www.elsevier.com/locate/ibmb
Juvenile hormone biosynthesis by corpora allata of larval tomato
moth, Lacanobia oleracea, and regulation by Manduca sexta
allatostatin and allatotropin
Neil Audsley
*, Robert J. Weaver, John P. Edwards
Central Science Laboratory, Sand Hutton, York YO41 1LZ, UK
Received 31 October 1999; received in revised form 31 December 1999; accepted 25 January 2000
Abstract
Juvenile hormone (JH) biosynthesis and the effects of synthetic Manduca sexta allatostatin (Mas-AS) and M. sexta allatotropin (Mas-AT) were investigated in isolated corpora allata (CA) of Vth stadium larvae of the tomato moth, Lacanobia oleracea. Reversed-phase high-performance liquid chromatography (RP-HPLC) of JH extracted from CA shows that larvae produce predominantly JH II and its corresponding acid. It appears that the acid homologue is a result of JH esterase activity in the CA (and other tissues) rather than the lack of JH acid methyltransferase. Mean rates of synthesis (100–200 fmol/pr/h) were inhibited ca. 70% by Mas-AS and stimulated in a dose-dependent manner up to three times by Mas-AT. However, Mas-AS had no significant effect on Mas-AT-stimulated rates of JH biosynthesis. Using RP-HPLC and an enzyme-linked immunosorbent assay (ELISA) to Mas-AT, a peak of Mas-AT-like immunoreactivity was detected in larval L. oleracea brain homogenates. Co-elution of this immunoreactive peak with synthetic Mas-AT suggests that this neuropeptide is also present in L. oleracea. Crown Copyright 2000 Published by Elsevier Science Ltd. All rights reserved.
Keywords: Allatostatin; Allatotropin; Corpora allata; Juvenile hormone; Juvenile hormone acid; Insect; Lacanobia; Manduca; Neuropeptide
1. Introduction
Very little is known about the nature of the biosynth-esis of juvenile hormone (JH) in the corpora allata (CA) of larval Lepidoptera, research in this area having been restricted to the tobacco hornworm, Manduca sexta (Schooley et al., 1973; Kramer and Law, 1980; Bhaska-ran, 1981; Sparagana et al., 1984; Bhaskaran et al. 1986, 1987; Janzen et al., 1991), the gypsy moth, Lymantria
dispar (Jones and Yin, 1989), the Egyptian cotton
leaf-worm, Spodoptera littoralis (Pfister-Wilhelm and Lanzr-ein, 1996), the wax moth, Galleria mellonella (Bogus and Scheller 1991, 1996), and more recently the tomato moth, Lacanobia oleracea (Audsley et al., 1999a,b). Consequently, the hormonal control of JH biosynthesis
Abbreviations: Manduca sexta allatostatin (Mas-AS) (pEVRFRQCYFNPISCF–OH); Manduca sexta allatotropin (Mas-AT) (GFKNVEMMTARGF–NH2).
* Corresponding author. Tel.: +44-1904-462628; fax: +44-1904-462111.
E-mail address: [email protected] (N. Audsley).
0965-1748/00/$ - see front matter. Crown Copyright2000 Published by Elsevier Science Ltd. All rights reserved. PII: S 0 9 6 5 - 1 7 4 8 ( 0 0 ) 0 0 0 3 9 - 4
by CA in larval Lepidoptera has received even less atten-tion and has only been investigated in M. sexta (Kataoka et al., 1989; Kramer et al., 1991; Janzen et al., 1991),
Pseudaletia unipuncta (Jansons et al., 1996), G. mel-lonella (Bogus and Scheller 1991, 1996) and L. oleracea
(Audsley et al., 1999b). From the pharate adult of M.
sexta two peptides were characterised which regulate JH
biosynthesis by CA in vitro: a stimulatory allatotropin (Mas-AT; Kataoka et al., 1989) and an inhibitory allatos-tatin (Mas-AS; Kramer et al., 1991). However, although Mas-AT stimulates JH biosynthesis in isolated adult female M. sexta and Heliothis virescens CA, it was reported to have no activity in larval M. sexta (Kataoka et al., 1989), leading to the suggestion that Mas-AT was adult-specific. In contrast, Mas-AS inhibits JH biosynth-esis in vitro by CA from both larval and adult female
M. sexta and adult female H. virescens (Kramer et al.,
1991). An allatotropic factor was partially purified from larval G. mellonella brains (Bogus and Scheller, 1996) which promotes in vitro and in vivo synthesis of JH.
Lepidoptera, namely P. unipuncta (Jansons et al., 1996),
L. oleracea (Audsley et al., 1998) and S. littoralis
(Audsley et al., 1999a), but to date no inhibitory activity of Mas-AS has been demonstrated in any of these larvae. Synthetic Mas-AS does, however, cause up to 60% inhi-bition of JH biosynthesis in CA of adult female P.
unipuncta (Jansons et al., 1996) and 50–60% inhibition
of CA output in adult male and female L. oleracea (Audsley et al., 1999a,b). Recently, Oeh et al. (1999) reported that Mas-AS is active on CA of adult female
Spodoptera frugiperda, but only on Mas-AT-stimulated
levels of JH production.
