THESIS
THE EFFECT OF
Cissus quadrangularis
EXTRACT
THERAPY ON SERUM ALKALINE PHOSPHATASE
CONCENTRATION IN
Rattus norvegicus
OSTEOPOROTIC BONE FRACTURE
By:
VIDI PURDIANINGRUM 061111125
FACULTY OF VETERINARY MEDICINE UNIVERSITAS AIRLANGGA
Has been assessed in Result Seminar
Date: August 14th2015
ASSESMENT COMMITTEE OF RESULT SEMINAR Chairman :Prof. Dr. Bambang S. L., drh., DEA.
Secretary :Ira Sari Yudaniayanti, drh., MP.
Member : Dr. Kadek Rachmawati, drh., M.Kes.
Supervisor : Prof. Dr. Fedik Abdul Rantam, drh.
THE EFFECT OFCissus quadrangularisEXTRACT THERAPY ON SERUM ALKALINE PHOSPHATASE CONCENTRATION IN
Rattus norvegicusOSTEROPOROTIC BONE FRACTURE
Vidi Purdianingrum
ABSTRACT
The purpose of this research was to determine the effect ofCissus quadrangularis extract therapy on serum alkaline phosphatase concentration in Rattus norvegicus osteoporotic bone fracture. This research used femaleRattus novergicusof Wistar strain. The rats were three months old with a weight range of 150-200 grams. Twenty-four rats were randomly divided into four groups, as follows T0(-) were non-ovariectomized rats that received 1.5 ml Na CMC as the negative control, T0(+) were ovariectomized rats that received 1.5 ml Na CMC as the positive control, T1 were ovariectomized rats that received raloxifene and T2 ovariectomized rats that received Cissus quadrangularis extract. Serum alkaline phosphatase were measured at week-2 and week-6 after osteotomy. Analysis of Variance did not show significant differences in the effect of Cissus
quadrangularis extract on serum alkaline phosphatase concentration in Rattus
norvegicusosteoporotic bone fracture (p>0.05).
ACKNOWLEDGEMENT
Alhamdulillah, my greatest gratitude for Allah S.W.T for blessing and
mercy that this thesis entitled THE EFFECT OF Cissus quadrangularis
EXTRACT THERAPY ON SERUM ALKALINE PHOSPHATASE
CONCENTRATION IN Rattus norvegicus OSTEOPOROTIC BONE
FRACTURE has been completed. Shalawat and salam always dedicated to
Prophet Muhammad S.A.W who brought us from darkness into the light.
In arranging this thesis, I would like to give my sincere gratitude to:
Prof. Hj. Romziah Sidik, drh., Ph.D. as the dean, Dr. Anwar Ma’ruf, drh.,
M.Kes. as the first vice dean, and Dr. Rr. Sri Pantja Madyawati, drh., M.Si. as the
head of academic division of the Faculty of Veterinary Medicine, Universitas
Airlangga.
My supervisor committee, Prof. Dr. Fedik Abdul Rantam, drh. and
Suzanita Utama, drh., M.Phil., Ph.D. for the guidance, advices, motivations, and
patience from the beginning up to the completion of this thesis. The advisor
committee, Prof. Dr. Bambang Sektiari L, drh., DEA., Ira Sari Yudaniayanti, drh.,
MP. and Dr. Kadek Rachmawati, drh., M.Kes. for all the advices and corrections.
Emy Koestanti Sabdoningrum, drh., M.Kes. as my academic counselor for
all help, patience, and guidance throughout the years. All the lecturers for the
knowledge, information, help, and encouragement.
My beloved father Sukamto, SE. and my beloved mother Endang
affection, advices, and everything they gave to me. My sisters and brothers for all
the love and motivations.
To my beloved college best friends Elsa, Gita, Inanda, Karina and Riri, my
beloved classmates in International Class 34+, Vanya, Benda, Grady, Gita, Ogen,
Karina, Anisah, Diana, Sally, Usy, Dewi, Bayu, Tika, Belga, Hening, Lilian, Ari,
Sony, Hadi, Inanda, Gheby, Ihsan, Riri, Dona, Kemala, Tri, Elsa, Imam and Pavi
for all motivations, informations, help, energy, prayers, and encouragement
whenever I feel down. My high school best friends Andien, Asma, Dita, Diana,
Lilik and Rina for the prayers, supports, love and laughters. All members of
ANDALAS and KMPV PW, thanks for all experiences and stories we shared.
Also, thanks to everyone that can’t be mentioned one by one who was very
helpful during my study.
I, as the author, understand that this writing is still lacking in several parts
and far from perfection. However, I sincerely hope that this research will be useful
for the advancement of science and may contribute to the veterinary medicine
world and the society.
Surabaya, August 7th 2015
CONTENTS
1.2 Statement of the Problem ... 3
1.3 Theoretical Basis ... 4
1.4 Aim of Research ... 5
1.5 Outcomes of Research ... 6
1.6 Hypothesis ... 6
CHAPTER 2 LITERATURE REVIEW ... 7
2.1 Bone ... 7
2.2 Osteoporosis ... 8
2.3 Estogen Deficiency ... 10
2.4 Bone Fracture ... 11
2. 4. 2 Bone Healing in Osteoporotic Fracture ... 13
2.5 Ovariectomy ... 13
2.6 Raloxifene ... 14
2.7 Alkaline Phosphatase... 15
2.8Cissus quadrangularis ... 16
2.8.1 Classification ofCissus quadrangularis(Shah, 2011)... 17
2.8.2 Habitat ... 17
2.8.3 Morfology ... 17
2.8.4 Chemical Contents ... 18
2.9 Rattus norvegicus ... 19
2.9.1 Classification ofRattus norvegicus(Ballenger, 2009)... 20
CHAPTER 3 MATERIALS AND METHODS ... 21
3.1 Research Location ... 21
3.2 Research Materials and Equipments ... 21
3.2.1 Material ... 21
3.2.2 Experimental Animal... 21
3.2.3 Research Chemical Material... 22
3.2.4 Research Equipments ... 22
3.3 Research Procedure ... 22
3.3.1 Preparation ofCissus quadrangularisEthanolic Extract .. 22
3.3.2 Treatment ... 23
3.3.3 Preparation of Osteoporotic Rat ... 24
3.3.3.1 Ovariectomy ... 24
3.3.3.2 Osteotomy ... 25
3.3.4 Samples Observation and Examination... 26
3.4 Experimental Design ... 26
3.5 Research Variables ... 27
3.5.1 Independent Variables ... 27
3.5.2 Dependent Variables ... 27
3.7 Research Flowchart ... 28
CHAPTER 4 RESULTS... 29
CHAPTER 5 DISCUSSION ... 31
CHAPTER 6 CONCLUSSION AND SUGGESTION ... 33
6.1 Conclussion ... 33
6.2 Suggestion ... 33
SUMMARY ... 34
REFERENCES ... 36
LIST OF TABLES
Pages
LIST OF FIGURES
Pages Figure 2.1 X-ray of the femur fracture model in ovariectomized rat
a 4 weeks. b 12 weeks (Dai and Hao, 2007)... 12
Figure 2.2 The leaves and stems ofCissus quadrangularisplant (Rao,
2007)... 18
LIST OF APPENDICES
Pages
Appendix 1. Preparation ofCissus quadrangularisethanolic extract ... 44
Appendix 2. Dosage Calculation... 45
Appendix 3. SPSS Data Analyzing... 49
Appendix 4. Data of Alkaline Phosphatase Laboratory Examination ... 53
ABBREVIATIONS AND SYMBOLS
ALP : Alkaline Phosphatase
ANOVA : Analysis of Variance
BALP : Bone specific Alkaline Phospatase
BR : Bone Remodelling
et al : et alii
HRT : Hormon Replacement Therapy
Iu/l : international unit per liter
NF-kB : Nuclear Factor kappa B
MSCs : Mesenchymal Stem Cell
PTH : Parathyroid Hormone
CHAPTER 1 INTRODUCTION
1. 1 Background of Research
Osteoporosis is characterized by low bone mass and micro-architectural
deterioration of bone tissue, leading to enhanced bone fragility and consequent
increase in fracture risk (Tuck and Francis, 2002). A recent study of the global
burden of osteoporotic fractures estimated that nine million new osteoporotic
fractures occurred during the year 2000. The number of individuals suffering from
the consequences of osteoporotic fractures in the year 2000 was conservatively
estimated to be fifty million worldwide (Johnell and Kanis, 2006).
