Malaysian Statistics On
MEDICAL DEVICES
Subramani V; Mohammad Ali K; Mohd. Roslan H; Jahizah H; Zakaria Z; Ramanathan R; Katijjahbe M.A; Muralitharan G; Suganthi C; Suarn Singh; Aizai A; Abdul Razak M; Rosemi S; Zaki Morad; Murali S; Rohna R;
Rahmat O; Mariam I. Ariza Zakaria Faridah Aryani Md. Yusof
2007
Malaysian Statistics On
MEDICAL DEVICES
Subramani V; Mohammad Ali K; Mohd. Roslan H; Jahizah H; Zakaria Z; Ramanathan R; Katijjahbe M.A; Muralitharan G; Suganthi C; Suarn Singh; Aizai A; Abdul Razak M; Rosemi S; Zaki Morad; Murali S; Rohna R;
Rahmat O; Mariam I. Ariza Zakaria Faridah Aryani Md. Yusof
Malaysian Statistics On Medical Devices 2007
August 2008
© Ministry of Health Malaysia
Published by:
The National Medical Device Survey
Level 3, MMA House 124, Jalan Pahang 53000 Kuala Lumpur Malaysia
Tel. : (603) 4043 9300 Fax : (603) 4043 9500 e-mail : [email protected]
Web site: http://www.crc.gov.my/nmds
This report is copyrighted. However it may be freely reproduced without the permission of the National Medical Device Survey. Acknowledgement would be appreciated. Suggested citation is Ariza Z, Faridah A, Lim T.O. (Eds). Malaysian Statistics On Medical Devices 2007. Kuala Lumpur 2008
This report is also published electronically on the website of the National Medical Device Survey at: http://www.crc.gov.my/nmds
Funding:
FOREWORD
The Ministry of Health Malaysia has embarked on a landmark project, the National Medical Device Survey (NMDS), to capture data on the availability and use of medical devices in both the government and private sectors in Malaysia and this report is an output of the project.
This NMDS report is very relevant in the present environment of ever increasing healthcare costs in both the government and private sectors. We are almost completely lacking in data on the availability and use of medical devices in the country. This publication will help in some ways to rectify the situation.
I am conident this publication will be a very useful reference to the government, the industry and the
public and I must congratulate those who are involved in the survey for successfully completing the project. I am looking forward to see that the data are regularly updated through follow-up surveys.
PREFACE
Data on the availability and use of medical devices is important to better understand healthcare provision in a country. Availability and access to medical technologies are likely to differ among countries and almost certainly unevenly distributed within a country. These differences could be because of several factors, such as demographic differences, differences in epidemiology of disease, differences in medical approaches or differences in economic conditions. This type of information allows for better decision-making in the allocation of resources and procurement of medical technologies. The use of this information can help to ensure access to and appropriate use of medical technology for better health outcomes.
To our knowledge, there has not been any published survey on the availability and utilization of medical devices in Malaysia and this aptly called National Medical Device Survey (NMDS) is, we
believe, the irst of its kind. However in carrying out this survey, in a country like Malaysia that does
not have single central database on the procurement or reimbursement for medical devices, the task of compiling data on devices are fraught with problems. Data needed to be collected from multiple sources and some of these sources were less than forthcoming in providing data due to apprehension on the actual or possible use of the data or possibly, some sources were too busy to be able or want to provide the data needed.
After the hurdle of data collection was surmounted, the next problem was data analysis. There was a need for intelligent and expert analysis to distill credible information out of all these data as the data from various sources were not always complete or clean or in the format or depth that was wanted. We
therefore regard this irst report from the NMDS as a proof of concept; it demonstrates such project
is indeed feasible. These initial efforts and experiences in conducting NMDS will stand us in good stead. NMDS is a work in progress, and as it improves its coverage and secure better cooperation from all relevant source data providers over time, we expect to publish more comprehensive reports in future.
We would like to thank all staff that has worked so hard in this survey. We would also like to thank all agencies and institutions who have helped in providing data and who have helped in one way or another.
Dr. Lim Teck Onn Mr. Zamane Abd. Rahman Mr. Chin Goo Chai
Chairperson Co-Chairperson Co-Chairperson
iv
ACKNOWLEDGEMENTS
The National Medical Device Survey would like to thank the following :
• All the medical doctors, medical assistants and nurses who participated in NMDS survey
• Participating private hospitals for cooperating with the survey
• The Faber Mediserve Sdn. Bhd., Radicare Sdn. Bhd. and Tongkah Medivest Sdn. Bhd., for their valuable assistance
1. Hospital Alor Gajah 2. Hospital Ampang 3. Hospital Bahagia 4. Hospital Balik Pulau 5. Hospital Baling 6. Hospital Banting 7. Hospital Batu Gajah 8. Hospital Batu Pahat 9. Hospital Bau 10. Hospital Beaufort 11. Hospital Beluran 12. Hospital Bentong 13. Hospital Besut 14. Hospital Betong 15. Hospital Bintulu
16. Hospital Bukit Mertajam 17. Hospital Changkat Melintang 18. Hospital Daro
19. Hospital Duchess of Kent 20. Hospital Dungun
21. Hospital Gerik 22. Hospital Gua Musang 23. Hospital Hulu Terengganu 24. Institute of Respiratory Medicine 25. Hospital Ipoh
26. Hospital Jasin 27. Hospital Jelebu 28. Hospital Jeli 29. Hospital Jempol 30. Hospital Jengka 31. Hospital Jerantut 32. Hospital Jitra 33. Hospital Kajang 34. Hospital Kampar 35. Hospital Kanowit 36. Hospital Kapit 37. Hospital Kemaman 38. Hospital Keningau 39. Hospital Kepala Batas 40. Hospital Kinabatangan 41. Hospital Kluang 42. Hospital Kota Belud 43. Hospital Kota Marudu 44. Hospital Kota Tinggi 45. Hospital Kuala Kangsar 46. Hospital Kuala Krai 47. Hospital Kuala Kubu Bharu 48. Hospital Kuala Lipis 49. Hospital Kuala Lumpur 50. Hospital Kuala Nerang 51. Hospital Kudat
52. Hospital Kulim 53. Hospital Kunak 54. Hospital Labuan 55. Hospital Lahad Datu 56. Hospital Langkawi 57. Hospital Daerah Lawas 58. Hospital Likas
59. Hospital Limbang 60. Hospital Daerah Lundu 61. Hospital Machang 62. Hospital Marudi 63. Hospital Melaka
67. Hospital Muadzam Shah 68. Hospital Mukah
69. Hospital Papar 70. Hospital Parit Buntar 71. Hospital Pasir Mas 72. Hospital Pekan 73. Hospital Permai 74. Hospital Pontian 75. Hospital Port Dickson 76. Hospital Pulau Pinang 77. Hospital Putrajaya 78. Hospital Queen Elizabeth
79. Hospital Raja Perempuan Zainab II 80. Hospital Rajah Charles Brooke Memorial 81. Hospital Ranau
82. Hospital Raub 83. Hospital Saratok
84. Hospital Umum Sarawak 85. Hospital Sarikei
86. Hospital Seberang Jaya 87. Hospital Segamat 88. Hospital Selama 89. Hospital Selayang 90. Hospital Semporna 91. Hospital Sentosa 92. Hospital Serdang 93. Hospital Seri Manjung 94. Hospital Serian 95. Hospital Setiu 96. Hospital Sibu 97. Hospital Sik 98. Hospital Simunjan 99. Hospital Sipitang 100. Hospital Slim River 101. Hospital Sri Aman
102. Hospital Sultan Abdul Halim 103. Hospital Sultan Haji Ahmad Shah 104. Hospital Sultan Ismail
105. Hospital Besar Sultanah Aminah 106. Hospital Sultanah Bahiyah 107. Hospital Pakar Sultanah Fatimah 108. Hospital Sultanah Nur Zahirah 109. Hospital Sungai Bakap
110. Hospital Sungai Buloh 111. Hospital Sungai Siput 112. Hospital Taiping 113. Hospital Tambunan 114. Hospital Tampin 115. Hospital Tanah Merah 116. Hospital Tangkak 117. Hospital Tanjong Karang 118. Hospital Tapah