Bhatt and Horodyski (1999) have demonstrated Mas-AT mRNA and immunoreactivity in M. sexta larval ner-vous system, but this peptide does not stimulate JH biosynthesis in larval CA of M. sexta (Kataoka et al., 1989) or L. oleracea (Audsley et al., 1999b). M. sexta allatotropin does, however, stimulate JH synthesis in CA of adult female M. sexta (Kataoka et al., 1989), L.
olera-cea (Audsley et al., 1999b) and S. frugiperda (Oeh et
al., 1999). Audsley et al. (1999a,b) suggest that the low rates of JH biosynthesis observed in larval L. oleracea and the apparent inactivity of Mas-AS and Mas-AT may be due to the assay procedures used to measure JH lev-els, rather than to an actual lack of bioactivity.
This study investigates these possibilities and deter-mines that JHs produced by CA of larval L. oleracea are converted to acid homologues by JH esterases, and reports for the first time the regulation of JH biosynthesis in the CA of a larval lepidopteran by both an allatostatin and allatotropin first characterised from M. sexta (Kataoka et al., 1989; Kramer et al., 1991). In addition to the identification of Mas-AS in L. oleracea (Audsley et al., 1998), evidence is presented that a Mas-AT immu-noreactive peptide is present in this noctuid.
2. Materials and methods
2.1. Experimental animals
L. oleracea were reared at 20°C and 65% relative humidity under a 16 h light/8 h dark photoperiod. Larvae were fed on a maize-flour-based noctuid artificial diet (Korano Ltd, Montalieu, France), and adults on a sol-ution of 10% sucrose, 2 g/l (w/v) methyl-4-hydroxyben-zoate and 2% (v/v) of mixed vitamins.
2.2. JH biosynthesis assay
Corpora allata were dissected from last-day Vth instar larval L. oleracea (Vth=penultimate stadium), and the rate of JH biosynthesis determined by the incorporation of a radiolabel using procedures derived from those developed by Pratt and Tobe (1974). Five pairs (pr) of CA were incubated at 30°C for 4 h in 100µl of
methion-ine-free tissue-culture medium (medium 199; Gib-coBRL, catalogue no. 041-90009 H) which contained Hanks salts and 25 mM HEPES buffer, pH 7.2, and to which was added 2% Ficoll, 0.1% bovine serum albumin (protease-free), 0.3% bacitracin, 5 mM calcium chloride, and 30 mM sodium acetate and 200µM methionine. To this was added 0.74 MBq/ml of [1-14C]-propionic acid
(sodium salt, 1.99 GBq/mmol; NEN Life Sciences). It is important to note that propionate does not incorporate into JH III (Schooley and Baker, 1985), and hence this homologue would not be measured by this method.
Synthetic peptides were added to individual incu-bation tubes in 1µl volumes of 70% acetonitrile (Mas-AS) or water (Mas-AT). Similar amounts of solvent alone were added to control CA incubations. To deter-mine the effects of Mas-AS on Mas-AT-stimulated lev-els of JH, both peptides were added at the start of the incubation period. At the end of the incubation period synthetic JH standards were added and samples extracted with 2×500µl ethyl acetate. The organic phase was dried under nitrogen and resuspended in 100µl of 70% ace-tonitrile for analysis by liquid chromatography.
2.3. High-performance liquid chromatography of JHs/JH acids
Reversed-phase high-performance liquid chromato-graphy (RP-HPLC) was performed using a Beckman System Gold chromatographic system, utilising a dual-pump programmable solvent module 126. Samples were loaded via a Rheodyne loop injector on to an Aquapore RP-300, 7µm analytical column (220 mm×4.6 mm; Anachem) fitted with a guard column (30 mm×4.6 mm) of similar packing material. The column was eluted with a linear gradient of 12–80% acetonitrile/5 mM HEPES, pH 7.4, over 20 min at a flow rate of 1 ml/min, and elu-tions were monitored at 245 nm by a System Gold diode-array detector module 168. Fractions (1 ml) were col-lected into 3 ml Ecoscint A (National Diagnostics) for counting on a Beckman LS 6000TA liquid-scintillation spectrometer. Columns were routinely flushed with methanol to remove residual contaminants.
2.4. Chemical conversion of JH to JH acid
The acid homologues of JH I, JH II and JH III were produced using the methods of Goodman (1984). Juven-ile hormone was incubated in the dark at 40°C for 4 h in methanol/1 N NaOH (1:1, v/v). At the end of the incu-bation period 1 N HCl was added to reduce the pH to ca. 5.0, and the mixture extracted with 2×500µl ethyl acetate. The organic phase was dried under N2and
2.5. JH esterase activity
The JH esterase activities of CA from last-day Vth instar larvae were determined by incubating 3H-JH III
(606.8 GBq/mmol, ca. 350 Bq per 5 CA pr; NEN Life Sciences) with CA under normal experimental con-ditions (e.g., in medium-199 for 4 h at 30°C). Corpora allata were either dissected clean of surrounding tissue or with some trachea/fat body attached. At the end of the incubation period the medium was extracted and sub-jected to RP-HPLC as described above to assess the rela-tive amounts of 3H-JH III remaining and 3H-JH III
acid produced.
