www.elsevier.com/locate/ibmb
Evidence of poly(ADP-ribosylation) in the cockroach
Periplaneta americana
Marco Denegri
a, Simonetta Lambiase
b, Chiara Donadoni
a, Laura Rossi
a,
A. Ivana Scovassi
a,*aIstituto di Genetica Biochimica ed Evoluzionistica CNR, Via Abbiategrasso 207, I-27100 Pavia, Italy
bDipartimento di Biologia Animale, Laboratorio di Entomologia, Universita` di Pavia, Via Taramelli 24, I-27100 Pavia, Italy Received 25 January 2000; received in revised form 16 March 2000; accepted 31 March 2000
Abstract
Poly(ADP-ribosylation) is a post-translational modification of nuclear proteins typical of most eukaryotic cells. This process participates in DNA replication and repair and is mainly regulated by two enzymes, poly(ADP-ribose) polymerase, which is respon-sible for the synthesis of polymers of ADP-ribose, and poly(ADP-ribose) glycohydrolase, which performs polymer degradation. The aim of this work was to investigate in the cockroach Periplaneta americana L. (Blattaria: Blattidae) the behaviour of poly(ADP-ribosylation). In particular, we addressed: (i) the possible modulation of poly(ADP-ribosylation) during the embryonic development; (ii) the expression of ribose) polymerase and glycohydrolase in different tissues; and (iii) the role of poly(ADP-ribosylation) during spermatogenesis. In this work we demonstrated that: (i) as revealed by specific biochemical assays, active poly(ADP-ribose) polymerase and glycohydrolase are present exclusively in P. americana embryos at early stages of development; (ii) an activity carrying out ribose) synthesis was found in extracts from testes; and (iii) the synthesis of poly(ADP-ribose) occurs preferentially in differentiating spermatids/spermatozoa.
Collectively, our results indicate that the poly(ADP-ribosylation) process in P. americana, which is a hemimetabolous insect, displays catalytical and structural features similar to those described in the holometabolous insects and in mammalian cells. Further-more, this process appears to be modulated during embryonic development and spermatogenesis.2000 Elsevier Science Ltd. All rights reserved.
Keywords: Poly(ADP-ribosylation); Insect development; Spermatogenesis; PARP; PARG
1. Introduction
Poly(ADP-ribosylation) is a post-translational modi-fication of proteins controlled mainly by the action of two enzymes: poly(ADP-ribose) polymerase (PARP; EC 2.4.2.30) and poly(ADP-ribose) glycohydrolase (PARG). This process modulates many biological func-tions like DNA repair, DNA amplification, cell cycle, transformation, carcinogenesis and cell death (for a review, see Althaus and Richter, 1987). PARP uses NAD as substrate for transferring ADP-ribose moieties to nuclear acceptor proteins (histones, non-histone
* Corresponding author. Tel.:+39-0382-546334/8; fax: + 39-0382-422286.
E-mail address: [email protected] (A. Ivana Scovassi).
0965-1748/00/$ - see front matter2000 Elsevier Science Ltd. All rights reserved. PII: S 0 9 6 5 - 1 7 4 8 ( 0 0 ) 0 0 0 7 6 - X
Among holometabolous insects, poly(ADP-ribosylation) reactions have been described in Ceratitis capitata (Cavalloro et al., 1988), Drosophila melanogaster (Uchida et al., 1993b)) and Sarcophaga peregrina (Masutani et al., 1994).
The aim of this work was to investigate the presence of poly(ADP-ribosylation) in the insect Periplaneta
americana (Blattaria: Blattidae), which differs from
those previously studied being a hemimetabolous. Fur-thermore, we focused on the possible modulation of poly(ADP-ribosylation) during the embryonic develop-ment and during spermatogenesis. We demonstrated for the first time in the hemimetabolous insect P. americana the presence of poly(ADP-ribosylation), which is strictly regulated during physiological processes.
2. Materials and methods
2.1. Animals
The insects were reared in a controlled environment at 26°C and about 65% RH; a natural daily rhythm of light and darkness was followed. They were fed on a mixture of fresh lettuce and bread crumbs and received water ad libitum. The age of the ootechae was estimated from the time of their deposition (age 0). We utilised embryos aged from day 4 to day 31, covering all the embryonic developmental stages (Lenoir-Russeaux and Lender, 1970; Lambiase et al., 1997). The ootechae were opened in cold PBS and the embryos were immediately frozen in liquid nitrogen. Embryos recovered until day 15 were deprived of the deutoplasm. Ventral nerve cord, abdominal muscles, gonads, accessory glands and abdominal fat bodies were collected from adult males and females sacrificed under anaesthesia with CO2, and were dissected in PBS.