The increase in bone turnover and enhanced bone fragility, with disruptive
and lytic changes in the bone architecture as observed in the histopathological
study following ovariectomy is indicative of the development of osteoporosis in
rats due to estrogen deficiency and mimics human postmenopausal osteoporosis
(Shirwaikaret al., 2003).
Estrogen deficiency accelerates the normal turnover of bone tissue, but the
net activity of bone resorbing cells (osteoclasts) is greater than that of bone
forming cells (osteoblasts). This gives rise to thinning of the cortices of bones,
thinning of trabecular bone and loss of trabecular elements (Kanis, 2010).
In the modern clinical practice for prevention and treatment of
postmenopausal osteoporosis, hormone replacement therapy (HRT), as well as
some drugs, such as raloxifene, bisphosphonates, calcium and vitamin D,
bone protective effects of these agents are well-confirmed, side effects, such as
hypercalcemia, increased risk of endometrial and breast cancer, vaginal bleeding
and hot flushes have also been reported (Jordan, 2001). Due to some severe side
effects or lack of efficacy of synthetic drugs, the potential efficacy of traditional
medicines has arouse the interest of scientists and doctors to seek the cues from
traditional medicines for treatment of some chronic and difficult diseases,
including the treatment for osteoporosis (Yanet al.,2006).
ALP is a membrane bound protein, synthesized by the cells of various
tissue. ALP in animal have five variants (intestinal, hepatic, bone, kidney and
placenta in rats). Bone and hepatic ALP are considered as isoforms, not as
isonzymes and there are some laboratory assays and kits able to determine only
the bone ALP (Paskalev et al., 2012). Serum ALP concentrations correlate well
with the process of fracture healing, ALP is considered a more spesific marker of
bone (Singhet al., 2013).
The measurement of serum markers of bone metabolism could assist in the
evaluation of the cellular function in the bone fracture healing process, providing
near real time information about the organic response to the lesion and to the
selected treatment. These assays could also provide a simple, accessible and
accurate method of assessing disease progression during the bone fracture healing
process (Allen, 2003; Breur et al., 2004). The process of fracture healing have a
positive correlation with the total alkaline phospatase (ALP) serum concentration
and could be used to determine the progress of fracture healing of the surgically
activity in serum, the most common being obstructive liver disease and metabolic
bone disease (Sarac and Saygili, 2007).
Cissus quadrangularis can influence bone by several mechanisms. At the
fracture site, it increases mucopolysaccharides and mineral (calcium) that is
deposited during the bone formation phase (Srisook et al., 2010). The fresh stem
and leaves of Cissus quadrangularis are used for the treatment of various
ailments. Pharmacological studies have revealed the bone fracture healing
property and antiosteoporotic effect of this plant and that 750 mg/kg of body
weight of ethanolic extract given to rats was effective in ovariectomy induced
osteoporosis (Shirwaikaret al., 2003). Experimental fracture animal models, local
and systemic administration of Cissus quadrangularis extract caused less tisssue
reactions, significantly accelerated complete new bone formation and reduced
healing time (Dekaet al., 1994).
Further experimentation in animal models is really needed to find out the
multiple factors implicated in osteoporosis, its obscure pathogenesis, the dramatic
decline in quality of life, high incidence of the disorder, financial cost and high
mortality (Lelovas et al.,2008). More research are needed to determine the ALP
serum concentration in osteoporotic fracture healing process of Rattus norvegicus
withCissus quadrangularisextract therapeutic.
1.2 Statement of the Problem
Can Cissus quadrangularis plant extract increase serum ALP
1.3 Theoretical Basis
Osteoporosis is a chronic, progressive disease characterized by low bone
mass, microarchitectural bone deterioration, and decreased bone strength that lead
to increased bone fragility and a consequent increase in fracture risk (Mauck and
Clarke, 2006).
The most common causes of osteoporosis are postmenopausal estrogen
deficiency, immobility or disuse and drug-related causes, including
corticosteroids, long-term heparin use, anti-epileptics and lithium. Both estrogen
and androgen are protective of bone mineral density by decreasing the rate of
bone remodelling. Reduction in estrogen after menopause effects to an increase in
bone turnover causing an imbalance between formation and resorption, an
increase in osteoclast lifespan and decrease in osteoblast lifespan and increase in
immature bone with incomplete mineralisation due to decreased time between
remodelling episodes. Estrogen depletion may cause delayed callus mineralization
and reduced sensitivity to applied mechanical strain. Glucocorticoid treatment is
the most common cause of secondary osteoporosis, exerting its effects on the
skeleton by decreasing the function and differentiation of osteoblasts (Giannoudis,
2007).
Osteoblasts are needed to produce large amounts of ALP, a
phosphate-splitting enzyme that is released into the osteoid to initiate the deposition of
minerals. Calcium hydroxyapatite, which comprises 70% of the bone mass,
crystallizes along the cavities in the three-dimensional collagen network. After
properties necessary to withstand external forces, support the body and protect the
internal organs (Potuet al,2009).
Studies examining the fracture healing process in osteoporotic bone are
very limited and scant which might be due to lack of a standard protocol for
osteoporosis induction. In a glance osteoporosis would result in bone fragility and
an increase in susceptibility to fracture (Namkung-Matthaiet al.,2001).
Experimental studies on the effect of osteoporosis on fracture healing have
been carried out on ovariectomized rats. These studies have shown that
ovariectomy significantly reduces bone mass and that the mechanical strength of
the bone after completion of healing appears to be reduced (Giannoudis et al.,
2007).
Potu (2009) studied that the Petroleum ether extract of Cissus
quadrangularis stimulates osteoblastogenesis and can be used as preventive or
alternative natural medicine for bone diseases such as osteoporosis and it might be
a potential candidate for prevention and treatment of postmenopausal
osteoporosis. The biological activity of Cissus quadrangularis on bone may be
attributed to the phytogenic steroids present in this plant.
1.4 Aim of Research
To find out the effect of Cissus quadrangularis plant extracts on
1.5 Outcomes of Research
The research can be used as a reference about the benefits of Cissus
quadrangularis plants as alternative medicine to improve the healing outcome of
osteoporotic fractures.
1.6 Hypothesis
Cissus quadrangularis plant extract can increase the serum ALP
CHAPTER 2 LITERATURE REVIEW
2. 1 Bone
Bone is a complex tissue of which the principal function is to resist
mechanical forces and fractures. Bone strength depends not only on the quantity
of bone tissue but also on the quality, which is characterized by the geometry and
the shape of bones, the microarchitecture of the trabecular bones, the turnover, the
mineral and the collagen (Viguet-Carrin,, 2006).
The structural properties of bone include geometry (size and shape) and
microarchitecture (eg, trabecular thickness and connectivity and cortical
thickness/porosity). The material properties of bone include mineralization
(mineral-to-matrix ratio and crystal size), collagen composition (type and
cross-links), and damage accumulation (such as microfractures). These components of
bone strength are affected by the bone turnover rate, in which old bone is resorbed
and new bone is created (Link, 2003; Felsenberg, 2005).
Normal bone develops using only two mechanisms, those are
intramembranous and endochondral bone formation. Intramembranous bone
formation is mediated by the inner periosteal osteogenic layer with bone
synthesized initially without the mediation of a cartilage phase. Endochondral
bone formation describes the synthesis of bone on a mineralized cartilage scaffold
after epiphyseal and physeal cartilage have shaped and elongated the developing
Bone modeling and remodeling are the result of the osteoblastic and
osteoclastic cell activities. The healing potential of bone, whether in a fracture or
fusion model, is influenced by variety of biochemical, biomechanical, cellular,
hormonal, and pathological mechanisms (Kalfas, 2001; Allen, 2003).
Bone tissue is subject to remodeling throughout the lifetime of the
individual. The continuous remodeling cycle is actuated by the bone remodeling
(BR) units (Camozzi, 2007). BR also plays an integral role in the union of
fractures which consists of removal of older bone tissue followed by callus
formation (NCCLS, 2004).