119. Hospital Tawau 120. Hospital Teluk Intan
121. Hospital Temenggung Seri Maharaja Tun Ibrahim 122. Hospital Tengku Ampuan Afzan
123. Hospital Tengku Ampuan Jemaah 124. Hospital Tengku Ampuan Rahimah 125. Hospital Tengku Anis
126. Hospital Tenom
127. Hospital Tuanku Ampuan Najihah 128. Hospital Tuanku Fauziah
129. Hospital Tuanku Ja’afar
PARTICIPANTS OF THE NATIONAL MEDICAL DEVICE SURVEY
vi
University & Armed Forces Hospitals
1. Pusat Perubatan Universiti Kebangsaan Malaysia 2. Pusat Perubatan Universiti Malaya
3. Hospital Universiti Sains Malaysia 4. Hospital Angkatan Tentera Lumut 5. Hospital Angkatan Tentera Terendak
Private Hospitals
1. Amanjaya Specialist Centre
2. Arunamari Specialist Medical Centre 3. Assunta Hospital
4. Az-Zahrah Islamic Medical Centre 5. Bukit Mertajam Specialist Hospital 6. Columbia Asia Medical Centre, Sarawak 7. Columbia Asia Medical Centre, Seremban
8. Columbia Asia Nursing and Rehabilitation Centre 9. Damai Medical and Heart Clinic Sdn. Bhd 10. Damai Service Hospital (Head Quarters) 11. Damai Specialist Centre Sdn. Bhd 12. Damansara Specialist Hospital 13. Darul Ehsan Medical Centre 14. Gleneagles Medical Centre, Penang 15. Hope Children Hospital, Farlim 16. Hope Children Hospital, Jln Gottlieb 17. Hope Children Hospital, Sg. Ara 18. INS Specialist Centre
19. Ipoh Specialist Hospital 20. Island Hospital
21. Johor Specialist Hospital 22. Kajang Medical Centre 23. Kajang Plaza Medical Centre 24. Kampung Baru Medical Centre 25. KCDC Hospital Sdn. Bhd 26. Kedah Medical Centre 27. Kempas Medical Centre 28. Kinta Medical Centre 29. Kota Bharu Medical Centre 30. Kuantan Medical Centre 31. Lam Wah Ee Hospital
32. Landmark Medical Centre Sdn. Bhd 33. Larut Medical Centre
34. Loh Guan Lye Specialist Centre 35. Mahkota Medical Centre
36. Medical Specialist Centre (JB) Sdn. Bhd 37. Metro Specialist Hospital
38. Miri City Medical Centre
39. Mount Miriam Hospital Cancer Centre 40. Multimedic Specialist & Maternity Centre 41. N. S. Chinese Maternity Hospital and Medical Centre
42. National Heart Institute 43. NCI Cancer Hospital
44. Normah Medical Specialist Centre 45. Pantai Ayer Keroh Hospital Sdn. Bhd 46. Pantai Cheras Medical Centre 47. Pantai Indah Hospital
48. Pantai Mutiara Hospital 49. Peace Medical Centre 50. Pelangi Medical Centre 51. Penang Adventist Hospital 52. Penawar Hospital
53. Perak Community Specialist Hospital 54. Perdana Specialist Hospital
55. Pusat Pakar Tawakal
56. Pusat Rawatan Islam Medical Centre 57. PUSRAWI Hospital Sdn. Bhd 58. PUSRAWI SMC Hospital Sdn. Bhd 59. Puteri Specialist Hospital
60. Putra Medical Centre, Alor Setar 61. Putra Medical Centre, Sungai Buloh
62. Putra Specialist Hospital (Melaka) Sdn. Bhd 63. Raflesia Medical Centre Sdn. Bhd
64. Rejang Medical Centre 65. Sabah Medical Centre 66. Sambhi Clinic Sdn. Bhd
67. Sentosa Medical Centre (KPJ Kajang Specialist Hosp.) 68. Siow Specialist Hospital
69. Sri Kota Specialist Medical Centre 70. Sri Manjung Specialist Centre Sdn. Bhd
71. Srigim Specialist Women and Children’s Hospital 72. Subang Jaya Medical Centre
73. Sunway Medical Centre 74. Taiping Medical Centre 75. Taj Hospital
76. Taman Desa Medical Centre 77. Tanjung Medical Centre 78. Timberland Medical Centre
AbOUT THE NATIONAL MEDICAL DEVICE SURVEY
The National Medical Device Survey (NMDS) is a service initiated and supported by the Ministry of Health (MOH) to collect information on the supply, procurement and utilization of medical devices in Malaysia. It is designed to quantify the current trends of availability and utilization of medical devices, as well as support the implementation of the proposed regulatory system for medical devices in Malaysia. In supporting this, the NMDS shall provide the functional capacity for the collection, analysis, reporting and dissemination of data on medical devices in Malaysia.
Sponsors and Organization of the NMDS
The NMDS is jointly sponsored by:
• The Engineering Services Division and the Medical Device Bureau of the MOH
• The Clinical Research Centre, National Institute of Health MOH.
The Pharmaceutical Research Unit of the Clinical Research Centre MOH undertakes the daily operation of NMDS. To ensure that the NMDS meets the needs and expectations of all interested parties, a Governance Board has been established to oversee the operations of the NMDS. All major groups involved in medical device issues in Malaysia such as the MOH, professional bodies, private healthcare providers and the medical device industries are represented on this board. The board, therefore works as a consultative forum and provide advice on issues pertaining to the NMDS and other aspects of quality use of medical devices in Malaysia.
Premise of the NMDS
Eficient functioning of device market depends in part on buyers’ access to information on suppliers,
products and prices. Safe and effective use of device depends in part on users’ access to information on technical performance and users’ instructions.
The objective of the NMDS is therefore to quantify the present state and time trends of medical device procurement and utilization at various level of our health care system, whether national, regional, local or institutional. It will provide a public information service to ensure that high quality, reliable and timely information on medical devices are available for promoting equitable access to, and safe and effective use of such devices in Malaysia.
Routinely compiled statistics on availability and use of medical devices have many applications, such as to:
1. Describe the demographic patterns of device availability and utilization to better understand device use in its natural clinical environment.
2. Estimate expenditure on medical devices, which constitutes a signiicant proportion of our public
and private healthcare costs.
3. Monitor and evaluate the cost-effectiveness of device-based treatments with respect to device
productivity and equity in provision, which may have signiicant impact on resource management
within our healthcare system.
4. Monitor users’ device-experience and evaluate the clinical effectiveness of medical devices, and
their associated health outcomes as well as factors inluencing those outcomes.
5. Support reporting of adverse events or product problems by suppliers or user facilities.
viii
NMDS GOVERNANCE bOARD
CHAIRPERSON: Dr. Lim Teck Onn,
Director, Clinical Research Center MOH.
CO-CHAIRPERSON: Mr. Zamane Abdul Rahman,
Director, Medical Device Bureau MOH.
CO-CHAIRPERSON: Mr. Chin Goo Chai.
Director, Engineering Services Division MOH
MEMbERS
Clinical Research Centre MOH Dr. Faridah Aryani Md. Yusof
Medical Device Bureau MOH Mr. Ahmad Sharif Hambali
Engineering Services Division MOH Pn. T. Sasikala Devi
Procurement Division MOH Pn. Hamidah Bidin
Malaysian Medical Association
-Malaysian Dental Association (MDA)
-Malaysian Private Dental Practitioner’s Association (MPDPA)
-Association of Private Hospitals Malaysia (APHM)
Dr. Hooshmana M Palany
Malaysian Medical Device Association Mr. Yong Tuan Heng
Association of Malaysian Medical Industries
MEMbERS OF NMDS EXPERT PANELS
Expert Panel Institution
1. Imaging and Diagnostic Facilities And Devices
Dr. Subramani A/L Venugopal (Chairperson) Hospital Tuanku Ja’afar Dr. Nik Fatimah Salwati Hospital Sultanah Bahiyah Dr. Hjh Salwah Hashim Hospital Pulau Pinang Dr. Zaharah Musa Hospital Selayang.
Dr. Harikrishna A/L Sivaganabalan Hospital Tengku Ampuan Rahimah Dr. Che Zubaidah Che Daud Institut Pediatrik, HKL.
Ir. Dr. Syed Mustafa Kamal Engineering Services Div, MOH
2. Nuclear Medicine Facilities And Devices
Dato’ Dr. Mohammad Ali Kadir (Chairperson) Hospital Pulau Pinang Dr. Lee Boon Nang Hospital Kuala Lumpur.