2.6. Tissue extraction and RP-HPLC separation
Brains (100) were dissected from mixed-aged VIth instar larval L. oleracea, sonicated (Soniprep 150; MSE) in ice-cold acetone and centrifuged at 13,200g for 20 min at 4°C. Acetone was discarded and the pellets were re-extracted (2×) in 75% ethanol/0.2 M HCl. The supernatants were then combined, diluted 10 times with 0.1% trifluoroacetic acid (TFA) and loaded on to a C18
Sep-pak cartridge (Waters), conditioned with bovine serum albumin. The cartridge was eluted stepwise with 5 ml of 20%, 45% and 60% acetonitrile/0.1% TFA. The 45% fraction was diluted to ,10% acetonitrile with 0.1% TFA and fractionated by RP-HPLC using the same system and column as described above, but eluted with a linear gradient of 20–50% acetonitrile/0.1% TFA over 30 min at a flow rate of 1 ml/min, and monitored at 214 nm. Thirty fractions (1 ml) were collected using a RediFRAC fraction collector, and aliquots dried on to a multiwell plate for enzyme-linked immunosorbent assay (ELISA).
2.7. ELISA for Manduca allatotropin
An indirect ELISA for Mas-AT was developed using primary antiserum raised against Mas-AT (Veenstra and Hagedorn, 1993) using similar methods to those reported for Mas-AS ELISA (Audsley et al., 1998).
Briefly, HPLC fractions and Mas-AT were dried on to multiwell plates (Sigma, UK; catalogue no. M4034) at 37°C and then incubated overnight at 4°C with 100µl of 0.1 M bicarbonate (coating) buffer (pH 9.6). Plates were washed three times with 150µl of 10 mmol/l phos-phate buffer/0.1% TWEEN-20 [phosphate-buffered saline (PBS)], blocking solution (150µl; 2% non-fat milk in PBS) was added, and the plates incubated for 90 min at 37°C. After a further PBS wash, 100µl of primary antiserum (1:10,000 dilution) was added to each well and the plates incubated for another 90 min at 37°C. Next, 100µl of goat anti-rabbit antiserum conjugated with horseradish peroxidase (1:3000 dilution in PBS) was added as secondary antibody after washing three
times with PBS. Plates were then incubated for 40 min at 37°C. After a final PBS wash (3×), 100µl of substrate solution (10 mg O-phenylenediamine, 10µl H2O2 in
25 ml citrate buffer, pH 5.0) was added to each well and incubated for 40 min at 37°C. The reaction was stopped by addition of 50µl of 1.0 N H2SO4 to each well and
optical density read at 492 nm on a Labsystems Multi-skan MCC/340.
2.8. Synthetic Manduca sexta allatostatin and allatotropin
M. sexta allatostatin was custom-synthesised using
solid-phase methodology (Fmoc procedure) on an Applied Biosystems model 431A automatic peptide syn-thesiser at the Advanced Biotechnology Centre, Charring Cross and Westminster Hospital Medical School, Lon-don. M. sexta allatotropin was purchased from Sigma, UK.
2.9. Statistical treatment
Results are presented as mean±standard error (SE). Treatments were considered significantly different when a Student’s t-test indicated a P value of ,0.05.
3. Results
3.1. JH biosynthesis by larval CA in vitro
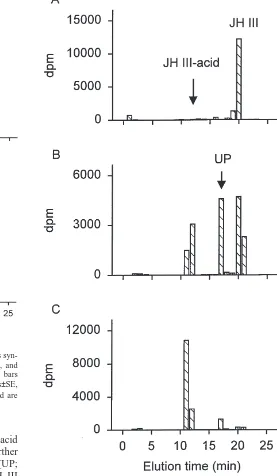
The radiolabelled JH homologues synthesised by CA from last-day Vth instar larvae in vitro are shown in [Fig. 1(A)] (marked by arrows) as disintegrations per minute (dpm) in each HPLC fraction. Under control conditions, larval CA synthesise predominantly JH II, some of which appears as JH II acid [Fig. 1(A)]. Very little radio-activity was detected in the JH I zone, although some appeared as JH I acid [Fig. 1(A)]. The total amount of JH synthesised (JH and JH acid) was 184.7±14.9 fmol/pr/h (mean±SE, n=6), of which ca. 90% was JH II.
In some incubations (when CA were not cleared of all contiguous tissue), all of the JH synthesised eluted as acid homologues [Fig. 1(B)]. Here the total amount of JH acid produced was 162.3±17.4 fmol/pr/h (mean±SE,
n=8), of which ca. 80% was JH II acid.
3.2. JH esterase activity of larval corpora allata
Fig. 2(A) shows the amount of radiolabel (dpm) and elution position of 3H-JH III under RP-HPLC
con-ditions. The arrow shows the elution position of 3H-JH
III acid produced by the chemical conversion of 3H-JH
Fig. 1. Separation by RP-HPLC of radiolabelled JH homologues syn-thesised by CA from last-day Vth instar larval L. oleracea (A), and the effect of tissue (B) on JH homologues produced. Hatched bars show the amount of JH (dpm) measured in each fraction (means±SE,
n=6). Elution positions of JH I, JH II, JH I acid and JH II acid are
shown by arrows.