2.2. Immunohistochemical assay
Testes from adult insects were dissected out, fixed in Carnoy mixture and then routinely processed for paraffin embedding. Deparaffinized and rehydrated 8-mm thick sections were processed for the immunocytochemical detection of PARP with the anti-poly(ADP-ribose) monoclonal antibody 10H (Kawamitsu et al., 1984) diluted 1:10 in PBS, followed by incubation with a biotin-conjugated anti-mouse antibody and a streptavid-ine-peroxidase complex (1 h each, Histomark products). Finally, the sections were treated with 0.03% 3,39 -diami-nobenzidine tetrahydrochloride in 50 mM Tris–HCl, pH 7.6 containing 0.02% H2O2. In the control sections, the primary antibody was omitted.
2.3. Poly(ADP-ribose) polymerase assay
Crude extracts were obtained by suspending 100 mg of embryos at different ages or from adult organs in 100
µl of 1.5 M NaCl, 50 mM Tris–HCl pH 7.5, 0.5 mM dithiothreitol, 1 mM phenylmethyl sulfonylfluoride, 1 mM EDTA, 10 mM sodium bisulfite, pH 7.5, and 1µM pepstatin. The suspension was sonicated twice for 20 s at 50 W in ice and centrifuged at 4°C for 10 min at 13,000 rpm. The supernatants were used immediately as enzyme extracts for PARP assay, activity gel and West-ern blot. (a) PARP assay was carried out as previously reported (Scovassi et al., 1986). One unit of enzymatic activity corresponds to the incorporation of 1 nmol of NAD into acid-insoluble material/h at 25°C. (b) PARP autoribosylation was evaluated by the activity gel tech-nique essentially as described by Scovassi et al. (1986). The radioactive gel was autoradiographed with Kodak AR film or exposed to the PhosphorImager 445 SI (Molecular Dynamics). (c) Western blot analysis was carried out following a standard procedure (Donzelli et al., 1999) with the exception that C-2-10 monoclonal antibody to PARP was diluted 1:7500.
2.4. Poly(ADP-ribose)glycohydrolase assay
2.4.1. Thin layer chromatography
32P-labeled polymer was synthesized by
automodifi-cation of partially purified calf thymus PARP (Bernardi et al., 1997). PARG exoglycosidic activity was evaluated by thin layer chromatography (TLC) on PEI-F cellulose plates developed in 99% methanol and, subsequently, in 0.3 M LiCl, 0.9 M acetic acid. After autoradiography, the radioactivity of the spots corresponding to the mono and poly(ADP-ribose) was quantified using a Phos-phorImager 445 SI. The hydrolysis ratio and PARG spe-cific activity were calculated according to Bernardi et al. (1997).
2.4.2. High resolution gel
blue (BPB), that comigrates with the octamer of ADP-ribose, and xylene cyanol (XC), that migrates with the 20-mer of ADP-ribose.
3. Results and discussion
Poly(ADP-ribosylation) has a relevant role during embryonic development and differentiation (Althaus and Richter, 1987). To test the behaviour and the possible modulation of this process during the P. americana development, we have analyzed PARP and PARG enzy-matic activities in extracts from embryos at different developmental stages, from the 4th to the 31st day. PARP activity was measured by a specific biochemical assay based on the incorporation of32P-NAD. The high-est value of PARP specific activity was found in samples recovered after 9 days (0.87 U/mg of proteins compared with 0.35 U/mg of proteins in both 14- and 31-day-old embryos). This observation was confirmed by the activity gel assay for PARP, based on the autoribosyl-ation property of the enzyme. We have revealed only one active band of 116 KDa, which was visible mainly in extracts from 9-day-old embryos (Fig. 1A). Western blot analysis showed an immunoreactive band of 116 KDa in all the extracts (Fig. 1B). These results indicate that PARP from P. americana exhibits structural and catalytic properties similar to PARP from HeLa cells.