3. Oncology Facilities And Devices
Dr. Mohd. Roslan Bin Haron (Chairperson) Hospital Sultan Ismail
Ass. Prof. Dr. Fuad Ismail Pusat Perubatan Universiti Kebangsaan Malaysia Dr. Ahmad Kamal Mohamed Subang Jaya Medical Centre
Dr. Vincent Phua Hospital Kuala Lumpur En. Mohd Farihan Jaffar Hospital Kuala Lumpur Puan Rubiah Mohd Pakah Hospital Kuala Lumpur Pn Mahzom Pawanchek Hospital Kuala Lumpur
4. Anaesthesiology and Intensive Care Facilities And Devices
Dato’ Dr. Jahizah Hj. Hassan (Chairperson) Hospital Pulau Pinang
Prof. Dr. Mazidar Mansor. Pusat Perubatan Universiti Malaya
Clinical Ass. Prof. Dr. Choy Yin Choy. Pusat Perubatan Universiti Kebangsaan Malaysia Dato’ Dr. Teh Keng Hwang Hospital Sultanah Bahiyah
Dr. Irene Cheah Hospital Kuala Lumpur Dr. Neoh Siew Hong Hospital Taiping
5. General Surgery Facilities And Devices
Dato’ Dr. Zakaria Bin Zahari (Chairperson) Hospital Kuala Lumpur Dr. Mohammed Saffari Mohammed Haspani Hospital Kuala Lumpur Dr. Mohd Mazri Yahya Hospital Kuala Lumpur
6. Orthopedic & Traumatology Facilities And Devices
Dato’ Dr. Ramanathan Ramiah (Chairperson) Hospital Ipoh
Dr. Mohammad Anwar Hau Abdullah Hospital Raja Perempuan Zainab II Dr. Kamariah Nor Mohd Daud Hospital Kuala Lumpur
Dr. Ng Yue Onn Hospital Ampang
x
7. Physiotherapy and Occupational Therapy Facilities And Devices
Cik Katijjahbe Mohd Ali (Chairperson) Pusat Perubatan Universiti Kebangsaan Malaysia Datin Hjh Asiah Bt Hashim Hospital Kuala Lumpur
Pn. Misnah Roslam Hospital Serdang Pn. Tan Wai Choo Hospital Sungai Buloh
Pn. Wong Swee Fong Hospital Tengku Ampuan Rahimah Pn. Lim Khee Li Hospital Kuala Lumpur
Pn. Hjh. Hamidah Hj. Arifin Hospital Selayang
Pn. Khuzaimah Abd. Aziz Hospital Kuala Lumpur Pn. Jamaliah Musa, Hospital Kuala Lumpur Pn. Zalila Kashim Hospital Kuala Lumpur Pn Aqilah Leela T. Narayanan Hospital Sultanah Aminah Pn. Zunaidah Abu Samah Hospital Kuala Lumpur Pn. Tan Foo Lan Hospital Kuala Lumpur
Pn Anil Kalsom Bte Musa Hospital Tengku Ampuan Rahimah
Pn. Rohana Mukahar Pusat Perubatan Universiti Kebangsaan Malaysia Dr. Julia Patrick Engkasan Pusat Perubatan Universiti Kebangsaan Malaysia Pn. Noormah Mohd Darus Health Technology Assesment Div., MOH
8. Obstetrics & Gynecology Facilities And Devices
Dr. Muralitharan Ganesalingam (Chairperson) Hospital Kuala Lumpur
Dr. Zaridah Shafie Hospital Tuanku Fauziah
Dr. Krishnakumar A/L Harikrishnan Hospital Tuanku Ja’afar Dr. R.P Japaraj Hospital Ipoh
9. Neurology Facilities And Devices
Dr. Suganthi Chinnasami Hospital Kuala Lumpur
Y. Bhg. Dato’ Dr. Md. Hanip Bin Raia Hospital Kuala Lumpur Dr. Santhi Datuk Puvanarajah Hospital Kuala Lumpur Dr. Mooi Chin Leong Hospital Kuala Lumpur
10 Psychiatry Facilities And Devices
11. Cardiology And Cardiothoracic Surgery Facilities And Devices
Dr. Aizai Azan (Chairperson) Institut Jantung Negara Dr. Surinder Kaur Institut Jantung Negara Dr. Alan Yean Yip Fong Hospital Umum Sarawak
Dr. Chong Wei Peng Pusat Perubatan Universiti Malaya Dr. Faisal Bin Ismail Hospital Serdang
Dr. Ernest Ng Hospital Serdang
Prof Dr. Sim Kui Hian Hospital Umum Sarawak Dr. Ong Tiong Kiam Hospital Umum Sarawak
12. Respiratory Medicine Facilities And Devices
Dato’ Dr. Abdul Razak Abdul Mutalif Hospital Pulau Pinang
Assoc. Prof. Dr. Roslina Abdul Manap Pusat Perubatan Universiti Kebangsaan Malaysia Dr. Tengku Saifudin Tengku Ismail Hospital Selayang
Dr. Noor Aliza Md Tarekh Hospital Sultanah Aminah Dr. George Kutty Simon Hospital Sultanah Bahiyah Dr. Ashari Yunus Institut Perubatan Respiratori Dr. Jamalul Azizi Abdul Rahman Hospital Queen Elizabeth Dr. Norhaya Mohd Razali Hospital Sultanah Nur Zahirah Assoc. Prof. Dr. Pang Yong Kek Pusat Perubatan Universiti Malaya Assoc. Prof. Dr. How Soon Hin Universiti Islam Antarabangsa Malaysia /
Hospital Tengku Ampuan Afzan Professor Dr. Liam Chong-Kin Pusat Perubatan Universiti Malaya Dr. Mat Zuki Bin Mat Jaeb Hospital Raja Perempuan Zainab II Dr. Zalwani Bt Zainuddin Hospital Tuanku Fauziah
Bgd Gen Dr. Mohd Ello Mohd Sued Hospital Angkatan Tentera Lumut
13. Gastroenterology Facilities And Devices
Dr. Hj. Rosemi Salleh (Chairperson) Hospital Raja Perempuan Zainab II Dato’ Dr. Muhammad Radzi Abu Hassan Hospital Sultanah Bahiyah
Dr. Hjh Rosaida Hj Md Saudi Hospital Kuala Lumpur
Dr. Sheikh Anwar Abdullah Pusat Perubatan Universiti Kebangsaan Malaysia
14. Nephrology Facility And Devices
Prof. Dato’ Dr. Zaki Morad (Chairperson) Ampang Puteri Specialist Hospital Dr. Ong Loke Meng Hospital Pulau Pinang
Dato’ Dr. Rozina Ghazalli Hospital Pulau Pinang Dr. Hooi Lai Seong Hospital Sultanah Aminah Dr. Goh Bak Leong Hospital Serdang
xii
15. Urology Facilities And Devices
Dr. Murali Sundram Abdullah (Chairperson) Hospital Kuala Lumpur Dr. Clarence Lei Chang Moh Normah Medical Centre
16. Dermatology Facilities And Devices
Dr. Rohna Ridzwan (Chairperson) Hospital Selayang Puan Sri Datin Dr. Suraiya Hani Tun Hussein. Hospital Kuala Lumpur Dr. Asmah Johar Hospital Kuala Lumpur
Dr. Choon Siew Eng Hospital Sultanah Aminah, Johor Bahru Dr. Najeeb Bin Mohd. Safdar Hospital Tuanku Jaafar
17. Otorhinolaryngology And Audiology Facilities And Devices
Prof. Madya Dr. Rahmat Omar (Chairperson) Pusat Perubatan Universiti Malaya Dato’ Dr. Abd. Majid Md. Nasir Hospital Kuala Lumpur
Dr. Junainah Sabirin Health Technology Assesment Div., MOH En. Mahamad Almyzan Awang Pusat Perubatan Universiti Kebangsaan Malaysia Cik Nor Shahrina Mohd Zawawi Pusat Perubatan Universiti Kebangsaan Malaysia
18. Ophthalmology And Optometry Facilities And Devices
Datin Dr. Mariam Ismail (Chairperson) Hospital Selayang Dr. Goh Pik Pin Hospital Selayang Dato’ Dr. Balaravi Pillai Hospital Ipoh
Dato’ Dr. Vasantha Kumar S. Thangasamy Hospital Tengku Ampuan Rahimah Dr. Hj. Abdul Mutalib Bin Othman Hospital Queen Elizabeth
NMDS PROjECT STAFF
Project Leader Dr. Faridah Aryani Md. Yusof
Clinical Research Manager Dr. Ariza Zakaria.