CA, 27.1% of3H-JH III was converted to3H-JH III acid
with 41.7% remaining as JH III [Fig. 2(B)]. A further 27.3% was converted to an unidentified product [UP; Fig. 2(B)]. This conversion of 3
H-JH III to 3
H-JH III acid was virtually complete in the presence of CA with tissue attached [Fig. 2(C)], with 84% of total dpm con-verted to JH III acid and only 3.7% remaining in the JH III co-eluting fraction. It became clear that the esterolytic activity was dependent on both CA and the amount of other tissue present.
3.3. The effect of Mas-AS on control rates of larval corpora allata JH biosynthesis
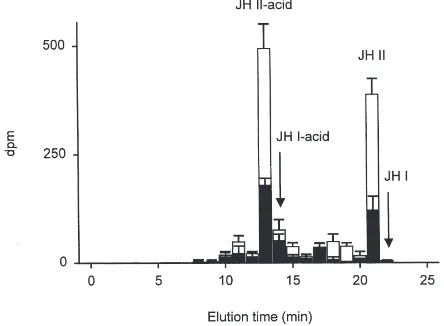
Under control conditions the incorporation of propi-onate into JH homologues by CA from last-day Vth instar larvae was low, with most radioactivity eluting in the JH II acid position, and smaller amounts in a zone corresponding to JH I acid (Fig. 3). Virtually no
radioac-Fig. 2. Distribution of radioactivity (dpm) in RP-HPLC fractions after separation of3H-JH III (A) in the absence of tissue (B) following incubation CA alone, and (C) CA+attached tissue. Corpora allata were from last-day Vth instar larval L. oleracea. Elution positions of JH III, JH III acid and an unknown product (UP) are shown.
tivity was detected in the JH I and JH II zones. The estimated total rate (JH I acid+JH II acid) under control conditions was 125.8±14.7 fmol/pr/h. On the addition of 10µM Mas-AS, JH I acid and JH II acid were signifi-cantly reduced by 60.8% (P=0.018) and 72.5% (P=0.001), respectively, to a total of 36.6±5.6 fmol/pr/h (means±SE, n=6; Fig. 3).
con-Fig. 3. RP-HPLC separation of JH homologues produced by last-day Vth instar larval L. oleracea CA, showing inhibition of JH synthesis by Mas-AS. Open bars represent control levels of radioactivity in each fraction and solid bars the amount in CA treated with 10µM Mas-AS (means±SE, n=6). JH I, JH I acid, JH II and JH II acid=juvenile hormone homologues.
trol rates. Control rate=142.8±11.5 fmol/pr/h; rate in the presence of 100 nM Mas-AS=134.2±4.2 fmol/pr/h (means±SE, n=4).
3.4. Stimulation of JH biosynthesis in larval CA by MAS-AT
Fig. 4 shows the increase in radioactivity (dpm) detected in each RP-HPLC fraction under control and Mas-AT (100 nM) stimulated conditions in extracts of incubations of CA from last-day Vth instar larvae. Radioactivity was distributed between the JH and the JH acid zones under control conditions in this series of
Fig. 4. RP-HPLC separation of JH homologues produced by last-day Vth instar larval L. oleracea CA, showing stimulation of JH synthesis by Mas-AT. Solid bars represent control levels of radioactivity in each fraction and open bars the amount in CA treated with 100 nM Mas-AT (means±SE, n=6–8).
Fig. 5. Dose–response relationship between concentration of Mas-AT and measurements of total JH produced by last-day Vth instar larval L. oleracea CA (means±SE, n=4–10), represented as percentage of maximum response.
experiments, and total radioactivity in the JH zones was significantly increased (183%, P,0.001) in glands treated with Mas-AT. The total JH and JH acid
syn-thesised under control conditions was
181.6±17.8 fmol/pr/h; in the presence of Mas-AT it was 513.7±31.2 fmol/pr/h (means±SE, n=6–8).
A dose-dependent relationship was observed for Mas-AT stimulation of total larval JH biosynthesis expressed as a percentage of a maximum response (achieved with 1µM Mas-AT) which is about a threefold increase over control values (Fig. 5). The stimulation by Mas-AT is linear between 0.1 and 10 nM Mas-AT (means±SE,
n=4–10).
3.5. Mas-AT-stimulated JH production by larval CA and the effects of Mas-AS
Last-day Vth instar larval CA incubated in the pres-ence of Mas-AT (1 and 10 nM) and Mas-AS (10µM) synthesised JH at levels that were not significantly dif-ferent to those stimulated by Mas-AT alone (Table 1). Inhibition of JH biosynthesis was only 6% (1 nM
Mas-Table 1
The effect of Mas-AT and combined effect of Mas-AT+Mas-AS on the rate of total JH biosynthesis by larval L. oleracea CA (means±SE,
n=4–6)
Treatment JH biosynthesis (fmol/pr/h)
No Mas-ATa 1 nM Mas-AT 10 nM Mas-AT
No Mas-AS 125.8±14.7 464.4±89.7 331.3±38.1 10µM Mas-AS 36.6±5.6 436.8±46.9b 268.3±13.5b
P value ,0.02 0.42 0.17
a Data from Section 3.3.
AT+10µM Mas-AS) or 19% (10 nM Mas-AT+10µM AS) compared with the stimulated rate with Mas-AT alone. In contrast, inhibition of total JH synthesis by Mas-AS in the absence of Mas-AT was greater than 60% and significant (P,0.02; Table 1, see also Section 3.3).