PARG activity was evaluated by a chromatographic assay able to discriminate between mono and poly(ADP-ribose), which was recently applied to the analysis of the enzyme during human cell apoptosis (Bernardi et al., 1997). As illustrated in Fig. 1C, the conversion of the synthetic32P-polymer into the monomer of ADP-ribose was carried out essentially in the extracts prepared from embryos recovered on day 9 and day 14 (hydrolysis ratios 0.122 and 0.082, respectively). The product of the reaction was identical to that of purified PARG (Fig. 1C) and of HeLa cells (not shown). The specific activity of PARG, calculated from the hydrolysis ratio, is reported in Fig. 1D. As it is clearly shown, 9-day-old embryos exhibited the highest PARG activity (34 mU/mg of protein), whereas the level of PARG in samples har-vested later on during the development was very low (3.5 and 5.8 mU/mg on day 15 and day 31, respectively). To further support the presence of an active PARG in P. americana embryos, we have separated on a 20% polyacrylamide gel the product of the reaction on syn-thetic 32P-poly(ADP-ribose) by the endogenous PARG present in the extracts. The autoradiography revealed that the enzyme from 9-day-old embryos was able to convert the long32P-polymers synthesized in vitro, into short polymers, as expected for an active PARG (Fig. 2). The reaction was carried out to a lesser extent by extracts prepared from 15-day-old embryos, while samples at later stages of development (31 days)
exhib-Fig. 1. Analysis of poly(ADP-ribose) polymerase (A, B) and gly-cohydrolase (C, D) in P. americana embryos. (A) In extracts from 9-, 15- and 31-day-old embryos, PARP autoribosylation was evaluated by the activity gel. HeLa cell extract: positive control. (B) Western blot analysis of PARP was carried out by using the monoclonal antibody C-2-10. HeLa: positive control. (C) Poly(ADP-ribose) degradation to monomers of ADP-ribose was evaluated by TLC in embryos at 9, 15 and 31 days of development. Purified PARG: positive control. Poly(ADP-ribose) hydrolysis ratio was reported. (D) PARG specific activity.
ited a very low capacity of degrading the substrate (Fig. 2).
Fig. 2. Evaluation of poly(ADP-ribose) hydrolysis performed by insect PARG. Radiolabelled poly(ADP-ribose) (1×105cpm), was syn-thesized in vitro as described in Section 2, and the products of the reaction of P. americana extracts on the32P-polymer were separated on a 20% polyacrylamide gel and autoradiographed. For each assay, 1×103cpm were loaded. Extracts were prepared from 9-, 15- and 31-day-old embryos. BPB and XC comigrate with an octamer and a 20-mer of ADP-ribose, respectively.
early stage, irrespective of the form of development (hemimetabolous vs holometabolous).
Furthermore, we investigated PARP distribution in different tissues from adults, males and females, by isol-ating muscles, ventral cord, fat bodies, accessory glands, testes and ovary. As illustrated in Fig. 3, we found an active PARP band exclusively in the extract prepared from testes. The presence in this tissue of a protein
syn-Fig. 3. Activity gel of PARP autoribosylation in different tissues from adult P. americana: m: abdominal muscle; n: ventral nerve cord; t: testis; o: ovary; f: abdominal fat body; a: accessory gland. HeLa cell extract was used as positive control.
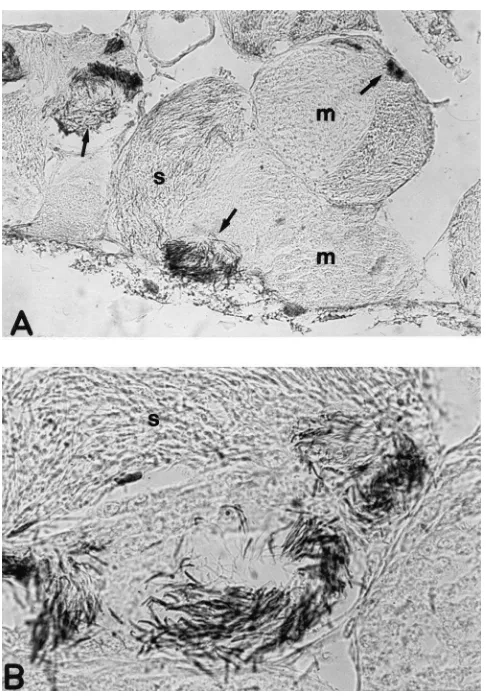
thesizing poly(ADP-ribose) was further supported by immunohistochemistry experiments performed on testis sections by using the monoclonal antibody 10H, which recognizes poly(ADP-ribose) (Kawamitsu et al., 1984). The positive staining revealed that testes are rich in poly(ADP-ribose) at different stages of spermatogenesis (Fig. 4). However, the positive signal was confined to the spermatocists with spermatids in the late differentiating phase, and to spermatozoa, while no staining was revealed in other components of the spermatocists. Moreover, only the head of the differentiating spermatids/spermatozoa appears to be labeled.