In the reversal phase, mononuclear cells line the resorptive cavity and form
a cement line (glycoprotein) that helps in attaching osteoblasts. Osteoblast
precursors are derived from the stromal mesenchymal cells and converted into
mature osteoblasts under the influence of many growth factors, hormones and
cytokines. Osteoblasts synthesize collagenous bone matrix and then complete its
mineralization leading to the formation of bone matrix proteins like collagen
type-1, osteopontin, osteocalcin, bone specific alkaline phosphatase (BALP) and bone
sialoprotein (Gallagher and Sai, 2010).
2. 2 Osteoporosis
Osteoporosis is a silent disease, reflected only in a low bone density, till a
fracture occurs. Much in the manner that asymptomatic conditions such as
respectively, a low bone density (reflecting poor bone health) predisposes to
osteoporotic fractures (Malhotra, 2008).
Osteoporosis characterized by reduced bone mineral density and an
alteration of bone micro-architecture that results in an increased risk of fracture
(Raisz 2005). Loss of bone mineral density is attributable to a pathological
imbalance between bone resorption and bone formation during the remodelling
process. Whereas the postmenopausal osteoporosis is mainly attributable to the
increased bone resorbing activity of osteoclasts caused by oestrogen deficiency,
senile osteoporosis is attributed to inadequate osteoblastic function (Beil et al.
2008). Various systemic and local factors, both in physiological than in
pathologicalconditions, can influence the strictly coupled activity of osteoblasts
and osteoclasts, determining an imbalance in bone remodelling in favour of
resorptive activity (Horwitz and Lorenzo 2002).
Several factors such as genetic, nutritional and lack of exercise etc., along
with aging have been shown to be risk factors in the aetiology of osteoporosis
(Malhotra, 2008). Osteoblastic activity and calcium absorption from the gut also
suffers with the age (Tanna, 2005). In addition to menopause and aging,
hereditary factors, lack of exercise or immobilization, lifestyle, prolonged steroid
administration, excessive diet, alcohol intake, smoking, thyroxin therapy and
geographical variations are the major causes of osteoporosis, among which
lifestyle changes, diet and oestrogen deficiency are modifiable factors, whereas
with corticosteroids, cyclosporins, cytotoxins or certain anticonvulsants like
phenytoin are the prime candidates for osteoporosis (Ferguson, 2004).
2. 3 Estrogen deficiency
Osteoporosis that associated with ovarian hormone deficiency following
menopause is by far the most common cause of age-related bone loss.
Postmenopausal osteoporosis has become a major problem with significant
morbidity and mortality (Reddy, 2003). Estrogen modulates the
mechano-sensitivity of bone cells. In the presence of estrogen, the expression of
prostaglandin as a response to mechanical strain was significantly enhanced,
which indicates that fractures in postmenopausal women may react differently to
the mechanical signal that occurs during fracture repair, compared to fractures in
premenopausal women or men (Joldersma et al., 2001). In addition, estrogen
deficiency after menopause has been associated with an accelerated loss of bone
and bone turnover, leading to a substantial increase in the risk for fracture
(Felsenberg, 2005).
Loss of estrogens increases the rate of bone remodeling by removing
restraining effects on osteoblastogenesis and osteoclastogenesis and also causes a
focal imbalance between resorption and formation by prolonging the lifespan of
osteoclasts and shortening the lifespan of osteoblasts. (Kini and Nandeesh, 2012).
Therefore, loss of estrogen in women after the menopause results in
increased osteoclast formation and survival (Krum et al., 2008). Estrogen also
has a role in regulating the response of bone to mechanical stimulation (Zamanet
al.,2006).
2. 4 Bone Fracture
2. 4. 1 Bone Healing in Normal Traumatic Fracture
Bone has a remarkable ability to repair itself to full structural and
functional effectiveness. The ideal end result of repair should be a complete
reconstitution of lamellar bone oriented along the longitudinal axis of the bone,
merged across the fracture site with a seamless incorporation into the adjacent
bone, and remodelled to the point of no indication as to where the fracture or
osteotomy was (Shapiro, 2008).
Fracture healing is the most remarkable of all repair processes in the body
since it results in the actual reconstitution of the injured tissue. The relation
between metabolic bone disease and fracture healing depends on the role of the
skeleton as a metabolic resource (Giannoudis et al., 2007). Extracellular matrix
metabolism plays a central role in the development of skeletal tissues and in most
orthopaedic diseases and trauma such as fracture healing (Fukuiet al.,2003).
Marsh and Li (1999), observed that bone fracture healing in animal model
can be divided into three phases. The inflammation phase is the first stage of
healing. Immediately upon fracture, a blood clot forms, allowing the influx of
inflammatory, clean up cells to the wound area. This is followed by a cytokine
begin to differentiate into specialized cells that build new bone tissue (osteoblasts)
and new cartilage (chondroblasts) (Marsh and Li, 1999).
The second, reparative stage begins about two weeks after the fracture
occurs. In this stage, proteins produced by the osteoblasts and chondroblasts begin
to consolidate into what is known as a soft callus. This soft, new bone substance
eventually hardens into a hard callus as the bone weaves together over a 6 to 12
week time period (Marsh and Li, 1999).
The final step of fracture repair is known as the remodeling phase. At this
stage the callus begins to mature and remodel itself. Woven bone is remodeled
into stronger lamellar bone by the action of both osteoblast bone formation cells
and osteoclast bone resorption cells (Marsh and Li, 1999).
Figure 2.1 X-ray of the femur fracture model in ovariectomized rat.a4 weeks.b
2. 4. 2 Bone Healing in Osteoporotic Fracture
The mechanical and biological factors that are involved in the healing
process of bone are certainly affected by age and osteoporosis. Alterations in bone
metabolism, like osteoporosis, seem to delay callus maturation and consequently
decelerate fracture healing (Giannoudiset al.,2007).
Endochondral bone formation and intramembrane bone formation acted in
the osteoporotic fracture healing, with the former playing a major role. But during
osteoporotic fracture healing process, endochondral bone formation decelerated,
and simultaneously, bone callus tissue remodeling (bone resorption more than
bone formation) accelerated and resulted in decline of callus quality. The
abnormal change of the organizational constitution, microstructure, bone mineral
metabolism, and bone mass in osteoporotic fracture repair could result in the
decrease of its mechanical strength. As compared with normal fracture healing,
the osteogenesis and endochondral ossification were delayed, whereas the hard
callus remodeling was accelerated, a faster bone turnover resulting in more bone
resorption and less bone formation. In addition, the collagen fibers in the hard
callus appeared loosely disorganized and irregular with regard to the direction of
the principal stress (Dai and Hao, 2007).
2. 5 Ovariectomy
Mice ovariectomy is analogized to resemble a dog or cat and women
which estrogen deficiency occurs in these conditions. According the study of Estai
was sufficient to cause significant bone loss in the rat model. Therefore, a period
of eight weeks post-ovariectomy will be implemented radiological examination in
the control group to see the changes in bone density and osteoporosis
examinations in adult rat models to make sure osteoporis have occurred.
Experimental studies on the effect of osteoporosis on fracture healing have
been carried out on ovariectomized rats. These studies have shown that
ovariectomy significantly reduces bone mass and that the mechanical strength of
the bone after completion of healing appears to be reduced (Giannoudis et al.,
2007). The ovariectomized rat is one of the excellent pre-clinical animal model
that precisely follows the clinical feature of the estrogen depleted human skeleton
and the response of therapeutic agents (Jee and Yao, 2001).
2. 6 Raloxifene
One such pharmacological measure, hormone replacement therapy (HRT),
initiated at the onset of menopause, has been demonstrated in numerous studies to
be capable of improving menopause-related symptoms, while at the same time
preventing the loss of bone mass associated with menopause (Brown and Josse,
2001). Nevertheless, due to its side effects, particularly relevant among which is
the potential risk of developing breast and endometrium cancer (Chlebowski,
2003). The use of this type of medication should not be consumed as first line
therapy for osteoporosis, reserving it instead for its current indication as treatment
of perimenopausal symptoms and always for the shortest time possible
Raloxifene, a member of the class of selective estrogen receptor
modulators (SERM), reproduces the beneficial effects of estrogens on the skeletal
system, without the negative effects estrogens on breast and endometrium (Reyet
al., 2009). Currently, it is used for prevention of osteoporosis in postmenopausal
women.