3.6. Mas-AT immunoreactivity in brain extract
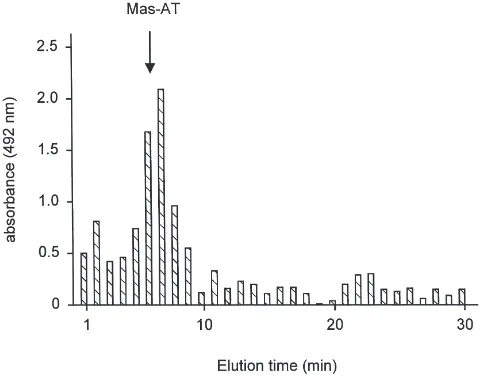
The Mas-AT-like immunoreactivity in individual HPLC fractions from an extract of 100 brains from mixed-aged VIth instar larval L. oleracea measured by ELISA (assayed at 10 brain equivalents) is shown in Fig. 6. The major immunoreactive fractions co-eluted with synthetic Mas-AT (indicated by arrow).
4. Discussion
This study has demonstrated that the very low rates of JH biosynthesis in CA of larval L. oleracea and the apparent lack of effect of Mas-AS and Mas-AT reported previously (Audsley et al., 1999a,b) were not due to inactivity of CA per se but rather to an inability to meas-ure rates of biosynthesis by the methods used. Measmeas-ure- Measure-ment of rates of JH biosynthesis by the incorporation of
l-[methyl-14C]-methionine can be compromised by
endogenous esterase activity, which will remove the incorporated radiolabelled methyl group. This fact, com-bined with the apparent low levels of incorporation of
l-[methyl-14C]-methionine into JH produced by L.
oler-acea larval CA (unpublished observations), possibly due
to an endogenous pool of methionine, may explain the low rates of synthesis (,1 fmol/pr/h) reported by Audsley et al. (1999a,b). To overcome these problems, and to enable the measurement of acid homologues pro-duced by esterase activity, [1-14C]-propionic acid, which
Fig. 6. Mas-AT-like immunoreactivity measured by ELISA in RP-HPLC fractions of mixed-aged VIth instar larval L. oleracea brains. Arrow indicates elution position of synthetic Mas-AT.
incorporates into the carbon skeleton of JH (Schooley and Baker, 1985), was used as a precursor to measure JH biosynthetic rates in the present experiments. Methyl ester and acid homologues of JH I and II were then sep-arated by RP-HPLC using a modification of methods described by Halankar and Schooley (1990). These methods (a combination of [1-14C]-propionic acid and
RP-HPLC) enabled measurement of JH biosynthetic rates up to 700 times greater than those reported by Audsley et al. (1999a,b) using methionine as the precur-sor coupled with normal-phase HPLC.
These results have clearly demonstrated that L.
olera-cea larval CA have inherent esterase activity, converting
.50% of exogenous 3H-JH III to the acid homologue
and an unknown product. Esterase activity was greater in the presence of CA with tissue attached, since vir-tually all 3H-JH III was converted to3H-JH III acid (Fig.
2). This was also evident for JHs biosynthesised by lar-val CA where at least 50% of JHs produced were acid homologues, presumably due to esterase activity rather than to JH acid synthesis. Tissue from larval Lepidoptera are known to contain JH esterases (Hanzlik and Ham-mock, 1988; Kallapur et al., 1996; de Kort and Granger, 1996) and esterase activity of CA has been demonstrated in larval M. sexta (Sparagana et al., 1984; Sparks et al., 1989; Janzen et al., 1991).
Secretion of JH acid by CA from last stadium larvae of M. sexta (Sparagana et al., 1984; Bhaskaran et al. 1986, 1987; Janzen et al., 1991) begins at day 0 and by day 4 JH acid is the sole product (Bhaskaran et al. 1986, 1987; Janzen et al., 1991). The use of a JH-specific ester-ase inhibitor s-benzyl-o-ethyl phosphoramidothiolate (BEPAT) in these studies demonstrated that some of the JH acid is synthesised de novo, rather than being pro-duced by hydrolysis of JH due to endogenous esterase activity. This is most likely a consequence of the loss of JH acid methyltransferase (MT) activity in older lar-val M. sexta CA, leading to a reduced level of esterifi-cation of JH acid to JH (Sparagana et al., 1984; Bhaska-ran et al. 1986, 1987; Janzen et al., 1991). Sparagana et al. (1984) suggest that JH I acid is converted to JH I by the imaginal discs in pre-pupal M. sexta. Adult male M.
sexta and Heliothis zea similarly lack JH acid MT and
shown to have significant JH esterase activity (Janzen et al., 1991). Regardless, the total rates of JH biosynthesis (JHs and JH acids) under control conditions (100– 200 fmol/pr/h) are similar to those reported for S.