Clinical Research Coordinator
-Research Assistants
-Economist Mr. Adrian Goh
Statistician Dr. Hoo Ling Ping
IT Manager Ms. Celine Tsai Pao Chien
Database Developer/ Administrator Ms Tang Roh Yu
Mr. Patrick Lum See Kai
Network Administrator Mr. Kevin Ng Hong Heng
Mr. Adlan Abd. Rahman
CONTENTS
FOREWORD . i
PREFACE . iii
ACKNOWLEDGEMENTS . iv
PARTICIPANTS OF THE NATIONAL MEDICAL DEVICE SURVEY . v
AbOUT THE NATIONAL MEDICAL DEVICE SURVEY . vii
NMDS GOVERNANCE bOARD . viii
MEMbERS OF NMDS EXPERT PANELS . ix
NMDS PROjECT STAFF . xiii
CONTENTS . xv
AbbREVIATIONS . xvi
METHODS . xvii
Chapter 1: Imaging And Diagnostic Facilities And Devices . 1
Chapter 2: Nuclear Medicine Facilities And Devices . 5
Chapter 3: Oncology Facilities And Devices . 9
Chapter 4: Anaesthesiology And Intensive Care Facilities And Devices . 11
Chapter 5: General Surgery Facilities And Devices . 15
Chapter 6: Orthopaedic And Traumatology Facilities And Devices . 21
Chapter 7: Physiotherapy And Occupational Therapy Facilities And Devices . 23
Chapter 8: Obstetrics & Gynaecology Facilities And Devices . 27
Chapter 9: Neurology Facilities And Devices . 31
Chapter 10: Psychiatry Facilities And Devices . 35
Chapter 11: Cardiology And Cardiothoracic Surgery Facilities And Devices . 39
Chapter 12: Respiratory Facilities And Devices . 43
Chapter 13: Gastroenterology Facilities And Devices . 47
Chapter 14: Nephrology Facilities And Devices . 53
Chapter 15: Urology Facilities And Devices . 57
Chapter 16: Dermatology Facilities And Devices . 63
Chapter 17: Otorhinolaryngology And Audiology Facilities And Devices . 69
Chapter 18: Ophthalmology And Optometry Facilities And Devices . 73
xvi AbbREVIATIONS
AbbI Advanced Breast Biopsy Instrumentation
APC Argon Plasma Coagulation
bAER Brainstem Auditory Evoked Response
bER Beyond Economic Repair
biPAP Bilevel Positive Airway Pressure
Cathlab Cardiac Catheterization Laboratory
CCU Coronary Care Unit
COPD Chronic Obstructive Pulmonary Disease
CPAP Continuous Positive Airway Pressure
CPM Continuous Passive Motion
CRC Clinical Research Centre
CRRT Continuous Renal Replacement Therapy
CT Computed Tomography
CTG Cardiotocography
CUSA Cavitron Ultrasonic Surgical Aspirator
DbE Double Balloon Enteroscope
DG Director General of Health, Ministry of Health, Malaysia
ECG Electrocardiography
ECMO Extra-Corporeal Membrane Oxygenator
ECT Electroconvulsive Therapy
EEG Electroencephalography
EMG Electromyography
EOG Electrooculography
EP Evoked Potential
ESWL Extracorporeal Shockwave Lithotripter
EUS Endoscopic Ultrasound
FESS Functional Endoscopic Sinus Surgery
Gb Governance Board
HAL Haemorrhoid Artery Ligation
HD Haemodialysis
HDU Haemodialysis Unit
HFOV High Frequency Oscillatory Ventilator
HKL Kuala Lumpur Hospital
HDR High Dose-Rate
IAbP Intra-Aortic Balloon Pump
ICU Intensive Care Unit
IMRT Intensity Modulated Radiotherapy
IVUS Intra Vascular Ultrasound
KKM Kementerian Kesihatan Malaysia
LINAC Linear Accelerator
LDR Low Dose-Rate
LVAD Left Ventricular Assist Device
MDb Medical Device Bureau
MMHD Malaysian Medical Health Directory
MOH Ministry of Health
MRI Magnetic Resonance Imaging
NC Not Classiied (cannot be classiied between
public and private sectors)
NCV Nerve Conduction Velocity
NCS Nerve Conduction Study
ND No Data
Nd:YAG Neodymium-Doped Yttrium Aluminium Garnet
NMDS National Medical Device Survey
OAE Otoacoustic Emission
ORL Otorhinolaryngology
PCA Patient Controlled Analgesia
PD Peritoneal Dialysis
PET Positron Emission Tomography
PICU Paediatric Intensive Care Unit
PSG Polysomnography
PUVA Psoralen combined with exposure to ultraviolet light A (UVA)
SDP Source Data Providers
SSEP SomatoSensory Evoked Potential
SWD Short Wave Diathermy
TCD Transcranial Doppler
TEE Trans-Oesophageal Echocardiography
TENS Transcutaneous Electrical Nerve Stimulation
UVA Ultraviolet light A
UVb Ultraviolet light B
VACS Vacuum Assisted Closure System
VEP Visual Evoked Potential
VT Video-Telemetry
METHODS
Introduction
The NMDS is designed, broadly speaking, to estimate the quantity and pattern of use of medical devices in Malaysia, as well as to estimate our expenditure on devices. This is an ambitious project, which requires multiple surveys targeting the various levels of the medical device supply chain and utilization in healthcare facilities in the country in order to capture all the required data to meet its
purpose. For this irst effort, we had therefore realistically targeted data sources that are absolutely
critical and accessible.
Hence, the statistics on the availability of medical device in this report are estimated based on data from only a limited number of surveys. In particular, the scope of the survey was limited to:
• Hospitals only, though for certain therapy areas (Nephrology, Cardiology), we were able to supplement the data from more specialized surveys conducted by the National Renal Registry (NRR) and National Cardiovascular Disease Database (NCVD)
• Asset device only. Data on disposable devices will have to await future survey.
Survey Population, Sampling And Response (Coverage) Rate
The survey conducted by NMDS and supplemented by those by NRR and NCVD, its survey population, its sampling unit and sample size, and the survey response or coverage rates are summarized in the table below.
# Site Classiication Survey population MaximumResponse Rate (%)
Minimum Response Rate (%)
1. Public sector 137 71 43
2 Private sector 79 55 37
* Note:
1. Public sector consistsof 132 MOH, 3 University and 2 Armed Forces hospitals. 2. Response rates vary between specialties.
The survey conducted by NMDS and supplemental surveys by NRR and NCVD were entirely by primary data collection. For MOH data, available database on device asset was also used to cross-check the data.
Data Management
The collected data, whether in databases, on paper or electronic data collection form, is compiled into a single database, appropriately processed and coded prior to statistical analysis.
xviii
The data processing steps for this initial version of NMDS data are presented as follows, in 2 phases:
Phase 1 Survey/Database Development and Data Collection
1. On the basis of the NMDS project’s Terms of Reference (ToR), some initial discussions were
held within CRC, supplemented with research on literature to develop the irst drafts of NMDS
survey forms.
2. Various existing databases were obtained and studied to extract learnings for application on the NMDS. Where relevant, these learnings were used to enhance/edit the structure and content of survey forms.
3. On the basis of the said information in 1 & 2, the irst draft of the survey forms were produced
and presented for challenge of adequacy in the required data ields/ variables for each specialty.
4. Draft survey forms were shared with internal and external referees and the feedback was collated
for the inalization of the survey forms.
In most cases, the project team had to consult with the experts in various disciplines to get a
better understanding of the variables of signiicance.
5. The survey forms were then inalized for approval, with the inal versions being made up of 28 medical specialties and 5 supportive specialties.
The variables required were divided into 3 sections: Section 1: Establishment Details.
Section 2: Diagnostic and Therapeutic Facility. Section 3: Associated Equipment of Interest.
6. Approval of the NMDS survey was granted by the Chairperson of the NMDS, allowing the team to proceed with actualizing the survey activities.
7. The approved survey forms were shared with the IT department for them to use as the basis for creating the NMDS Database. The intention was for the Database to be ready for populating by the time the completed survey forms had been returned by the SDPs.
8. Upon approval, survey forms were sent out to the Directors of relevant identiied SDPs to seek
voluntary participation into the survey. SDPs were identiied from the Malaysian Medical Health
Directory (MMHD) and counter-checked by staff making the calls.
9. The project team was dependent on the SDP sites to ensure the survey forms were appropriately distributed within their institutions. The project team then followed up with each individual specialty. The follow up was dependent on the availability of the specialty at the site with the MMHD used as reference. Where there are uncertainties, the NMDS project team contacts the SDP directly to ascertain the availability of specialties.
A “service provider” of a particular specialty was deined as any participating site that provides
specialists services respective to the discipline and includes sites that only provide visiting specialist services. It does not require a resident specialist to be available at the site.
Data Processing of Survey Data
1. Survey data was entered into the NMDS Database that had been created by the IT department based
on the inalized survey forms (refer to Phase 1: 7 above). Drop down options similar to that in the
survey form of each specialty was part of the database design to minimize data entry errors.
Prior to data entry, personnel were fully briefed on how to use the database and enter the data via a training event which included a demonstration.
Personnel were supervised whilst doing the irst few entries to make sure suficient competency had
been developed to minimize if not eliminate errors.
A standard document on steps and precautions on Data entry was mailed to each personnel. Those who were unable to decipher the feedback were advised to enquire from senior staff.
Each entry is recorded for quality assurance purposes.
2. Visual review and manual assessment of entries are performed to capture erroneous, inconsistent or inaccurate entries in the survey forms. These typically occur when information is entered under the “Others” or “Shared Equipment” section. Follow-up with the SDP is performed where required.
Where data was provided using brand names, online searches or follow-up with the SDP was conducted to guide any required editing of the survey form data.
3. The populated Database then underwent Edit Checks, with the database entries being meticulously crosschecked against the original survey forms.
4. It was then decided to exclude further data processing of a subset of devices reported by the SDPs due to limitations in resources. The criteria for selection of devices to be reported per specialty were outlined by priority for treatment and quality of survey data available. Selection was done by the NMDS project team with support from the Chairperson.
Only data on functioning medical devices were processed and reported. Functioning medical devices
were deined as medical equipment that were used in daily procedures at the participating site and
excludes:
• equipment deemed as in Beyond Economic Repair as well as
• equipment which, although were still in working condition, were non-operational (not used) at the site.