The effects of raloxifene on bone and the determinants of bone strength
(turnover, quantity, and quality) have been well studied in the last several years.
According to the research by Bjarnasonet al.,(2001) in a subgroup of participants
in the Multiple Outcomes of Raloxifene Evaluation study, those patients with the
most important reductions of bone formation markers (bonespecific alkaline
phosphatase and osteocalcin) were precisely the ones who presented a greater
decrease in the risk of vertebral fracture at the 3 year endpoint; no such correlation
was seen with bone resorption markers.
2.7. Alkaline Phosphatase
ALP serum is a member of a family of zinc metalloprotein enzymes that
function to split off a terminal phosphate group from an organic phosphate ester.
Many things may cause increases of ALP activity in serum, the most common
being obstructive liver disease and metabolic bone disease. The highest total ALP
values have been attributed to an increased bone isoenzyme level due to Paget
disease or rickets/osteomalasia. The enzyme activity, which is localized in the
plasma membrane of osteoblasts before extracellular release, correlates with the
Causes of high bone ALP include bone growth, healing fracture, acromegaly,
osteogenic sarcoma, or bone metastases, leukemia, myelofi brosis, and rarely
myeloma; so ALP is used as a tumor marker. Hyperthyroidism, by its effects upon
bone, may also elevate ALP (Sarac and Sagili, 2007)
ALP in animal have five variants ( intestinal, hepatic, bone, kidney and
plascenta in rats). ALP can be a detection of spesific serum biomakers of bone
formation and clinically useful in evaluating the progres of healing process.
Osteoblast secrete large quantities of ALP, which is involved in the process of
bone matrix formation and its calcium. ALP is believed to either increase the
concentration of local inorganic phospate or neutralize inorganic pyrophosphate,
an inhibitor of hydroxyapatite crystal formation (Whelan et al.,2010). The range
of normal values of rat is 56.8-128 iu/l (international unit/liter) (Johnson, 1996).
2. 8 Cissus Quadrangularis
Cissus quadrangularisis available throughout the year. The plant has been
reported to possess wound healing (Mohanty et al., 2010), antiostoporotic
(Shirwaikaret al., 2003), antioxidant (Chidambaraet al.,2003), antipseudomonal
and antibacterial (Kashikar and George, 2006), ulcer protective (Jainu and Devi,
2006a), antiplasmodial (Bah et al., 2007) and anti-inflammatory (Jainu et al.,
2.8.1 Classification ofCissus quadrangularis (Shah, 2011)
Cissus quadrangularis grows natively in hot, dry regions of India, such as
the Deccan peninsula and also can be found on the lower slopes of the Western
Ghats and widespread across drier areas of Arabia and Africa (Justin Raj, 2011).
2.8.3. Morphology
Cissus quadrangularis is a succulent shrubby climbers reaches a height of
1.5 m. Stems sharply 4-angled, jointed at nodes, internodes are 8 to 10 cm long
and 1.2 to 1.5 cm wide, tendrils simple long and slender emerging from the
opposite side of the node. Leaves simple, lamina ovate or reniform, ±5 cm wide,
crenate-serrate, base truncate-cordate; petiole ±2 cm long. Flowers is umbellate
stamens 4. Berries globose, ±0.7 cm in diameter, apiculate, red on ripening,
1-seeded (Panda, 2004).
Cissus quadrangulariscan be identified with the fleshy quadrangular stem
and nodes at intervals. At nodes we can see a leaf and a tendril (Figure 2.1)
Figure 2.2The leaves and stems ofCissus quadrangularisplant (Rao, 2007) 2.8.4 Chemical Contents
Phytochemical screening ofCissus quadrangularisrevealed high contents
of ascorbic acid, carotene, anabolic steroidal substances, and calcium. The stem
contains two asymmetric tetracyclic triterpenoids, and two steroidal principles.
The presence of β-sitosterol, δ-amyrin,δ-amyrone, and flavanoids (quercetin)
having different potential metabolic and physiological effects has also been
reported (Jakikasem, 2000; Jainu and Devi, 2004).
Cissus quadrangularis can influence bone by several mechanisms. At the
fracture site, it increases mucopolysaccharides and mineral that is deposited
during the bone formation phase. In addition, it also reduces bone resorption,
maybe by inhibiting the activation of nuclear factor kappa B (NF-kB) (Srisooket
in the long bones. Cissus quadrangularis is also anti-inflammatory (Panthong et
al., 2007), so it may reduce the formation of proinflammatory cytokines that
stimulate bone resorption, thereby reducing bone loss. It may also act as an
estrogen receptor agonist as the Friedlin rich fraction of Cissus quadrangularis
increases estrogen in rats (Aswaret al.,2010).
2. 9 Rattus norvegicus
The Norway rat is a social, colonial and mostly nocturnal rodent (Olds and
Olds, 1979). Mean adult mass is 150 – 300 g, and mean total length (body plus
tail) is 37–60 cm (Olds and Olds, 1979; Moors, 1990).
Rattus novergicus is generally robust and heavily built. The tail length is
always less than head and body length in adult rat, and sometimes equal to head
and body length in young rat. The ear is short and, when drawn forward, does not
reach the eyes. Dorsal fur color varies slightly from dark brownish to ochre, and
the dorsal hair bases are grayish. Tail slightly bicolored and covered with short,
sparse, dirty, whitish hairs. The outer and inner surfaces of ears are covered with
short, sparse, blackish hairs. The upper sides of the feet are covered with tiny
whitish hairs, the nail is pigmented, and the soles of the fore and hind feet are
completely naked. The hairs on ventral fur are white but the bases are grayish.
The line of demarcation along the flanks is fairly distinct. Rattus norvegicus has
12 mammae, those are two pairs pectoral, one pair abdominal and three pairs
2.9.1 Classification ofRattus norvegicus (Ballenger, 2009) Kingdom : Animalia
Phylum : Chordata
Class : Mammal
Order : Rodentia
Suborder : Sciurugnathi
Family : Muridae
Subfamily : Murinae
Genus : Rattus
Species :Rattus norvegicus.
CHAPTER 3 MATERIALS AND METHODS
3.1 Research Location
Research conducted in several places. The animals were maintained under
standard husbandry conditions in Animal Holding Unit Universitas Airlangga
Surabaya. The ovariectomy and osteotomy procedure was done in Hospitalization
Room of Educational Veterinary Hospital Universitas Airlangga Surabaya.Cissus
quadrangularis leaf was performed extraction in the Assessment Service Unit of
Pharmacy Faculty Universitas Airlangga Surabaya. The examination of blood
serum ALP was performed in Balai Besar Laboratorium Kesehatan Daerah
Surabaya. This research was done from March 2015 until July 2015.
3.2 Research Materials and Equipments
3.2.1 Material
The test material used in this research are stems and leaves of the Cissus
quadrangularis plant which is collected from Purwodadi botanical garden,
Pasuruan.
3.2.2 Experimental Animal
Experimental animals used in this study were twenty-four (24) female
Rattus novergicus of Wistar strain. The rats were three months old with a weight
range of 150-200 grams under controlled conditions. Rats were in health
animals were allowedad libitumaccess drinking water and were fed a commercial
diet.
3.2.3 Research Chemical Material
The extraction process used absolute ethanol, aquadest, hexane and ethyl
acetate. For the treatment be used Raloxifene (Elly Lily production, USA). 0.5%
Na CMC, 70% alcohol, 96% alcohol, aquabidest, ketamine, xylazine, povidone
iodine and enrofloxacin (antibiotics).
3.2.4 Research Equipments
The research was using animal cage; Ohaus balance; digital balance;
rotavapur; knife; autoclave; gloves; masker; ovariectomy and osteotomy surgical
equipment set; Vernier caliper; intramedullary pin 1 and 1.2 mm; 1, 3, 5 ml
disposable syringes; tube and centrifuge.