lit-toralis larvae (ca. 50–200 fmol/pr/h; Pfister-Wilhelm and
Lanzrein, 1996). Jones and Yin (1989) also found similar rates in IVth stadium larval L. dispar, and rates of up to 630±110 fmol/pr/h in VIth stadium larvae, although the major homologue was JH III. In contrast, Jansons et al. (1996) report JH synthesis rates of ca. 1.5 fmol/pr/h in 0–4 h VIth instar larval P. unipuncta. Rather surpris-ingly, Bogus and Scheller (1991, 1996) report rates of JH biosynthesis of up to 140 pmol/pr/h in last instar lar-val G. melonella. In L. oleracea JH II+JH II acid would appear to be the major homologues produced; however, [1-14C]-propionic acid does not incorporate into JH III
(Schooley and Baker, 1985) so levels of this homologue would not be measured by these means. From measure-ments of whole body larval L. oleracea JH titres, Edwards et al. (1995) found that JH III was virtually undetectable, whilst JH II titres were the highest. Using
l-[methyl-14C]-methionine radiochemical assays, much
higher rates of JH biosynthesis by CA have been reported for larval M. sexta (2.1±0.4 pmol/pr/h; Kramer and Law, 1980 and 4.36±0.53 pmol/pr/h; Audsley et al., 1999a). Similarly, Bhaskaran et al. (1986) measured rates of 1.34±0.13 pmol/pr/h in day 0 Vth instar larval
M. sexta, 65% of which was JH II, 24% JH I and 11%
JH III, whereas Janzen et al. (1991) report rates of up to ca. 1 pmol/pr/h for each of JH I and JH III as meas-ured by JH radioimmunoassay. The variations in JH homologues measured in incubations of lepidopteran lar-val CA may be a consequence of the type of JH assay used, the radiolabel incorporated, the incubation con-ditions employed, as well as any species- or strain-spe-cific differences.
Juvenile hormone biosynthesis by CA of early Vth stadium larvae of M. sexta is inhibited by .90% by Mas-AS (Kramer et al., 1991; Audsley et al., 1999a), but Mas-AT has no effect on similarly staged insects (Kataoka et al., 1989). Both these peptides are active on CA of newly emerged adult female M. sexta (Kataoka et al., 1989; Kramer et al., 1991). Audsley et al. (1999b) originally found that neither Mas-AS nor Mas-AT had any effect on JH biosynthesis by CA from last-day Vth instar larval L. oleracea, but these results may have been compromised by esterase activity, as discussed above. Using [1-14C]-propionic acid as the precursor for JH,
both Mas-AS and Mas-AT affected rates of JH biosynth-esis in vitro by CA from last-day Vth instar larval L.
oleracea. This is the first report of the dual regulation,
by a defined allatostatin and allatotropin, of JH biosynth-esis in CA of a larval lepidopteran other than M. sexta, and the first evidence that Mas-AT is not adult-specific as was proposed by Kataoka et al. (1989).
In larval G. mellonella an allatotropic factor from the
brain, which stimulates JH synthesis in vitro and in vivo, was partially purified and estimated to have a molecular weight of approximately 20 kDa (Bogus and Scheller, 1996). Due to its apparent size, this factor is unlikely to be structurally related to Mas-AT (Kataoka et al., 1989), which has a mass of 1486.8.
As observed with adult female L. oleracea (Audsley et al., 1999a), Mas-AS (at a dose of 10µM) does not fully inhibit JH biosynthesis by larval CA, but still has a significant effect (ca. 70% reduction over control rates). However, in L. oleracea, it is possible that a 70% reduction of JH biosynthesis results in titres of JH below the threshold at which this hormone is physiologically active and, as such, Mas-AS is an effective inhibitor. Alternatively, greater levels of inhibition (as observed for Mas-AS on M. sexta CA; Kramer et al., 1991; Audsley et al., 1999a,b) may only be observed after a pre-incubation of CA with Mas-AS before JH biosyn-thetic rates are measured.
Although Mas-AT has no apparent stimulatory activity on CA from larval M. sexta (Kataoka et al., 1989), this peptide does increase JH biosynthesis by CA of larval L. oleracea up to threefold in a dose-dependent manner. In larval L. oleracea, Mas-AT may be more important in regulating CA activity than Mas-AS. In support of this theory, the stimulatory actions of Mas-AT appear to override the inhibitory effects of Mas-AS when larval CA are incubated in the presence of both peptides, even with a sub-maximal dose of Mas-AT and 10,000×greater dose of Mas-AS. In contrast, Oeh et al. (1999) report that in adult female S. frugiperda Mas-AS is only active on CA stimulated by Mas-AT. Addition-ally, measurements of Mas-AS-like immunoreactivity in brain extracts of L. oleracea (Audsley et al., 1998; Audsley et al., 1999a) do not correlate well with the JH titres reported by Edwards et al. (1995), which brings into question the role of this inhibitory neuropeptide in the regulation of JH levels. Similarly, expression of the gene for Mas-AS did not reflect JH biosynthetic rates in
P. unipuncta (Jansons et al., 1996). Brain levels of
Mas-AT at different developmental stages have still to be compared with JH titres and/or biosynthetic rates, though haemolymph titres and CA levels of both Mas-AS and Mas-AT may give a clearer picture as to how these two peptides may regulate JH biosynthesis.
are low or absent. Results of this study suggest that Mas-AT could be the overriding regulator of CA activity in larval L. oleracea. Thus, rather than glands being “turned off” by Mas-AS when JH is not required, the CA may be “turned on” by Mas-AT as needed. Hence, the removal or degradation of Mas-AT may be more important than the inhibition of synthesis by Mas-AS in reducing JH levels.