5. Data is then exported to Datamed Bio-Statistics for further data processing.
This included a speciic focus on estimation of missing data from poorly populated survey forms or
non-responsiveness.
xx Statistical Methods
In this report, the quantity of the availability of a device is expressed as in absolute count as well as in number per million inhabitants. The latter statistics are calculated as follows:
Where T is an estimate of the total quantity of the device available in the country in the year under consideration
P is the mid-year population of Malaysia or the relevant geographic region where the survey was conducted
T the total is estimated from the sample of relevant health care facilities as follows:
The total is estimated by T =
Σ
Wi TiWhere;
Ti is the value of the quantity of device available in the ith facility in the year
Wi is the sampling weight of the ith facility
Wi = (B/b) * (bi/ Ь)
Where B is total number of beds in the population, b is number of beds of the responding hospitals (sample), bi is number of beds in the ith facility, and Ь the mean number of beds in the population.
The sampling weight for each sampling unit or unit of analysis therefore has the following components:
1. Probability of selection.
The basic weight is obtained by multiplying the reciprocals of the probability of selection at each step of sampling design.
2. Adjustment for non-response.
The response rate was less than 100% for hospital surveys; an adjustment to the sampling weight is
required. The non-response adjustment weight is a ratio with the number of units in the population as the numerator and the number of responding sampling units as the denominator. The adjustment reduces the bias in an estimate to the extent that non-responding units have same characteristics as responding units. Where this is unlikely, some adjustments took into account differences in some
relevant characteristics between responding and non-responding units that may inluence drug
utilization, such as bed strength, staff strength, scope of services for hospitals etc.
Finally, adjustments are also made to the statistical estimates to approximate known values from existing device asset database and from key informants, where these are available.
T
Number of device/million population =
P
T
Number of device/million population =
CHAPTER 1
IMAGING AND DIAGNOSTIC FACILITIES AND DEVICES
Edited by :
Dr. Subramani a/l Venugopal1
With contributions from :
Dr. Che Zubaidah Che Daud2, Dr. Harikrishna a/l Sivaganabalan3, Dr. Hjh. Salwah Hashim4, Dr. Nik
Fatimah Salwati5, Dr. Zaharah Musa6
1 Hospital Tuanku Ja’afar, 2 Hospital Kuala Lumpur, 3 Hospital Tengku Ampuan Rahimah, 4 Hospital Pulau Pinang, 5 Hospital Sultanah Bahiyah, 6 Hospital Selayang
REPORT
The National Medical Devices Survey (NMDS), a service initiated and supported by the Ministry of Health, and coordinated by the CRC, gives complete information on the availability of devices and
services in all states in Malaysia. This information is crucial for future planning and inancing of
equipment and manpower.
The current survey can be considered a good starting point, but some minor changes will be made for
future data collection, in order to better relect the Imaging and Diagnostic facilities, equipment and
services that are available. Accurate compilation of data in this NMDS survey will greatly facilitate the planners to buy the right thing for the right place at the right time.
With capital expenditure for Imaging and Diagnostic being very high, coupled with rapid advances in technology, it is imperative that we have accurate records of the devices available, so that they can be optimally utilized, with minimal or no duplication of services, for a given population.
Almost all larger hospitals (both public and private) in the country are now fairly well equipped
with general radiography, luoroscopy, ultrasound, mammography, computed tomography (CT) and
magnetic resonance scanners. Angiography services are available in most tertiary centers. Basic and vascular interventional radiology is fast developing into a necessity. Smaller or primary hospitals and health facilities are also fairly well equipped with radiological services commensurate with the clinical services provided.
However, the rapid explosion of technology has thrown so many new machines and techniques, which need careful evaluation, before being adopted in Malaysia.
2
Table 1: Available Therapeutic and Diagnostic Facilities in Imaging and Diagnostic Medicine
Population Radiology Centre
No in million No % pmp
Malaysia 26.64 108 100 4
Sector
Negeri Sembilan 0.96 2 2 2
Pahang 1.45 7 6 5
Perak 2.28 11 10 5
Terengganu 1.04 3 3 3
Pulau Pinang 1.49 11 10 7
Sabah 3 13 12 4
Sarawak 2.36 12 11 5
Selangor & W.P. Kuala Lumpur 6.43 21 19 3
Table 2: Available Medical Devices in Imaging and Diagnostic Medicine
Population
Selangor & W.P
Population Total Mammography
Conventional Mammography
Digital Mammography
Mammography with Stereotactic
biopsy System
No in million No % pmp No % pmp No % pmp No % pmp
Malaysia 26.64 95 100 4 37 100 1 9 100 0 37 100 1
Sector
Public - 48 51 10 27 2 22 27 73
Private - 47 49 27 73 7 78 10 27
State
Johor 3.17 9 9 3 3 8 1 0 0 0 5 13 2
Kedah & Perlis 2.11 7 7 3 1 3 0 1 11 0 4 11 2
Kelantan 1.53 2 2 1 1 3 1 0 0 0 1 3 1
Melaka 0.73 4 4 5 4 11 5 0 0 0 0 0 0
Negeri Sembilan 0.96 4 4 4 2 5 2 0 0 0 1 3 1
Pahang 1.45 4 4 3 2 5 1 0 0 0 1 3 1
Perak 2.28 8 8 4 3 8 1 0 0 0 4 11 2
Terengganu 1.04 1 1 1 0 0 0 0 0 0 1 3 1
Pulau Pinang 1.49 12 13 8 5 14 3 1 11 1 5 13 3
Sabah 3 6 6 2 2 5 1 1 11 0 3 8 1
Sarawak 2.36 8 8 3 5 14 2 1 11 0 2 5 1
Selangor & W.P
Kuala Lumpur 6.43 30 32 5 9 24 1 5 56 1 10 27 2
Population Ultrasound with Doppler
No in million No % pmp
Malaysia 26.64 387 100 15
Sector
Public - 203 52
Private - 184 48
State
Johor 3.17 31 8 10
Kedah & Perlis 2.11 31 8 15
Kelantan 1.53 21 5 14
Melaka 0.73 12 3 16
Negeri Sembilan 0.96 11 3 11
Pahang 1.45 13 4 9
Perak 2.28 25 6 11
Terengganu 1.04 6 2 6
Pulau Pinang 1.49 37 10 25
Sabah 3 31 8 10
Sarawak 2.36 29 7 12
CHAPTER 2
NUCLEAR MEDICINE FACILITIES AND DEVICES
EXPERT PANEL MEMbERS
Chairperson: Dato’ Dr. Mohamed Ali Abdul Khader1
Members : Dr. Lee Boon Nang2, Dr. Ng Chen Siew 3, Dr. Felix Sundram4, Assoc. Prof Sazilah A. Sarji 5
1 Hospital Pulau Pinang, 2 Hospital Kuala Lumpur, 3 Hospital Sultanah Aminah, 4 Subang Jaya Medical Centre, 5 University Malaya Medical Centre
INTRODUCTION
Since the early 1960’s, when nuclear medicine services irst started in Malaysia, its scope has
expanded from just providing diagnostic services to the present therapeutic and also interventional nuclear medicine. With the introduction of Positron Emission Tomography (PET), the setting up of cyclotron facility and the use of targeted delivery agents for imaging and therapy, the possibility of
achieving earlier, more accurate and more speciic diagnosis, it promises signiicant improvements
in clinical outcomes. This increasing insight into the molecular origins of disease, the visualization of pathological changes at the cellular and biochemical level, before their anatomical changes occur shall without doubt reshape the whole pattern of healthcare.
Worldwide, the ield of nuclear medicine has developed tremendously and has become an established medical specialty and has expanded to various ields of subspecialisations and also integration with
other medical specialties to provide more comprehensive patient management.
Nuclear Medicine involves the use of radioactive isotopes (radioisotopes) to prevent, diagnose, and treat disease.
Scope of Nuclear Medicine
Nuclear Medicine Service has 3 major sections: a) Clinical Nuclear Medicine
b) Nuclear Pharmacy
c) Nuclear Medicine Physics
a) Clinical Nuclear Medicine
The Clinical Nuclear Medicine section is the mainstay of the department providing the diagnostic and therapeutic aspect of nuclear medicine. This section incorporates the nuclear medicine physicians,
medical oficers, nurses and the technologists.
b) Section of Nuclear Pharmacy
The nuclear pharmacy section is managed by a pharmacist trained in nuclear medicine and handles the quality control, and the preparation of radiopharmaceuticals for nuclear medicine procedure.
c) Nuclear Medicine Physics Section
6
REPORT
The nuclear medicine service in the Ministry of Health is to be setup as nuclear medicine department on a regional basis. These centres will play a bigger role as training centres and also develop a chosen subspecialty area to focus on at a tertiary level later on.