These results have been obtained by the same anti-body recently used against the polymers of ADP-ribose by Lankenau et al. (1999) to demonstrate the synthesis of poly(ADP-ribose) in Drosophila testes in response to
γ-irradiation. Remarkably, for the first time our data sup-port the presence of poly(ADP-ribose) in insect testes under physiological conditions and not after a DNA-damaging agent. Furthermore, our findings corroborate previous results obtained on PARP activity in rat testes (Quesada et al., 1989), and on poly(ADP-ribosylation) during rooster spermatogenesis (Corominas and Mez-quita, 1985).
In conclusion, our study provided the first evidence that Blattaria, which belong to the hemimetabolous form of insects, display a “classical” poly(ADP-ribosylation), as previously described for the holometabolous insects
C. capitata (Cavalloro et al., 1988), D. melanogaster
(Uchida et al., 1993b) and S. peregrina (Masutani et al., 1994). As for Drosophila and mammalian testes, the pro-cess appears to be modulated during the embryonic development and is mainly active in the early steps, which are characterized by a high rate of cell division. Among the different adult tissues examined, we have found the presence of poly(ADP-ribose) in testes, prob-ably because of the high rate of duplicative events in this tissue.
Acknowledgements
Fig. 4. Immunolocalization of poly(ADP-ribose) in testis from adult
P. americana. Longitudinal sections were stained by
immunohistoch-emistry with the monoclonal antibody 10H against the polymer of ADP-ribose. (A) Spermatocists with synchronous cellular populations at different maturation stages; arrows: positive area in the testis; mag-nification: 100×. (B) Late differentiating spermatids and head of sper-matozoa are positive for poly(ADP-ribose); magnification: 500×; m: meiocytes; s: spermatids.
PARG and PARP enzymes and with C-2-10 antibody to PARP, and to Prof. A. Bu¨rkle (DKFZ, Heidelberg, Germany) for 10H antibody. We thank Prof. C. Pellicci-ari and Prof. U. Laudani (University of Pavia, Italy) for invaluable discussions. The technical support of Alberto Tronconi is acknowledged. M.D. and L.R. are students from Scuola di Specialita` in Genetica Applicata and Dot-torato in Fisiopatologia Sperimentale of the University of Pavia, respectively.
References
Althaus, F.R., Richter, C., 1987. ADP-ribosylation of proteins. Mol. Biol. Biochem. Biophys. 37, 1–237.
Beneke, S., Meyer, R., Bu¨rkle, A., 1997. Isolation of cDNA encoding full-length rat (Rattus norvegicus) poly(ADP-ribose) polymerase. Biochem. Mol. Biol. Int. 43, 755–761.
Bernardi, R., Rossi, L., Poirier, G.G., Scovassi, A.I., 1997. Analysis of
poly(ADP-ribose) glycohydrolase activity in nuclear extracts from mammalian cells. Biochim. Biophys. Acta 1338, 60–68. Brightwell, M.D., Leech, C.E., O’Farrell, M.K., Whish, W.J.D., Shall,
S., 1975. Poly(adenosine diphosphate ribose) polymerase in
Physa-rum polycephalum. Biochem. J. 147, 119–129.
Cavalloro, R., Scovassi, A.I., Izzo, R., Bertazzoni, U., 1988. ADP-ribosyl transferase in Ceratitis capitata WIED cells and embryos. In: Kuroda, Y., Kurstak, E., Maramorosch, K. (Eds.), Invertebrate and Fish Tissue Culture. Japan Scientific Societies Press/Springer-Verlag, Tokyo/Berlin, pp. 53–57.
Corominas, M., Mezquita, C., 1985. Poly(ADP-ribosylation) at suc-cessive stages of rooster spermatogenesis. J. Biol. Chem. 260, 16269–16273.
de Murcia, G., Me´nissier de Murcia, J., 1994. Poly(ADP-ribose) poly-merase: a molecular nick sensor. Trends Biochem. Sci. 19, 172– 176.
Donzelli, M., Bernardi, R., Negri, C., Prosperi, E., Padovan, L., Lavi-alle, C., Brison, O., Scovassi, A.I., 1999. Apoptosis-prone pheno-type of human colon carcinoma cells with a high level amplification of the c-myc gene. Oncogene 18, 439–448.
Hanai, S., Uchida, M., Kobayashi, S., Miwa, M., Ueda, K., 1998. Gen-omic organization of Drosophila poly(ADP-ribose) polymerase and distribution of its mRNA during development. J. Biol. Chem. 273, 11881–11886.