Using an indirect ELISA to Mas-AT combined with RP-HPLC of L. oleracea larval brain extract, the HPLC fraction with Mas-AT-like immunoreactivity co-elutes with synthetic Mas-AT suggesting that this peptide is present in larval brains. Similarly, a Mas-AS immunore-active peptide which co-eluted with synthetic Mas-AS was isolated from larval L. oleracea brain extracts (Audsley et al., 1998) and found to have a molecular mass in close agreement with Mas-AS characterised by Kramer et al. (1991). Oeh et al. (1999) have recently isolated a Mas-AT-like peptide from adult S. frugiperda brains, and have shown this peptide to be active on adult female CA.
In conclusion, CA of larval L. oleracea synthesise detectable levels of JH which are affected by endogen-ous esterase activity. The results of this study also show that the regulators of CA activity in M. sexta (i.e., Mas-AS and Mas-AT) are most likely major components involved in the regulation of JH biosynthesis in another lepidopteran family. However, it would appear that in larval L. oleracea, Mas-AT is the more significant CA regulating factor. Whether there is also a switch from JH ester secretion to JH acid production as in M. sexta, and whether or not any further inhibitory peptide is involved in the regulation of JH biosynthesis in L.
olera-cea, as with the reported allatinhibin of M. sexta
(Bhaskaran et al., 1990), remains to be determined. It remains also to be seen what role if any is played by neuropeptides of the cydiastatin and helicostatin families (Duve et al., 1997a,b) in the regulation of larval noc-tuid CA.
Acknowledgements
The authors acknowledge support from the Pesticides Safety Directorate, MAFF. We thank Professor D.A. Schooley (University of Nevada, USA) for helpful suggestions, and we are grateful to Professor J. Veenstra (University of Bordeaux, France) for supplying Mas-AT polyclonal antisera.
References
Audsley, N., Weaver, R.J., Edwards, J.P., 1998. Enzyme linked immu-nosorbent assay for Manduca sexta allatostatin (Mas-AS), isolation and measurement of Mas-AS immunoreactive peptide in Lacanobia
oleracea. Insect Biochem. Mol. Biol. 28, 775–784.
Audsley, N., Weaver, R.J., Edwards, J.P., 1999a. Juvenile hormone synthesis by corpora allata of tomato moth Lacanobia oleracea (Lepidoptera: Noctuidae), and the effects of allatostatins and allato-tropin in vitro. Eur. J. Entomol. 96, 287–293.
Audsley, N., Weaver, R.J., Edwards, J.P., 1999b. The significance of
Manduca sexta allatostatin in the tomato Lacanobia oleracea. In:
Sandman, C.A., Strand, F.L., Beckman, B., Chronwall, B.M., Flynn, F.W., Nachman, R.J. (Eds.), Neuropeptides, structure and function in biology & behaviour. Ann. N.Y. Acad. Sci, 897 pp. 330–341.
Bhaskaran, G., 1981. Regulation of corpora allata in last Manduca
sexta larvae. In: Bhaskaran, G., Friedman, S., Rodriguez, J.G.
(Eds.), Current Topics in Insect Endocrinology and Nutrition. Ple-num Press, New York, pp. 53–82.
Bhaskaran, G., Sparagana, S.P., Barrera, P., Dahm, K.H., 1986. Changes in corpus allatum function during metamorphosis of the tobacco hornworm Manduca sexta: regulation at the terminal step in juvenile hormone biosynthesis. Arch. Insect Biochem. Physiol. 3, 321–338.
Bhaskaran, G., Dahm, K.H., Jones, G.L., Peck, K., Faught, S., 1987. Juvenile hormone acid synthesis and HMG–CoA reductase activity in corpora allata of Manduca sexta prepupae. Insect Biochem. 17, 933–937.
Bhaskaran, G., Sparagana, S.P., Dahm, K.H., Barrera, P., Peck, K., 1988. Sexual dimorphism in juvenile hormone synthesis by corpora allata and in juvenile hormone acid methyltransferase activity in corpora allata and accessory sex glands of some Lepidoptera. Int. J. Invert. Rep. Dev. 13, 87–100.
Bhaskaran, G., Dahm, K.H., Barrera, P., Pacheco, J.L., Peck, K., Muszynska-Pytel, M., 1990. Allatinhibin, a neurohormonal inhibi-tor of juvenile hormone biosynthesis in Manduca sexta. Gen. Comp. Endocrinol. 78, 123–136.
Bhatt, T.R., Horodyski, F.M., 1999. Expression of the Manduca sexta allatotropin gene in cells of the central and enteric nervous systems. J. Comp. Neurol. 403, 407–420.
Bogus, M.I., Scheller, K., 1991. Activation of juvenile hormone syn-thesis in vitro by larval brains of Galleria mellonella. Zool. Jb. Physiol. 95, 197–208.
Bogus, M., Scheller, K., 1996. Allatotropin released by brain controls larval molting in Galleria mellonella by affecting of juvenile hor-mone synthesis. Int. J. Dev. Biol. 40, 205–210.
Duve, H., Johnson, A.H., Maestro, J.L., Scott, A.G., Crook, N., Win-stanley, D., Thorpe, A., 1997a. Identification, tissue localisation and physiological effect in vitro of a neuroendocrine peptide in the codling moth Cydia pomonella (Tortricidae: Lepidoptera). Cell Tissue Res. 289, 73–83.