In Malaysia, the nuclear medicine services began its operation as a unit in the department of Radiotherapy in Kuala Lumpur Hospital and between in the next 30 years only 3 additional centres began its operation all within the Klang valley. It is only in the 1990’s that more centres were setup due to the advancement of nuclear medicine technologies both in the hardware and computerization. In the year 2007 there are a total of 12 nuclear medicine centers providing various degrees of services ranging from purely diagnostic to therapy and the more recent sophisticated Positron Emission Tomography.
However the provision of nuclear medicine services are provided by nuclear medicine setup under various jurisdictions like in the department of Radiology, Department of Internal Medicine, Department of Oncology and in one center under the department of Biomedical Imaging. Until 2005 there was no independent nuclear medicine department in the Ministry of Health.
To facilitate planned expansion, a National Nuclear Medicine Meeting was held on 2nd to 5th May 2002 in Johor Bahru, oficiated by the Director General of Health, Malaysia. During this meeting, the major
stakeholders involving nuclear medicine attended where various issues, problems, weaknesses were
identiied and extensively discussed. Recommendations and target for achievement were prioritized.
All regional nuclear medicine centres would be equipped in phases with:
• Hot lab providing dispensing of radioisotopes
• Diagnostic nuclear medicine services
• Therapeutic nuclear medicine services
• Therapeutic nuclear medicine wards
• Positron Emission Tomography (PET) services
The nuclear medicine facilities and devices data collated are as below :
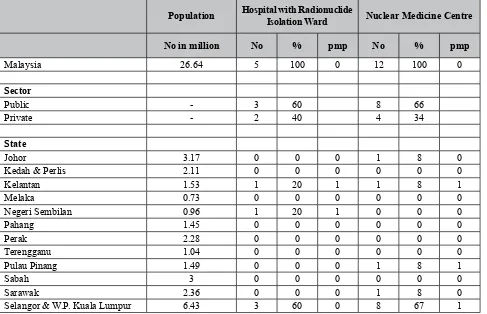
Table 1: Available Therapeutic and Diagnostic Facilities in Nuclear Medicine
Population Hospital with Radionuclide
Isolation Ward Nuclear Medicine Centre
No in million No % pmp No % pmp
Malaysia 26.64 5 100 0 12 100 0
Sector
Public - 3 60 8 66
Private - 2 40 4 34
State
Johor 3.17 0 0 0 1 8 0
Kedah & Perlis 2.11 0 0 0 0 0 0
Kelantan 1.53 1 20 1 1 8 1
Melaka 0.73 0 0 0 0 0 0
Negeri Sembilan 0.96 1 20 1 0 0 0
Pahang 1.45 0 0 0 0 0 0
Perak 2.28 0 0 0 0 0 0
Terengganu 1.04 0 0 0 0 0 0
Pulau Pinang 1.49 0 0 0 1 8 1
Sabah 3 0 0 0 0 0 0
Sarawak 2.36 0 0 0 1 8 0
Table 2: Available Medical Devices in Nuclear Medicine
Population Total Gamma Camera Units
Single head Gamma Camera
2-head Gamma Camera
3-head Gamma Camera
No in million No % pmp No % pmp No % pmp No % pmp
Malaysia 26.64 12 100 0 2 100 0 9 100 0 1 100 0
Sector
Public - 10 83 2 100 7 78 1 100
Private - 2 17 0 0 2 22 0 0
State
Johor 3.17 1 8 0 0 0 0 1 11 0 0 0 0
Kedah & Perlis 2.11 0 0 0 0 0 0 0 0 0 0 0 0
Kelantan 1.53 2 17 1 1 50 1 1 11 1 0 0 0
Melaka 0.73 0 0 0 0 0 0 0 0 0 0 0 0
N. Sembilan 0.96 0 0 0 0 0 0 0 0 0 0 0 0
Pahang 1.45 0 0 0 0 0 0 0 0 0 0 0 0
Perak 2.28 0 0 0 0 0 0 0 0 0 0 0 0
Terengganu 1.04 0 0 0 0 0 0 0 0 0 0 0 0
Pulau Pinang 1.49 3 25 2 1 50 1 2 22 1 0 0 0
Sabah 3 0 0 0 0 0 0 0 0 0 0 0 0
Sarawak 2.36 2 17 1 0 0 0 2 22 1 0 0 0
Selangor & W.P
Kuala Lumpur 6.43 4 33 1 0 0 0 3 33 0 1 100 0
References :
1. Nuclear Medicine in the 21st Century: Contributing To Better Health Care, MOH DG Technical Report
CHAPTER 3
ONCOLOGY FACILITIES AND DEVICES
Edited by:
Ass. Prof. Dr. Fuad Ismail1
With contributions from:
Dr. Ahmad Kamal Mohamed2, Dr. Mohd Roslan B. Haron3, Dr. Vincent Phua4, En. Mohd Farihan
Jaffar4, Puan Rubiah Pakah4, Pn. Mahzom Pawanchek4
1 Pusat Perubatan Universiti Kebangsaan Malaysia, 2 Pusat Perubatan Subang Jaya, 3 Hospital Sultan Ismail, 4 Hospital Kuala Lumpur.
REPORT
Radiotherapy is one of the cornerstones of cancer therapy, both for cure and palliation. Radiotherapy services are equipment based with 2 distinct modalities, teletherapy and brachytherapy.
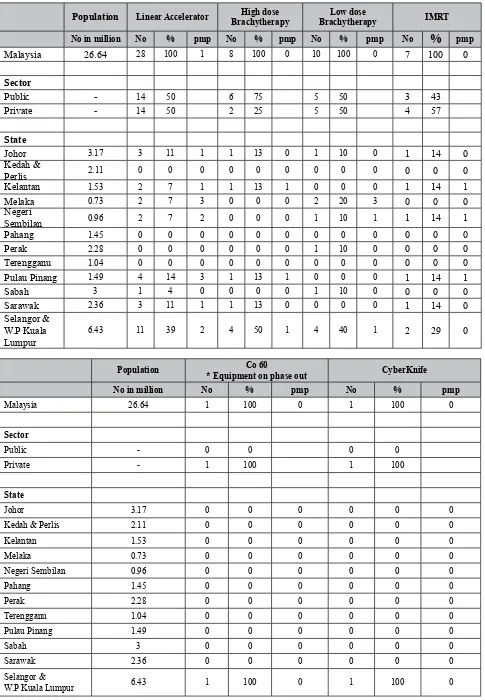
Teletherapy is currently based on Linear Accelerators (LINAC) with older cobalt units available in a few hospitals. There are 5 cobalt units in the country. These are technically still working but are not used as they are being phased out to be replaced by linacs. The only working unit is in a private centre in Selangor.
Linacs form the backbone of radiotherapy with the capability to deliver 2-D & 3-D treatment as standard. There are a total of 30 linacs in Malaysia with a ratio of 1.17 per million population (pmp). This is far lower than the recommended ratio in UK of 4 PMP. Apart from the lack of equipment, there is also a great imbalance in the distribution of linacs in Malaysia with almost half concentrated in the Klang Valley (Selangor & Wilayah Persekutuan). Large states such as Kedah, Perak, Pahang and Terengganu still lack radiotherapy centres hence have no available equipment. As radiotherapy is given over several weeks, the lack of facilities results in patients having to move to another state for a protracted period of time for treatment. The other states have between 1-4 linacs each.
Improvements in technology have resulted in improved treatment delivery by improved radiation dosimetry. Five (5) radiotherapy centres are able to offer more sophisticated radiotherapy using Intensity Modulated Radiotherapy (IMRT). Other specialized equipment includes one (1) cyberknife device and linac based stereotactic devices (4), all in Klang Valley except one.
Brachytherapy is an important modality in radiotherapy especially in gynaecological malignancies. Both High Dose-Rate (HDR) and Low Dose-Rate (LDR) are equally effective. The overall capacity in the country is adequate for gynaecological treatment but due to unequal distribution, there is no services in some states namely Kedah, Pahang & Terengganu. Klang Valley has overcapacity with about half the available brachytherapy devices.
10
Table 1: Available Therapeutic and Diagnostic Facilities in Oncology
No Data to Date
Table 2: Available Medical Devices in Oncology
Population Linear Accelerator High dose brachytherapy
* Equipment on phase out CyberKnife
CHAPTER 4
ANAESTHESIOLOGY AND INTENSIVE CARE
FACILITIES AND DEVICES
Edited by:
Dato’ Dr. Jahizah Hassan1, Professor Marzida Mansor2, Associate Professor Choy Yin Choy3, Dr.
Mary Suma Cardosa4, Dr. Irene Cheah5, Dr. Neoh Siew Hong6, Dato’ Dr. KH Teh 7.
1 Hospital Pulau Pinang, 2 Pusat Perubatan Universiti Malaya, 3 Pusat Perubatan Universiti Kebangsaan Malaysia, 4 Hospital Selayang, 5 Hospital Kuala Lumpur, 6 Hospital Ipoh, 7 Hospital Sultanah Bahiyah.