3.3 Research Procedure
3.3.1 Preparation ofCissus quadrangularisEthanolic Extract
Cissus quadrangularis fleshy stems and leaves were washed, cut into
small pieces, air-dried and ground into powder. The powder was then extracted
3.3.2 Treatment
All rats were allow to adapt in their cages for ten days, which aims to
reduce the level of stress and given ad libitum drinking water and feed.
Twenty-four rats were randomly divided into Twenty-four groups as follows:
T0 (-) : group of non-ovariectomized rats which received 1.5 ml 0.5%
NaCMC as the negative control (intact control).
T0 (+) : group of ovariectomized rats which received 1.5 ml 0.5%
NaCMC as the positive control (ovariectomized control).
T1 : group of ovariectomized rats which received 5.4 mg/kg BW
raloxifene
T2 : group of ovariectomized rats which received 750 mg/kg BW
Cissus quadrangularisextract.
The ovariectomy was performed at the tenth day of adaptation, except the
T0 (-) which is the negative control group. The osteotomy performed to all rats
after osteoporosis confirmed by radiological examination eight weeks after
ovariectomy. The treatment started to be given the next day after osteotomy for
six weeks. Rats in T0(-) and T0(+) group received 1.5 ml 0.5% NaCMC per-oral
as placebo. T1 group was treated using Raloxifene 5,4 mg/kg of body weight and
T2 group was treated using Cissus quadrangularis extract 750 mg/kg of body
weight. Raloxifene and Cissus quadrangularis extract doses were based on
previous research of Potu et al. (2009) which studied the effect of Cissus
3.3.3 Preparation of Osteoporotic Rat 3.3.3.1 Ovariectomy
Rats were weighed to determine the anesthetic dose with a weight range of
150-200 grams. Anesthesia was performed using a combination of ketamine (50
mg/kg of body weight) and xylazine (10 mg/kg of body weight) (Flecknell, 2009).
(Flecknell, 2009).
Mice were shaved and cleaned with 70% alcohol on the ventral-lateral area
at the level of the lower poles of the kidney before a single longitudinal skin
incision was made (Alagwu and Nneli, 2005). The shaved area was sterilized with
povidone iodine. Mice were placed in dorsal recumbency positionand and drapped
from the umbilicus to the edge of the pelvis.
A single longitudinal skin incision was made, the skin and linea alba was
retracted laterally toward one side and the ovary exposed through a thin muscle
mass just below the dorsal muscle mass. Each incision should be minimum length
to allow the extrusion of ovary. Ligation of the upper horn of the uterus, including
the arteries with chromic catgut was carried out (Alagwu and Nneli, 2005). The
abdominal cavity was irrigated using antibiotics (ampicillin) to prevent infection.
The incision was closed with sutures under aseptic technique. After the
operation is complete, the surgery area was swabbed with povidone iodine and
closed with hypafix. 5% Enrofloxacin (Baytril, Bayer) at a dose of 10 mg/kg of
body weight was given intramuscularly daily for two days. Povidone iodine was
3.3.3.2 Osteotomy
After the rats were confirmed by radiological examination as having
osteoporosis, rats from all groups were osteotomized in the diaphysis of the femur
with the installation of intramedulary pin. The length of the pin that would be
needed was measured with Vernier caliper, based on the length of the femur from
radiograph result to determine the length needed. Osteotomy was conducted under
anesthesia using a combination of ketamine (50 mg/kg of body weight) and
xylazine (10 mg/kg of body weight) (Flecknell, 2009). Mice were shaved along
the lateral of femur to the patella and cleaned with 70% alcohol. The shaved area
was sterilized with povidone iodine 10%. Rats were drapped from the umbilicus
to the edge of the pelvis. Incision was made along the lateral line cranio of major
trochanter to the patella bone. Bone stem was separated from the surrounding
muscles. The middle of the diaphysis of the femur was cut by using bone saw.
Intramedulary pin was inserted into the medullary canal of the femur
through the end of the broken bone fragments at the distal and driven until the pin
fixed. The part of proximal bone fragment was taken. The top end of the pin
which is mounted in the medullary canal of the distal part of the bone fragments
be pushed earlier into the proximal bone fragment of the medullary canal until the
bone connected perfectly.
The incision was closed with sutures under aseptic technique. After the
operation is complete, the surgery area was swabbed with povidone iodine and
body weight was given intramuscularly daily for two days. Povidone iodine was
applied topically on the wound to prevent wound infection (Estai, 2011).
The Cissus quadrangularis extract therapy, NaCMC and raloxifene was
given to each group for six weeks.
3.3.4 Samples Observation and Examination
Observation and examination of the blood sampling in this study
performed twice. The first examination was done two weeks after osteotomy.
Three rats were taken from each treatment groups and anesthetized for
intracardiac puncture.
The second examination was done six weeks after osteotomy. Examination
results in six weeks is in addition to be compared among treatment groups with
the two week post osteotomy.
Rat blood sample was taken 3 ml with disposable syringe 5 ml. The blood
samples was put into a test tube and centrifuged at 3000 rpm for 5-10 minutes
until the serum come out. Serum ALP concentration examination was done in
Balai Besar Laboratorium Kesehatan Daerah Surabaya using spectrophotometric
method (Bowers and McComb, 1966).
3.4 Experimental Design
The experimental design used completely randomized control group
3.5 Research Variables 3.5.1 Independent Variable
Independent variable used on this research was Cissus quadrangularis
extract and raloxifene drug.
3.5.2 Dependent Variable
Dependent variables in this research was the serum ALP concentrations.
3.5.3 Controlled Variable
Controlled variable in this research was the animal species, the strain, age,
sex, body weight, cages, animal feed, water, maintenance and the environmental
condition for the animal.
3.6 Data Analysis
The result data of serum ALP concentrations were analyzed using
ANOVA (Steel and Torrie, 1993). Analysis was performed with SPSS 20.0 for
3.7 Research Flowchart
T0 (+)
Osteotomy
Ovariectomy
Osteoporosis confirmation Rats adapted for ten days
T0 (-) T1 T2
Placebo (T0 (-)) Placebo (T0 (+)) Raloxifene (T1) CQ extract (T2)
Blood samples collection on week-2 and week-6 after osteotomy
CHAPTER IV RESULTS
Twenty-four rats consisted of six non-ovariectomized rats which received
1.5 ml 0.5% NaCMC (T0 (-)), six ovariectomized rats which received 1.5 ml 0.5%
NaCMC (T0(+)), six ovariectomized rats which received Raloxifene (T1) and six
ovariectomized rats which received Cissus quadrangularis extract (T2). All rats
were osteotomized eight weeks after ovariectomy.
The research followed by blood sampling and laboratory examinations
using spectrophotometric method (IFCC, 1983). Data of serum ALP
concentrations presented in the following table:
Table 4.1 Serum Alkaline phosphatase Concentrations (U/L, means ± SD) of ovariectomized rats 2 and 6 weeks after osteotomy
Group
T2 269.00ª ± 121.06 229.33ª ± 66.51
Comparison data of alkaline phosphatase serum concentration of week-2
and week-6 presented in the following figure:
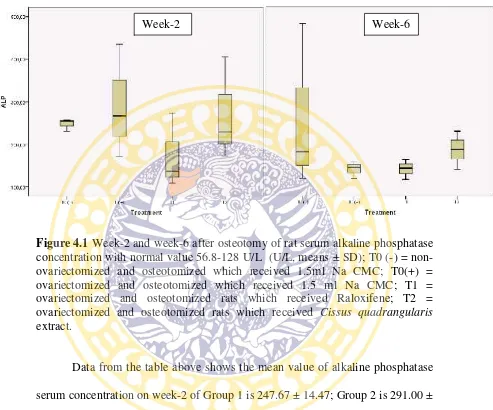
Figure 4.1Week-2 and week-6 after osteotomy of rat serum alkaline phosphatase concentration with normal value 56.8-128 U/L (U/L, means ± SD); T0 (-) = non-ovariectomized and osteotomized which received 1.5ml Na CMC; T0(+) = ovariectomized and osteotomized which received 1.5 ml Na CMC; T1 = ovariectomized and osteotomized rats which received Raloxifene; T2 = ovariectomized and osteotomized rats which received Cissus quadrangularis extract.