Duve, H., Johnson, A.H., Maestro, J.L., Scott, A.G., Winstanley, D., Davey, M., East, P.D., Thorpe, A., 1997b. Lepidopteran peptides of the allatostatin superfamily. Peptides 18, 1301–1309.
Edwards, J.P., Corbitt, T.S., McArdle, H.F., Short, J.E., Weaver, R.J., 1995. Endogenous levels of insect juvenile hormones in larval, pupal and adult stages of the tomato moth, Lacanobia oleracea. J. Insect Physiol. 41, 641–651.
Goodman, W.G., 1984. Semipreparative synthesis and purification of juvenile hormone acids by high performance liquid chromato-graphy. J. Chromatogr. 294, 447–451.
Halankar, P.P., Schooley, D.A., 1990. Reversed-phase liquid chroma-tography separation of juvenile hormone and its metabolites, and its application for an in vivo juvenile hormone catabolism study in
Manduca sexta. Anal. Biochem. 188, 394–397.
Hanzlik, T.N., Hammock, B.D., 1988. Characterisation of juvenile hor-mone hydrolysis in early larval development of Trichoplusia ni. Arch. Insect Biochem. Physiol. 9, 135–156.
Jansons, I., Cusson, M., McNeil, J.N., Tobe, S.S., Bendena, W.G., 1996. Molecular characterisation of a cDNA from Pseudaletia
unipuncta encoding the Manduca sexta allatostatin peptide
Janzen, P., Menold, M., Granger, N.A., 1991. Effects of endogenous esterases and an allatostatin on the products of Manduca sexta lar-val corpora allata in vitro. Physiol. Ent. 16, 283–293.
Jones, G.L., Yin, C.-M., 1989. Juvenile hormone biosynthesis by cor-pus cardiacum–corcor-pus allatum complexes of larval Lymantria
dis-par. Comp. Biochem. Physiol. 92A, 9–14.
Kallapur, V.L., Majumder, C., Roe, R.M., 1996. In vivo and in vitro-tissue specific metabolism of juvenile hormone during the last stad-ium of the cabbage looper, Trichoplusia ni. J. Insect Physiol. 42, 181–190.
Kataoka, H., Toschi, A., Li, J.P., Carney, L., Schooley, D.A., Kramer, S.J., 1989. Identification of an allatotropin from adult Manduca
sexta. Science 243, 1481–1483.
De Kort, C.A.D., Granger, N.A., 1996. Regulation of JH titres: the relevance of degradative enzymes and binding proteins. Arch. Insect Biochem. Physiol. 33, 1–26.
Kramer, S.J., Law, J.H., 1980. Control of juvenile hormone production: the relationship between precursor supply and hormone synthesis in the tobacco hornworm, Manduca sexta. Insect Biochem. 10, 569–575.
Kramer, S.J., Toschi, A., Miller, C.A., Kataoka, H., Quistad, G.B., Li, J.P., Carney, L., Schooley, D.A., 1991. Identification of an allatos-tatin from the tobacco hornworm Manduca sexta. Proc. Nat. Acad. Sci., USA 88, 9458–9462.
Oeh, U., Lorenz, M.W., Dyker, H., Loesel, P., Hoffmann, K.H., 1999. Interaction between Manduca allatotropin and Manduca allatostatin in the fall armyworm Spodoptera frugiperda. In: VIIth Inter-national Conference on the Juvenile Hormones, Jerusalem, Israel, Abstract.
Pfister-Wilhelm, R., Lanzrein, B., 1996. Precocious induction of meta-morphosis in Spodoptera littoralis (Noctuidae) by the parasitic wasp Chelonus inanitus (Braconidae): identification of the parasit-oid larva as the key regulatory element and the host corpora allata as the main targets. Arch. Insect Biochem. Physiol. 32, 511–525. Pratt, G.E., Tobe, S.S., 1974. Juvenile hormone radiobiosynthesised by corpora allata of adult female locusts in vitro. Life Sci. 14, 575–586.
Sparks, T.C., Allen, L.G., Schneider, F., Granger, N.A., 1989. Juvenile hormone esterase activity from Manduca sexta corpora allata in
vitro. Arch. Insect Biochem. Physiol. 11, 93–108.
Schooley, D.A., Baker, F.C., 1985. Juvenile hormone biosynthesis. In: Kerkut, G.A., Gilbert, L.I. (Eds.). Comprehensive Insect Physi-ology Biochemistry and PharmacPhysi-ology, vol. 7. Pergamon Press, Oxford, pp. 363–389.
Schooley, D.A., Judy, K.J., Bergot, B.J., Hall, M.S., Siddall, J.B., 1973. Biosynthesis of the juvenile hormones of Manduca sexta: labelling pattern from mevalonate, propionate, and acetate. Proc. Nat. Acad. Sci. USA 70, 2921–2925.
Sparagana, S.P., Bhaskaran, G., Dahm, K.H., Riddle, V., 1984. Juven-ile hormone production, juvenJuven-ile hormone esterase, and juvenJuven-ile hormone acid methyltransferase in corpora allata of Manduca
sexta. J. Exp. Zool. 230, 309–313.