INTRODUCTION
The data on medical devices for Anaesthesiology and Intensive Care was collected over duration of one-year using survey forms that were sent to both public and private hospitals in Malaysia. The availability of the therapeutic and diagnostic facilities in anaesthesia was based on the number of Intensive Care Units (ICU), High Dependency Units, Anaesthetic Clinics and Acute Pain Services in Malaysia.
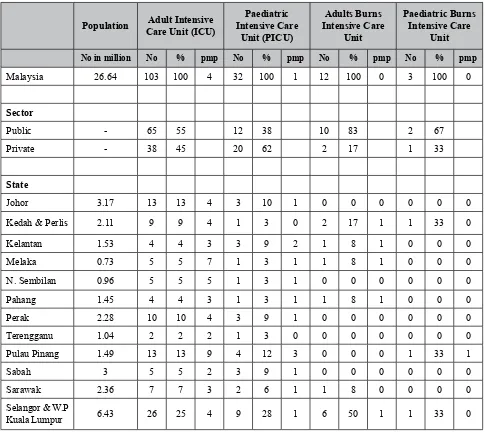
Table 1: Available Therapeutic and Diagnostic Facilities in Anaesthesiology
Population Adult Intensive Care Unit (ICU)
Paediatric Intensive Care
Unit (PICU)
Adults burns Intensive Care
Unit
Paediatric burns Intensive Care
Unit
No in million No % pmp No % pmp No % pmp No % pmp
Malaysia 26.64 103 100 4 32 100 1 12 100 0 3 100 0
Sector
Public - 65 55 12 38 10 83 2 67
Private - 38 45 20 62 2 17 1 33
State
Johor 3.17 13 13 4 3 10 1 0 0 0 0 0 0
Kedah & Perlis 2.11 9 9 4 1 3 0 2 17 1 1 33 0
Kelantan 1.53 4 4 3 3 9 2 1 8 1 0 0 0
Melaka 0.73 5 5 7 1 3 1 1 8 1 0 0 0
N. Sembilan 0.96 5 5 5 1 3 1 0 0 0 0 0 0
Pahang 1.45 4 4 3 1 3 1 1 8 1 0 0 0
Perak 2.28 10 10 4 3 9 1 0 0 0 0 0 0
Terengganu 1.04 2 2 2 1 3 0 0 0 0 0 0 0
Pulau Pinang 1.49 13 13 9 4 12 3 0 0 0 1 33 1
Sabah 3 5 5 2 3 9 1 0 0 0 0 0 0
Sarawak 2.36 7 7 3 2 6 1 1 8 0 0 0 0
Selangor & W.P
12
Population High Dependency Unit Anaesthetic Clinic Acute Pain Service
No in million No % pmp No % pmp No % pmp
Selangor & W.P.
Kuala Lumpur 6.43 13 24 2 5 17 1 10 26 2
The data showed that Malaysia has 4 adult, 1 paediatric and 0 adult and paediatric burns ICU per million population (pmp) as shown in Table 1. The distribution of the adult intensive care is almost
equal between the private and public hospitals; however there are more paediatric intensive care
units available in the private sector. In the case of adult and paediatric ICUs, majorities are in the
public sectors. With regards to distribution of ICU throughout Malaysia it is not surprising to ind
that majority of the intensive care services are located in places where there are major public or private hospitals. The availability of adult ICU for instance varies from 26% in Selangor and W.P Kuala Lumpur to 2% in Terengganu. This is probably due to maldistribution of human resources and
availability of private hospitals, which is probably inluenced by population’s income, geographical
demarcation and urbanization. The paediatric ICU availability follows the similar trend as the adult
ICU and in fact, their services are even more deicient as compared to adult ICU. We have 1 paediatric
ICU pmp. Their availability ranges from as low as 0% in Terengganu to 20% in Selangor& WP Kuala
Lumpur. Adult and paediatric burns ICU are the most deicient of all the intensive care facilities.
More of these services should be made available in the near future.
High dependency unit is available in most of the states as a step down unit. It is encouraging to note that the anaesthetic clinic services have taken off in Malaysia, both in the public and private sectors.
This relects that a proportion of patients schedule for elective surgery were seen prior to surgery
for optimization. This anaesthetic clinic services will certainly reduce cancellation rate of elective surgery and improves patient care and safety. In terms of medical devices, there are not many devices are needed in the anaesthetic clinic. Perhaps in future when more anaesthetists are trained to do
speciic echocardiography to assess the cardiovascular status of patients preoperatively, then, this
statistic on anaesthetic clinic may be more relevant.
Table 2: Available Medical Devices in Anaesthesiology
Selangor & W.P
14
Population Level 1 Infusion Pump With or Without Hotline
No in million No % pmp
Malaysia 26.64 491 100 18
Sector
Public - 293 60
Private - 198 40
State
Johor 3.17 46 9 15
Kedah & Perlis 2.11 42 9 20
Kelantan 1.53 29 6 19
Melaka 0.73 12 2 16
Negeri Sembilan 0.96 22 5 23
Pahang 1.45 19 4 13
Perak 2.28 77 16 34
Terengganu 1.04 35 7 34
Pulau Pinang 1.49 41 8 27
Sabah 3 23 5 8
Sarawak 2.36 7 1 3
Selangor & W.P Kuala Lumpur 6.43 138 28 21
The data in Table 2 concentrate on ventilators, which comprise of anaesthesia ventilator, adult portable ventilator, ICU ventilator, high frequency adult ICU ventilator and paediatric and neonatal high frequency oscillatory. These equipment are the common items available in the facilities in Table
1 but the igures may not be a true relection of the services rendered to the population as some of
these equipment may not being used due to shortage of anaesthetists and nurses. Similarly, the same
reasoning applies to the data on anaesthesia ventilators (anaesthetic machines) and lexible ibre optic
intubation scopes. In future, it may be more useful to have a statistics on the number of operation theatres in each hospitals surveyed as these two devices are mostly available in the operating theatre (OT) rather than in the intensive care. With regards to the anaesthesia ventilators, we are assuming that these ventilators are the ones that are attached to the anaesthetic machines.
The main data collection on the device used for acute pain service is patient controlled analgesia (PCA) infusion pump. The pumps are widely available in both private and public hospitals in a reasonable numbers, indicating that the services are being provided in both sectors. The last equipment in Table
2 is level 1 infusion pump with or without hotline. The igures combined the generic and speciic for
rapid infusion. The hotline is widely available in most hospital. However Level 1infusion pumps are not widely available.
This is our irst effort in trying to compile our own data on medical devices for our fraternity; therefore the data may not relect the actual numbers, as many of the private hospitals are not included. The range of equipment covered is also inadequate and more detailed deinitions and information should
CHAPTER 5
GENERAL SURGERY FACILITIES AND DEVICES
EXPERT PANEL MEMbERS
Chairperson : Dato’ Dr. Dato’ Dr. Zakaria Bin Zahari 1
Members : Dr. Mohammed Saffari Mohammed Haspani1, Dr. Mohd Mazri Yahya1
1 Hospital Kuala Lumpur
Table 1: Available Therapeutic and Diagnostic Facilities in General Surgery
Population
Day Case Surgery
Unit High Dependency (Surgical) Unit
Surgical Neonatal Intensive Care
Unit (SNICU)
Anorectal Physiology Laboratory
No in
million No % pmp No % pmp No % pmp No % pmp
Malaysia 26.64 63 100 2 41 100 2 9 100 0 1 100 0
Sector
Public - 27 43 17 41 7 78 1 100
Private - 36 57 24 59 2 22 0 0
State
Johor 3.17 4 6 1 2 5 1 1 11 0 0 0 0
Kedah &
Perlis 2.11 6 10 3 5 12 2 1 11 0 0 0 0
Kelantan 1.53 3 5 2 3 7 2 1 11 1 0 0 0
Melaka 0.73 2 3 3 3 7 4 1 11 1 0 0 0
N. Sembilan 0.96 1 2 1 2 5 2 1 11 1 0 0 0
Pahang 1.45 4 6 3 3 7 2 0 0 0 0 0 0
Perak 2.28 7 11 3 4 10 2 0 0 0 0 0 0
Terengganu 1.04 0 0 0 1 3 1 0 0 0 0 0 0
P. Pinang 1.49 5 8 3 5 12 3 1 11 1 0 0 0
Sabah 3 5 8 2 2 5 1 1 11 0 0 0 0
Sarawak 2.36 7 11 3 3 7 1 0 0 0 0 0 0
Selangor & W.P.
16
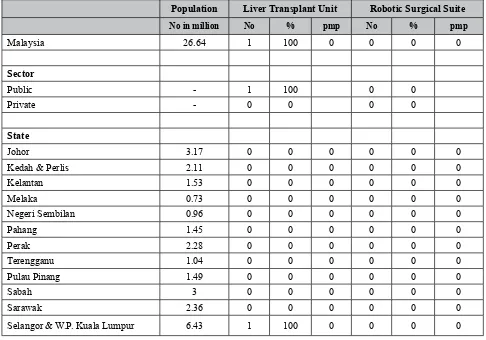
Population Liver Transplant Unit Robotic Surgical Suite
No in million No % pmp No % pmp
Table 2: Available Medical Devices in General Surgery
Population For Sentinel Node
And Parathyroid
Selangor & W.P.