Data from the table above shows the mean value of alkaline phosphatase
serum concentration on week-2 of Group 1 is 247.67 ± 14.47; Group 2 is 291.00 ±
133.63; Group 3 is 173.33 ± 88.30 and Group 4 is 269.00 ± 121.06. The mean
value of alkaline phosphatase serum concentration on week-6 of Group 1 is
342.00 ± 292.11; Group 2 is 161.00 ± 28.48; Group 3 is 161.67 ± 35.12 and
Group 4 is 229.33 ± 66.51. From table 4.1 it can be seen that in serum ALP
treatment there is no significant difference among groups of all treatment amd the
healing process time needed, either at week-2 nor at week- 6 (p> 0.05).
CHAPTER V DISCUSSION
Rats that were used in this research had the criteria of active movement,
shiny fur and bright eyes. All rats hadad libitumaccess to drinking water and fed
a commercial diet. The environment that used in this study have been conditioned.
The dosages of treatment used in this research are using effective dosages based
on previous study (Potu, 2009).
The normal range of serum alkaline phosphatase concentration in rats was
56.8-128 iu/l (± 87) (Johnson, 1996). Serum alkaline phosphatase concentration in
group of ovariectomized, osteotomized rats received Cissus quadrangularis
extract showed higher mean value on week-2 than week-6. The mean value on
week-2 is 269.00 ± 121.06 while the mean value on week-6 is 229.33 ± 66.51.
Hoemann, et al. (2008) has been studied that there is two stage developmental
process including a 1-2 week initiation phase during which cells slowly
proliferate, express ALP activity and other bone specific genes, and produce and
assemble a collagen matrix. The mineral phase generally deposited in these
osteoblast cultures is a calcium-phosphate, substitued hydroxyapatite.
The maximum concentration of total ALP occur on three weeks after
surgery, then gradually decreased and returned to initial value (Nakagawa et al.,
2006). The result of this study proved thatCissus quadrangulariscan increase the
osteoblast needed to produce alkaline phosphatase. The decrease of alkaline
phosphatase on week-6 means the bone healing fractures are confirmed similar
The reseach showed that there is no significant differences (p > 0.05) from
ALP serum concentration of six intact rats with 1.5 ml Na CMC treatment, six
ovariectomized rats with 1.5 ml Na CMC treatment, six ovariectomized rats with
Raloxifene treatment and six ovariectomized rats with Cissus quadrangularis
extract treatment which all rats are also osteotomized eight weeks post
ovariectomized.
It is currently accepted that bone ALP is a specific marker of bone
formation. Considerable increases of blood total and bone ALP activities were
reported after operative treatment of fractures in men (Kurdy, 2000; Nakagawa,
2006). It was observed that the serum activities of ALP and BALP presented
statistically significant differences between the non-union and the fracture healing
groups, with higher values in the fracture healing group during the post-operative
period regarding to the non-union group. This difference had also been observed
for ALP in an earlier study by (Komnenou et al., 2005; Sousa. 2011). Based on
the results of this study, ALP is still possible as markers of osteoblast activity
decrease during the bone remodelling to the bone due to osteoporosis but the
CHAPTER VI CONCLUSSION AND SUGGESTION
6.1. Conclussion
Based on the research that has been done, it can be concluded as follows:
Cissus quadrangularis can increase the ALP. ALP is still possible as markers of
osteoblast activity increases to repair damage to the bone due to osteoporosis but
the diagnosis can not stand alone without other diagnoses were complementary.
6.2. Suggestion
1. Alkaline phosphatase serum concentration can not be used as a single
diagnostic tool for osteoporotic bone fracture, should be supported by
other diagnostic tests, such as radiology, examination osteocalcin or
osteopontin examination.
2. Bone alkaline phosphatase should also be monitored as it is more
sensitive bone metabolism parameter than alkaline phosphatase.
3. Further research about Cissus quadrangularis effect on the healing
process of osteoporotic bone fracture in ovariectomized rats based on
SUMMARY
Vidi Purdianingrum. The Effect of Cissus quadrangularis on Serum Alkaline Phosphatase Concentration in Rattus norvegicus Osteoporotic Bone Fracture. This present research was conducted under the guidance of Prof. Dr. Fedik Abdul Rantam., drh. as the first supervisor, Suzanita Utama. drh., M.Phil.,
Ph.D. as the co-supervisor and Prof. Dr.Bambang Sektiari L, drh., DEA. as the
head of examiners.
Osteoporosis is characterized by low bone mass and micro-architectural
deterioration of bone tissue, leading to enhanced bone fragility and consequent
increase in fracture risk. The alkaline phosphatase (ALP) enzyme is essential for
mineralization of bone. ALP is produced by osteoblast to make an alkaline
environment (optimum pH of 10) on the osteoid which needed by calcium to
deposit and bind with phosphate leading to calcification of new bone. Cissus
quadrangularis high contents of ascorbic acid, carotene, anabolic steroidal
substances, and calcium. At the fracture site, it increases mucopolysaccharides
and mineral that is deposited during the bone formation phase.
This research be done from March 2015 to July 2015. Material used in this
research are stems and leaves of the Cissus quadrangularis plant which is
collected from Purwodadi botanical garden, Pasuruan. The extraction was
conducted in Assessment Service Unit of Pharmacy Faculty of Airlangga
University. The experimental animal used female Rattus novergicus of Wistar
Twenty-four rats randomly devided into four groups and allow to adapt in their
cagesfor ten days, as follows T0 (-) are non-ovariectomized rats that received 1.5
ml Na CMC as the negative control, T0 (+) are ovariectomized rats that received
1.5 ml Na CMC as the positive control, T1 are ovariectomized rats that received
raloxifene, T2 are ovariectomized rats that received Cissus quadrangularis
extraction. Three rats will be taken from each treatment groups and going to be
done serum ALP concentration examination on 2 and 6 weeks after osteotomized.
Serum ALP concentration examination be done in Balai Besar Laboratorium
Kesehatan Daerah Surabaya.
The result data was analyzed using ANOVA (Steel and Torrie, 1993).
Data showed that there is no significant differential between groups of all
treatment and the healing process time needed (p >0.05). Cissus quadrangularis
tends to increase the serum ALP concentrations. ALP is still possible as markers
of osteoblast activity increases to repair damage to the bone due to osteoporosis
but the diagnosis can not stand alone without other diagnoses were
REFERENCES
Alagwu, E.A. and Nneli, R.O. 2005. Effect of Ovariectomy on the Levels of Plasma Sex Hormones in Albino Rats. Niger J. Physiol Sci. 20: 90-94
Allen, M.J. 2003. Biochemical markers of bone metabolism in animals: Uses and limitations. Vet.Clin. Path. 32: 101-113.
Aswar, U.M., S. Bhaskaran., V, Mohan and S.L, Bodhankar. 2010. Estrogenic Activity of Friedelin Rich Fraction (IND-HE) Separated from Cissus
quadrangularis and its Effect on Female Sexual Function. J.Pharmacogn.
Res. 2: 138-145.
Bah, S., A.K. Jager., A. Adsersen., D. Diallo and B.S. Paulsen. 2007. Antiplasmodial and GABA (A)-benzodiazepine receptor binding activities of five plants used in traditional medicine in Mali, West Africa. J Ethnopharmacol. 110: 451-457.
Ballenger, L. 2009. Rattus norvegicus Norway Rats. Education Research Initiative. University of Michigan.
Beil, F.T., S. Seitz., M. Priemel., F. Barvencik., C. Von Domuras., J.M. Rueger., M. Amling and P. Podoga. 2008. Pathophysiology and pathomorphology of osteoporosis. Eur J Trauma Emerg Surg 6: 527–534.
Bjarnason, N.H., S. Sarkar and T. Duong. 2001. Six and twelve month changes in bone turnover are related to reduction in vertebral fracture risk during 3 years of raloxifene treatment in post-menopausal osteoporosis. Osteoporos Int. 12: 922-30.