Ittel, M.E., Garnier, J.M., Jeltsch, J.M., Niedergang, C.P., 1991. Chicken poly(ADP-ribose) synthetase: complete deduced amino acid sequence and comparison with mammalian enzyme sequence. Gene 102, 157–164.
Kawamitsu, H., Hoshino, H., Okada, H., Miwa, M., Momoi, H., Sugi-mura, T., 1984. Monoclonal antibodies to poly(adenosine diphos-phate ribose) recognize different structures. Biochemistry 23, 3771–3777.
Lambiase, S., Grigolo, A., Laudani, U., Sacchi, L., Baccetti, B., 1997. Pattern of bacteriocyte formation in Periplaneta americana (L.) (Blattaria: Blattidae). Int. J. Insect Morphol. Embryol. 26, 9–19. Lankenau, S., Bu¨rkle, A., Lankenau, D.-H., 1999. Detection of
poly(ADP-ribose) synthesis in Drosophila testes uponγ-irradiation. Chromosoma 108, 44–51.
Lenoir-Russeaux, J.J., Lender, T., 1970. Table de de´veloppement embryonnaire de Periplaneta americana (L.): Insecte. Dictyopte`re. Bull. Soc. Zool. Fr. 95, 737–751.
Masutani, M., Nozaki, T., Hitomi, Y., Ikejima, M., Nagasaki, K., de Prati, A.C., Kurata, S., Natori, S., Sugimura, T., Esumi, H., 1994. Cloning and functional expression of poly(ADP-ribose) polymerase cDNA from Sarcophaga peregrina. Eur. J. Biochem. 220, 607– 614.
Okolie, E.E., Onyezili, N.I., 1983. ADP-ribosyltransferase in Plasmod-ium (malaria parasites). Biochem. J. 209, 687–693.
Ozawa, Y., Uchida, K., Uchida, M., Ami, Y., Kushida, S., Okada, N., Miwa, M., 1993. Isolation of cDNAs encoding the catalytic domain of poly(ADP-ribose) polymerase from Xenopus laevis and cherry salmon using heterologous oligonucleotide consensus sequences. Biochem. Biophys. Res. Commun. 193, 119–125.
Panzeter, P.L., Althaus, F.R., 1990. High resolution size analysis of ADP-ribose polymers using modified DNA sequencing gels. Nucleic Acids Res. 18, 2194.
Quesada, P., Farina, B., Jones, R., 1989. Poly(ADP-ribosylation) of nuclear proteins in rat testis correlates with active spermatogenesis. Biochim. Biophys. Acta 1007, 167–175.
Rickwood, D., Osman, M.S., 1979. Characterisation of poly(ADP-Rib) polymerase activity in nuclei from the slime mould Dictyostelium
discoideum. Mol. Cell. Biochem. 27, 79–84.
Scovassi, A.I., Izzo, R., Franchi, E., Bertazzoni, U., 1986. Structural analysis of poly(ADP-ribose) polymerase in higher and lower euka-ryotes. Eur. J. Biochem. 159, 77–84.
and nucleoli of Tetrahymena pyriformis. FEBS Letters 93, 297– 300.
Uchida, K., Morita, T., Sato, T., Ogura, T., Yamashita, R., Noguchi, S., Suzuki, H., Nyunoya, H., Miwa, M., Sugimura, T., 1987. Nucle-otide sequence of a full-length cDNA for human fibroblast poly(ADP-ribose) polymerase. Biochem. Biophys. Res. Commun. 148, 617–622.
Uchida, K., Uchida, M., Hanai, S., Ozawa, Y., Ami, Y., Kushida, S., Miwa, M., 1993a. Isolation of the poly(ADP-ribose) polymerase-encoding cDNA from Xenopus laevis: phylogenetic conservation of the functional domains. Gene 137, 293–297.
Uchida, K., Hanai, S., Ishikawa, K., Ozawa, Y., Uchida, M., Sugimura, T., Miwa, M., 1993b. Cloning of cDNA encoding Drosophila poly(ADP-ribose) polymerase: leucine zipper in the auto-modifi-cation domain. Proc. Natl. Acad. Sci. USA 90, 3481–3485. Werner, E., Sohst, S., Gropp, F., Simon, D., Wagner, H., Kroger, H.,
1984. Presence of ribose) polymerase and poly(ADP-ribose) glycohydrolase in the dinoflagellate Crypthecodinium