Population
Operating Table With Yellow-Fin boots And jacknife
Positioning
Selangor & W.P.
18
Selangor & W.P.
Kuala Lumpur 6.43 0 0 0 0 0 0 3 75 0 19 26 3
Selangor & W.P.
Population
Vacuum Assisted Closure System
(VACS)
Dermatome Robotic Surgery System
No in million No % pmp No % pmp No % pmp
Malaysia 26.64 22 100 1 51 100 2 2 100 0
Sector
Public - 11 50 34 67 2 100
Private - 11 50 17 33 0 0
State
Johor 3.17 1 5 0 3 6 1 0 0 0
Kedah & Perlis 2.11 0 0 0 8 15 4 0 0 0
Kelantan 1.53 4 18 3 4 8 3 0 0 0
Melaka 0.73 1 5 1 1 2 1 0 0 0
Negeri Sembilan 0.96 0 0 0 1 2 1 0 0 0
Pahang 1.45 0 0 0 2 4 1 0 0 0
Perak 2.28 1 5 0 5 10 2 0 0 0
Terengganu 1.04 0 0 0 2 4 2 0 0 0
Pulau Pinang 1.49 1 5 1 6 12 4 0 0 0
Sabah 3 1 5 0 2 4 1 0 0 0
Sarawak 2.36 10 45 4 4 8 2 1 50 0
Selangor & W.P.
CHAPTER 6
ORTHOPAEDIC AND TRAUMATOLOGY
FACILITIES AND DEVICES
Edited by:
Dr. Kamariah Nor MD1., Dr. Mohammad Anuar H.A2., Dr. Ng YO3, Dato’ Dr. Ramanathan R4, Dr.
Lee JK 5 and Dr. Mahathar AW.1.
1 Hospital Kuala Lumpur, 2 Hospital Raja Perempuan Zainab II, 3 Hospital Ampang, 4 Hospital Ipoh, 5 Pusat Perubatan Pantai Bangsar.
REPORT
External ixation is the commonest mode of treatment for open fractures. Generally all hospitals with orthopedic services will have at least one external ixator set. So, generally in Malaysia there are 333 sets of external ixators, which equate roughly to 13 sets per million population. However, in Sabah, there are only 15 sets of external ixators that are generally inadequate for the 3 million population. The distribution of external ixators is balanced between the public and private sector. However in
the public sector, the distributions were high in urban areas and Wilayah Persekutuan.
Overall, there is some mal-distribution of external ixators, with higher concentration in Penang (22
PMP) but low concentration in Sabah. As for Ilizarov set, there are higher numbers in Sarawak (5PMP) as compared to other states (1-2 PMP). The panel recommends that each hospital which
provides orthopaedic and trauma services should have at least one set of external ixators (each for
upper limb, lower limb and mini set).
Currently there are only 116 sets of upper limb external ixators (4 set PMP), which is not enough to
deal with the increasing number of upper limb injuries.
The Panel also suggests a more proper deinition and clariication of external ixator sets. The speciication can be varies from different companies with different capacities. In addition, data on
trained personnel available to operate the devices in that particular hospital may be useful. This will indicate whether the devices are being used optimally or if they can be mobilized to other hospitals if trained personnel are not available or have been transferred.
Table 1: Available Therapeutic and Diagnostic Facilities in Orthopedic & Traumatology
22
Table 2: Available Medical Devices in Orthopedics & Traumatology
Population Total External Fixator Unit
Selangor & W.P
Kuala Lumpur 6.43 96 30 15 45 29 7 36 31 6 16 24 2
Population Illizarov Unit
No in million No % pmp
Malaysia 26.64 47 100 2
Sector
Negeri Sembilan 0.96 1 2 1
Pahang 1.45 1 2 1
Perak 2.28 4 9 2
Terengganu 1.04 2 4 1
Pulau Pinang 1.49 2 4 1
Sabah 3 3 6 1
Sarawak 2.36 11 23 5
CHAPTER 7
PHYSIOTHERAPY AND OCCUPATIONAL THERAPY
FACILITIES AND DEVICES
Contributors:
Datin Hjh Asiah Bt Hashim1, Cik Katijjah Be Mohd Ali2, Pn. Misnah Roslam3, Pn. Tan Wai Choo4,
Pn. Wong Swee Fong5, Pn. Lim Khee Li1, Pn. Hjh. Hamidah Hj. Arifin6, Pn. Khuzaimah Abd. Aziz1,
Pn. Jamaliah Musa1, Pn. Zalila Kashim1
1 Hospital Kuala Lumpur, 2 Pusat Perubatan Universiti Kebangsaan Malaysia, 3 Hospital Serdang, 4 Hospital Sungai Buloh, 5 Hospital Tengku Ampuan Rahimah, 6 Hospital Selayang
REPORT
The data portrayed in this report are data from the NMDS (2007) as well as data collated by the expert panel of this discipline (2008 data). Whilst the data collected in 2007 includes input from the private sector institutions, those of 2008 below are all from the public hospitals and health clinics in Malaysia. Figures from the private sectors, University Hospitals and Arm Forces Hospitals in 2008 were not obtained.
The indings are as
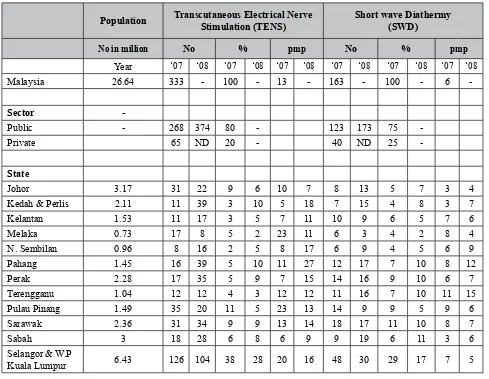
follows:-There is an increase of number of device per million population (PMP) from year 2007 to 2008.
MEDICAL DEVICE PMP
Transcutaneous Electrical Nerve Stimulation (TENS) From 13 to 14
Short wave Diathermy (SWD) From 6-9
Pressure Feedback* From 2-3
*Note :
The pressure feedback survey done in 2008 refers to a simple device that is commonly used to measure the core muscle work of cervical and lumbar region.
There is decrease of number of device per million population (PMP) from year 2007 to 2008 :
MEDICAL DEVICE PMP
Ultrasound* From 11-7
Laser From 3-2
Continuous Passive Motion Exerciser From 2-0
*Note:
There is a doubt whether the Neuromuscular Ultrasound Therapy stimulation system is referred to just an ultrasound machine which is commonly used in the physiotherapy department and Klinik Kesihatan in Malaysia.
The number of devices shown is not proportionate to the population of the respective states. It is most likely based on the demand of the cases seen and the services provided by the hospitals. The Klang valley is the most populated but does not have the most number of devices such as SWD, TENS, Ultrasound, laser, pressure feedback and CPM.
Conclusion:
The igures obtained for devices compared with the population (PMP) are very far from desirable
24
Table 1: Available Therapeutic and Diagnostic Facilities in Physiotherapy and Occupational Therapy
No Data To Date
Table 2: Available Medical Devices in Physiotherapy and Occupational Therapy
Population Transcutaneous Electrical Nerve Stimulation (TENS)
Short wave Diathermy (SWD)
No in million No % pmp No % pmp
Year ‘07 ‘08 ‘07 ‘08 ‘07 ‘08 ‘07 ‘08 ‘07 ‘08 ‘07 ‘08
Malaysia 26.64 333 - 100 - 13 - 163 - 100 - 6
-Sector
-Public - 268 374 80 - 123 173 75
-Private 65 ND 20 - 40 ND 25
-State
Johor 3.17 31 22 9 6 10 7 8 13 5 7 3 4
Kedah & Perlis 2.11 11 39 3 10 5 18 7 15 4 8 3 7
Kelantan 1.53 11 17 3 5 7 11 10 9 6 5 7 6
Melaka 0.73 17 8 5 2 23 11 6 3 4 2 8 4
N. Sembilan 0.96 8 16 2 5 8 17 6 9 4 5 6 9
Pahang 1.45 16 39 5 10 11 27 12 17 7 10 8 12
Perak 2.28 17 35 5 9 7 15 14 16 9 10 6 7
Terengganu 1.04 12 12 4 3 12 12 11 16 7 10 11 15
Pulau Pinang 1.49 35 20 11 5 23 13 14 9 9 5 9 6
Sarawak 2.36 31 34 9 9 13 14 18 17 11 10 8 7
Sabah 3 18 28 6 8 6 9 9 19 6 11 3 6
Selangor & W.P