Bowers, G.N.Jr. and McComb, R.B. 1966. A Continuous Spectrophotometric Method for Measuring the Activity of Serum Alkaline Phosphatase. J.Clin. Chem. 12.
Breur, G.J., M.J. Allen., S.J. Carlson and D.C. Richardson. 2004. Markers of bone metabolism in dog breeds of different size. Res. Vet. Sci. 76: 53-55.
Brown, J.P. and Josse R.G. 2002. Clinical practice guidelines for the diagnosis and management of osteoporosis in Canada. J. Can Med Assoc. 167: 1-34.
Chidambara, M.K.N., A. Vanitha., S.M. Mahadeva and G.A. Ravishan kar. 2003. Antioxidant and antimicrobial activity of Cissus quadrangularis L. J Med Food. 6: 99-105.
Chlebowski, R.T., S.L. Hendrix. and R.D, Langer. 2003. WHI Investigators. Influence of estrogen plus progestin on breast cancer and mammography in healthy postmenopausal women: the Women's Health Initiative Randomized Trial. JAMA. 289: 3243-53.
Dai, Ke-Rong and Hao, Yong-Qiang. 2007. Quality of Healing Compared Between Osteoporotic Fracture and Normal Traumatic Fracture. Techniques and Applications. 531-541.
Deka, D.K., Lahon, L.C., Saikia, J. and Mukit, A. 1994. Effect of Cissus
quadrangularis in Accelerating Healing Process of Experimentally
Fractured Radius-Ulna of Dog: A Preliminary Study. Indian J. Phar. 26: 44-45.
Djojoseobagio, S. 1990. Fisiologi Kelenjar Endokrin. Departemen Pendidikan dan Kebudayaan. Direktorat Jendral Pendidikan Tinggi. Pusat Antar Univeristas Ilmu Hayati IPB Bogor. 162-163.
Estai, M. A., Suhaimi, F., Soelaiman, I.-N., Shuid, A. N., and Das, S. 2011. Bone Histomorphometric Study Of Young Rats Following Oestrogen Deficiency. African Journal Of Biotechnology. 12064-12070.
Felsenberg, D. and Boonen, S. 2005. The bone quality framework: determinants of bone strength and their interrelationships, and implications for osteoporosis management. J. Clin Ther. 7:1-11.
Ferguson, N. 2004. Osteoporosis in Focus. London, UK: Pharmaceutical Press.
Flecknell, P. 2009. Laboratory Animal Anasthesia 3rd ed. Elsevier inc. London. 183-186.
Fukui, N., Y. Zhu and W.J. Maloney. 2003. Stimulation of BMP-2 expression by pro-inflammatory cytokines IL-1 and TNF-alpha in normal and osteoarthritic chondrocytes. J Bone Joint Surg. 85: 59−66.
Giannoudis, P., C. Tzioupis., T. Almaki and R. Buckley. 2007. Fracture Healing In Osteoporotic Fractures: Is It Really Different? A Basic Science Perspective. International Journal of the Injured. 38: S90-S99.
Hoemann, C.D., H. El-Gabalawy and M.D. McKee. 2008. In Vitro Osteogenesis Assays: Influence of the Primary Cell Source on Alkaline Phosphatase Activity and Mineralization. J. Pat Bio. 57: 318-323.
Horwitz, M.C. and Lorenzo, J.A. 2002. Local regulators of bone: Il-1, TNF, lymphotoxin, interferon-γ, IL-8, Il-10, IL-4, the LIF/IL-6 family, and additional cytokines. In: Bilezikian JP, Raisz LG, Rodan GA (eds) Principle of biology. Academic Press, San Diego. 961–977.
IFCC. 1983. IFCC Methods for the Measurement of Catalytic Concentration of Enzymes, Part 5. IFCC Method for Alkaline Phosphatase. J.clin Chem. Clin. Biochem. 731- 748.
Jainu, M. and Devi, C.S. 2004. Effect of Cissus quadrangularis on gastric mucosal defensive factors in experimentally induced gastric ulcer- a comparative study with Sucralfate. J. of medicinal food. 7: 372-376.
Jainu, M. and Devi, C.S. 2006a. Gastroprotective action of Cissus quadrangularis extract against NSAID induced gastric ulcer: Role of proinflammatory cytokines and oxidative damage. Chemico-Biological Interactions. 161: 262-270.
Jainu, M., Mohan, K.V. and Devi, C.S. 2006b. Protective effect of Cissus quadrangularis on neutrophil mediated tissue injury induced by aspirin in rats. J Ethnopharmacol. 104: 302-305.
Jainu, M., Mohan, K.V. and Devi, C.S. 2006c. Gastroprotective effect of Cissus quadrangularis extract in rats with experimentally induced ulcer. Indian J Med Res. 123: 799-806.
Jakikasem, S., P. Limsiriwong., T. Kajsongkarm and T. Sontorntanasart. 2000. Phytochemical Study ofCissus quadrangularis. Thai J Pharm Sci. 24-25.
Jee, W.S.S. and Yao, W. 2001. Overview: animal models of osteopenia and osteoporosis. J. Musculoskeletal and Neuronal Interactions. 1: 193-207.
Johnson, D.C. 1996. Exotic Companion Medicine Handbook for Veterinarians. Lake Worth, FL: Zoological Education Network.
Joldersma, M., J. Klein-Nulend and A.M. Oleksik. 2001. Estrogen enhances mechanical stress-induced prostaglandin production by bone cells from elderly women. Am J Physiol Endocrinol Metab. 280: 436−442.
Jordan, V.C. 2001. The past, present, and future of selective estrogen receptor modulation. Ann NY Acad Sci. 949: 72-79.
Justin Raj, S. 2011. Pharmacognostic and Traditional Properties of Cissus Quadrancularis Linn: An overview. Vol 2. International Journal of Pharma and Bio Sciences. India
Kalfas, I.H. 2001. Principles of Bone Healing. J. Neurosurg. Focus. 10: 1
Kamnenou, A., M. Karayannopoulou., Z.S. Polizopoulou., T.C. Constantinidis and A. Dessiris. 2005. Correlation of Serum Alkaline Phosphatase Activity with the Healing Process of Long Bone Fractures in Dogs. J. Vet. Clin. Pathol. 34: 35-38.
Kanis, J.A. 2010. Osteoporosis. J. Medical Sciences. 3: 124-130.
Kashikar, N.D. and George, I. 2006. Antibacterial activity of Cissus quadrangularis Linn. Indian J Pharm Sci. 68: 245-247.
Kerr, M.G. 2002. Veterinary Laboratory medicine, Second edition, Blackwell science.
Kini, U. and Nandeesh, B.N. 2012. Physiology of Bone Formation, Remodeling, and Metabolism. J. Clinical Chemistry. 3: 31-48.
Krum, S. A., C.G.A. Miranda., P.V. Hauschka., J.S. Carroll., T.F. Lane., L.P. Freedman and M. Brown. 2008. Estrogen Protects Bone by Inducing Fas Ligand in Osteoblasts to Regulate Osteoclast Survival. J. EMBO. 27: 535-545.
Kurdy, N.M. 2000. Serology of abnormal fracture healing: the role of PIIINP, PICP, and BsALP. J. Orthop. Traumatol. 14: 48-53.
Lelovas, P. P., T.T. Xanthos., S.E. Thoma., G.P. Lyritis., I.A. Dontas. 2008. The laboratory rat as an animal model for osteoporosis research. Comparative Medicine. 58: 424-430.
Link, T.M. and Majumdar, S. 2003. Osteoporosis imaging. Radiol Clin North Am. 41: 813-839.
Malhotra, N and Mithal, A. 2008. Osteoporosis in Indians. Indian J Med Res. 127: 263-268.
Marsh, D.R. and Li, G. 1999. The biology of fracture healing: Optimising outcome, British Medical Bulletin. 55: 856-869.
Mauck, K.F. and Clarke, B.L. 2006. Diagnosis, screening, prevention, and treatment of osteoporosis. Mayo Clin Proc. 81: 662-672.
Mohanty, A., P.K. Sahu and C. Das. 2010. Wound healing activities of methanolic extract of Cissus quadrangularis on albino rat. International Journal of Drug Formulation & Research. 1: 176-184.
