchyma formation in roots of Zea mays during hypoxia or nitrogen-starvation. Plant Physiol. 105, 861–865
9 Burdick, D.M. (1989) Root aerenchyma development in Spartina patens in relation to flooding. Am. J. Bot. 76, 777–780
10 Justin, S.H.F. and Armstrong, W. (1987) The anatomical characteristics of roots and plant response to soil flooding. New Phytol. 106, 465–495 11 Ray, J.D. et al. (1999) Introgressing root aerenchyma into maize. Maydica 44,
113–117
12 Jackson, M.B. et al. (1985) Aerenchyma (gas-space) formation in adventitious roots of rice (Oryza sativa L.) is not controlled by ethylene or small partial pressures of oxygen. J. Exp. Bot. 36, 1566–1572
13 Clark, R.B. et al. (1998) Eastern gamagrass (Tripsacum dactyloides) root penetration into and chemical properties of claypan soils. Plant Soil 200, 33–45 14 He, C.J. et al. (1996) Ethylene biosynthesis during aerenchyma formation in
roots of Zea mays subjected to mechanical impedance and hypoxia. Plant Physiol. 112, 1679–1685
15 Bacanamwo, M. and Purcell, L.C. (1999) Soybean root morphological and anatomical traits associated with acclimation to flooding. Crop Sci. 39, 143–149
16 He, J.B. et al. (1999) Survival tactics of Ranunculus species in river floodplains. Oecologia 118, 1–8
17 Moog, P. (1998) Flooding tolerance of Carex species. I. Root structure. Planta 207, 189–198
18 Ober, E.S. and Sharp, R.E. (1996) A microsensor for direct measurement of O2partial pressure within plant tissues. J. Exp. Bot. 47, 447–454
19 Armstrong, W. et al. (1994) Microelectrode and modelling study of oxygen distribution in roots. Ann. Bot. 74, 287–299
20 Wang, T.W. and Arteca, R.N. (1992) Effects of low O2root stress on ethylene biosynthesis in tomato plants (Lycopersicon esculentum Mill cv Heinz 1350). Plant Physiol. 98, 97–100
21 Shiu, O.Y. et al. (1998) The promoter of LE-ACS7, an early flooding-induced 1-aminocyclopropane-1-carboxylate synthase gene of the tomato, is tagged by a Sol3 transposon. Proc. Natl. Acad. Sci. U. S. A. 95, 10334–10339 22 Zarembinski, T.I. and Theologis, A. (1993) Anaerobiosis and plant growth
hormones induce two genes encoding 1-aminocyclopropane-1-carboxylate synthase in rice (Oryza sativa L.). Mol. Biol. Cell. 4, 363–373
23 He, C.J. et al. (1992) Enhanced sensitivity to ethylene in nitrogen- or phosphate-starved roots of Zea mays L during aerenchyma formation. Plant Physiol. 98, 137–142
24 Elliott, G.A. et al. (1993) Effects of phosphate and nitrogen application on death of the root cortex in spring wheat. New Phytol. 123, 375–382 25 Justin, S.H.F.W. and Armstrong, W. (1991) Evidence for the involvement of
ethene in aerenchyma formation in adventitious roots of rice (Oryza sativa L.). New Phytol. 118, 49–62
26 He, C.J. et al. (1996) Transduction of an ethylene signal is required for cell death and lysis in the root cortex of maize during aerenchyma formation induced by hypoxia. Plant Physiol. 112, 463–472
27 Subbaiah, C.C. et al. (1994) Elevation of cytosolic calcium precedes anoxic gene expression in maize suspension-culture cells. Plant Cell 6, 1747–1762 28 Ecker, J.R. (1995) The ethylene signal transduction pathway in plants. Science
268, 667–675
29 Kieber, J. (1997) The ethylene response pathway in Arabidopsis. Annu. Rev. Plant Physiol. Plant Mol. Biol. 48, 277–296
30 Greenberg, J.T. (1996) Programmed cell death: a way of life for plants. Proc. Natl. Acad. Sci. U. S. A. 93, 12094–12097
31 Mittler, R. (1998) Cell death in plants. In When Cells Die (Locksin, R.A. et al., eds), pp. 144–174, Wiley–Liss, NY, USA
32 Wang, H. et al. (1996) Apoptosis: a functional paradigm for programmed plant cell death induced by a host-selective phytotoxin and invoked during development. Plant Cell 8, 375–391
33 Groover, A. and Jones, A.M. (1999) Tracheary element differentiation uses a novel mechanism coordinating programmed cell death and secondary cell wall synthesis. Plant Physiol. 119, 375–384
34 Saab, I.N. and Sachs, M.M. (1996) A flooding-induced xyloglucan endo-transglycosylase homolog in maize is responsive to ethylene and associated with aerenchyma. Plant Physiol. 112, 385–391
Malcolm C. Drew*and Chuan-Jiu He are in the Dept of Horticultural Sciences, Texas A&M University, College Station, TX 77843, USA; Page W. Morgan is in the Dept of Soil and Crop Sciences, Texas A&M University, College Station, TX 77843, USA.