Moors, P.J. 1990. Norway rat. In: The handbook of New Zealand Mammals. (King, C.M., Ed.) Oxford University Press, Aukland. 192-206.
Muñoz-Torres, M., G. Alonso and R.P. Mezquita. 2003. Prevención y tratamiento de la osteoporosis. J. Endocrinol Nutr. 50: 1-7.
Nakagawa, H., Kamimura, M., Takahara, K., Hashidate H., Kawaguchi, A., Uchiyama, S. and Miyasaka, T. 2006. Changes in Total Alkaline Phosphatase Level After Hip Fracture: Comparison Between Femoral Neck and Trochanter Fractures. J. Orthop. Sci. 11: 135-139.
Namkung-Matthai, H., R. Appleyard., J. Jansen., J. Hao Lin., S. Maas-Tricht., M. Swain., R.S. Mason., G.A. Murrell., A. Diwan., T. Diamond. 2001. Osteoporosis Influences The Early Period Of Fracture Healing In A Rat Osteoporotic Model. J. Bone. 28: 80-86
NCCLS. 2004. Application of biochemical markers of bone turnover in the assessment and monitoring of bone disease. Approved guideline. NCCLS document C48-A (ISBN !-56238-539-9) NCCLS, 940 West Valley Road, Suite 1400, Wayne, Pennsylvania 19087-1898 USA.
Olds, R.J. and Olds, J.R. 1979. A colour atlas of the rat dissection guide. Wolfe Medical Public. Ltd. Italy.
Panda, S., and A. P. Das, 2004. Flora of Sambalpur, Dishen Singh Mahendra Pal Singh, Dehradun.
Panthong, A., W. Supraditaporn., D. Kanjanapothi., T. Taesotikul and V. Reutrakul. 2007. Analgesic, anti-inflammatory and venotonic effects of Cissus quadrangularis Linn. J Ethnopharmacol. 110: 264-270.
Paskalev, Priyanka, V. and Rekha, V. 2012. Analgesic, anti-inflammatory and antipyretic activity of Cissus quadrangularis. J Pharm Sci Res. 2: 64-71.
Potu, B.K., K.M. Bhat., M.S. Rao., G.K. Nampurath., M.R. Chamallamudi., S.R. Nayak and M.S. Muttigi. 2009. Petroleum Ether Extract Of Cissus
quadrangularis (Linn.) Enhances Bone Marrow Mesenchymal Stem Cell
Proliferation And Facilitates Osteoblastogenesis. Clinical Science. 64: 993-998.
Raisz LA (2005) Pathogeneis of osteoporosis: concepts, conflicts, and prospects. J Clin Invest 115: 3318–3325.
Rao, M.S., B.P. Kumar., N.V.B. Swamy and G.N. Kutty. 2007 Cissus quadrangularis Plant Extract Exhances the Development of Cortical Bone and Trabeculae in the Fetal Femur. J. Pharmacologyonline 3: 190-202.
Reddy, P.N. and Lakshmana, M. 2003. Prevention of Bone Loss in Calcium Deficient Ovariectomized Rats by OST-6 a Herbal Preparation. J. Ethno. Pharm. 84: 259-264.
Rey, J. R. C., E.V. Cervino., M.L. Rentero., E.C. Crespo., A.O. Alvaro and M. Casillas. 2009. Raloxifene: Mechanism of Action, Effects on Bone Tissue, and Applicability in Clinical Traumatology Practice. J. The Open Orthopaedics. 3: 14-21.
Sabri, M., Nurhidayat., K. Sigit., B.P. Priosoeryanto and W. Manalu. 2011. Kualitas Tulang Tikus Betina Normal yang diberi Ekstrak Sipatah-patah pada Masa Pertumbuhan. J. Veteriner; 12: 113-119.
Sarac, F. and Sagili, F. 2007. Causes of High Bone Alkaline Phospatase. J.Biotechnol & Biotechnol (abstr.): 21.
Shapiro, F. 2008.Bone Development and its Relation to Fracture Repair. The Role of Mesenchymal Osteoblasts and Surface Osteoblasts. J. European Cells and Materials. 15: 53-76.
Shirwaikar, A., S. Khan and S. Malini. 2003. Antiosteoporotic Effect of Ethanol Extracts of Cissus quadrangularis Linn on Ovariectomized Rat. Journal of Ethnophramacolog. 89: 245-50.
Singh, N., Singh, V., Singh, R. K., Pant, A. B., Pal, U. S., Malkunje, L. R., Mehta, Gagan. 2013. Osteogenic Potential Of Cissus Qudrangularis Assessed With Osteopontin Expression. National Journal Of Maxillofacial Surgery. Vol 4 Issue 1.
Sousa, C., H. Abreu., C. Viegas., J. Azevedo,, R. Reis,, M. Gomes and I. Dias. 2011. Serum total and bone alkaline phosphatase and tartrate-resistant acid phosphatase activities for the assessment of bone fracture healing in dogs. Arq. Bras. Med. Vet. Zootec. 63: 1007-1011.
Srisook, K., M. Palachot., N. Mongkol., E. Srisook and S. Sarapusit. 2010. Antiinflammatory effect of ethyl acetate extract from Cissus quadrangularis Linn may be involved with induction of heme oxygenase-1 and suppression of NF-kappaB activation. J. Ethnopharmacol., 133: 1008-1014.
Steel, R., and Torrie, J. 1993. Prinsip Dan Prosedur Statistika (Suatu Pendekatan Biometrik). Jakarta: Pt. Gramedia Pustaka Utama.
Tanna, N. 2005. Osteoporosis and its Prevention. J. Pharm. 275: 521- 24.
Tuck, S.P. and Francis, R.M. 2002. Osteoporosis. Postgrad Med J. 78: 526-532.
Viguet-Carrin, S., P. Garnero and P.D. Delmas. 2006. The role of collagen in bone strength. J. Osteoporos Int. 17: 319–336
Whelan D.B, Bhandari, M., Stephen, D., Kreder, H., Mc Kee, M.D., Zdero, R. and Scemitsch, E.H. 2010. Development fo the radiograpic union score for tibial fractures for the assesment of tibial fracture healing after the medullary fixation. J Trauma. 68: 629-632.
Yigit, N., E. Colak., M. Sozen and S. Ozkurt. 1998. Tr. J. of Zoology. 22: 203-212.
Yudaniayanti, Is., L. Nangoi and J. Soepraptini. 2014. Correlation of Serum ALP Activity with the Healing Process of Femoral Fractures in Rats Used Cissus quadrangularis Extract as Therapy. J. Phys Pharm Adv. 4: 298-302.
Appendix 1. Preparation ofCissus quadrangularisethanolic extract
The fleshy stem and leaves of Cissus quadrangularis are washed, cut into
small pieces and air dried. Dried Cissus quadrangularis are mashed and ground
until it turned into powder. The dry powder then was extracted using maceration
method.
In maceration method, the dry powder was soaked with ethanol 96% and
then sonicated for 10 minutes. The next step is allow to incubate for one night and
then filtered using bugner funnel. The remaining residue was added with ethanol
96%, and then stirred and filtered again. Maceration was performed three times,
the result solution were pooled and evaporated using rotavapour, so the condensed
Appendix 2. Dosage Calculation
Dosage Calculation for 1stand 2nd weeks
1. The dilution process was done once every two days. From the calculation
of Cissus quadrangularisextract dilution, the extract amount given to the
rats (Rattus norvegicus)with weigh±200 g was:
Cissus quadrangularis: 750 mg/kg BW= 150mg/200 g BW
Raloxifene: 5.4 mg/kg BW = 1.08mg/200 g BW
2. Cissus quadrangularis(CQ) extract needed for 3 days:
22 rats x 3 days x CQ dose = 22 x 3 x 150 mg = 9900 mg = 9.9 g
- Aquadest volume needed: 22 rats x 3 days x 1.5 ml = 99 ml
- 0.5 % Na CMC :
0.5 g/100 ml = a/99
a = 99 x 0.5 / 100
a = 0.495 g = 495 mg
- 495 mg Na CMC was poured to petri disc and added with 99 ml
aquadest 80oC and the 9.9 g CQ extract slowly and stir well. The