www.elsevier.com / locate / bres
Research report
Heme oxygenase (HO)-1 expressing macrophages / microglial cells
accumulate during oligodendroglioma progression
a ,
*
a a bMartin H. Deininger
, Richard Meyermann , Katrin Trautmann , Frank Duffner ,
b c a
Ernst H. Grote , Juergen Wickboldt , Hermann J. Schluesener
a
Institute of Brain Research, University of Tuebingen, Medical School, Calwer Strasse 3, D-72076 Tuebingen, Germany
b
Department of Neurosurgery, University of Tuebingen, Medical School, Tuebingen, Germany
c
Department of Neurosurgery, Asklepios Klinik Schildautal, Seesen, Germany
Accepted 13 June 2000
Abstract
Heme oxygenase (HO-1, HSP32) catalyzes the oxidation of heme to biliverdin and carbon monoxide, a putative neurotransmitter. In the brain, HO-1 expression has been associated with neuroprotection during oxidative stress and hypoxia. However, consecutive downstream mediation is involved in neoangiogenesis and consequent neoplastic outgrowth. We have analyzed HO-1 expression in 69 oligo-dendroglioma tissue samples, in rat intracranially transplanted C6 gliomas, and neuropathologically unaltered control brains by immunohistochemistry. Double labeling experiments confirmed the nature of HO-1 expressing cells. Reverse transcription–polymerase chain reaction was used to demonstrate HO-1 gene expression. HO-1 immunoreactivity was predominantly observed in macrophages / microglial cells. The number of HO-1 expressing macrophages / microglial cells was significantly lower in primary oligodendrogliomas than in their matched relapses (P,0.0001) and lower in primary anaplastic oligodendrogliomas than in their relapses (P50.0006). Prominent accumulation of HO-1 expressing macrophages / microglial cells was observed in perinecrotic areas of both experimental rat and human glioblastoma relapses. HO-1 expressing neurons, macrophages / microglial cells and astrocytes were scattered in areas of infiltrative tumor growth. Surprisingly, HO-1 mRNA was detected in only one glioblastoma multiforme relapse. We conclude from these data that HO-1 expressing macrophages / microglial cells accumulate during oligodendroglioma progression in areas of focal necrosis. However, overall biological function of this phenomenon remains to be determined. 2000 Elsevier Science B.V. All rights reserved.
Theme: Disorders of the nervous system
Topic: Neuro-oncology
Keywords: Oligodendroglioma; Rat C6 glioblastoma; Heme oxygenase-1; Immunocytochemistry; RT–PCR
1. Introduction neuronal populations. HO-2 is much more widely
ex-pressed. It is present in mitral cells in the olfactory bulb,
HO-1 (HSP32) is oxidative stress-inducible [15] and pyramidal cells in the cortex and hippocampus, granule
catalyzes oxidation of heme to biologically active mole- cells in the dentate gyrus, many neurons in the thalamus,
cules: iron, a gene regulator, biliverdin, an antioxidant and hypothalamus, cerebellum and caudal brainstem [32].
carbon monoxide. Consecutive downstream mediation is While the constitutively expressed HO-2 has been reported
involved in vasodilation, stimulation of guanylate cyclase, to be exclusively regulated by glucocorticoids, the
induc-and neuronal transmission [7]. ible HO-1 isozyme is associated with a wide range of
In normal brain, HO-1 is present at the limit of pathological conditions in the mammalian brain. Induction
immunodetection and is discretely localized in selected of HO-1 expression has been associated with
neuroprotec-tion during hyperthermia in glial cells [4] and during hypoxia [23]. Consequently, HO-1 expression in neurons,
*Corresponding author. Tel.:149-7071-298-2283; fax:1
49-7071-294-astrocytes and macrophages was observed in a wide range
846.
E-mail address: [email protected] (M.H. Deininger). of experimental diseases of the rodent brain such as
traumatic injury [5], ischemia [21] and in human Alzheim- numerous multinucleated cells, high mitotic activity,
vas-er’s disease [26]. Moreover, HO-1 expression in infiltrating cular proliferation and areas of focal necrosis. Seven
macrophages has been associated with disease severity in patients with oligodendroglioma received radiotherapy
atherosclerosis [33] and constitutes a marker of oxidative following the resection of the primary tumor, and 14
stress in asthma [8]. In brain tumors, elevated HO-1 patients received no post surgical treatment. In five
pa-expression was observed, but spatial and cellular expres- tients, no postsurgical therapy was mentioned thus
sug-sion patterns remain unresolved [6,22]. gesting that no post surgical therapy was applied. Thirteen
In order to provide a pathological basis for the in- patients with anaplastic oligodendroglioma received
volvement of HO-1 in oligodendrogliomas, we have radiotherapy after resection of the primary tumor, three
analyzed its expression in 69 oligodendroglioma tissue radiotherapy and chemotherapy with ACNU and VM26,
samples, in rat intracranially transplanted C6 gliomas, four and three received no postsurgical treatment.
Involved-rat brains and four neuropathologically unaltered human field radiotherapy was applied at doses of 36–60 Gy.
brains by immunohistochemistry. Twenty-six primary
WHO grade II oligodendrogliomas and 16 primary WHO 2.2. Human control brains
grade III anaplastic oligodendrogliomas were included.
Nineteen grade II tumors progressed, 10 were again grade Four control brains were obtained from autopsies at the
II oligodendrogliomas, and nine had progressed to higher Institute of Brain Research in Tuebingen (Table 2). All
grade lesions. Eight anaplastic oligodendrogliomas pro- tissues were fixed in buffered 4% formalin (pH 7.4) and
gressed, five were again WHO grade III tumors, and three embedded in paraffin by routine methods.
had progressed to glioblastoma multiforme. Double
label-ing experiments confirmed the nature of HO-1 expresslabel-ing 2.3. Cell culture
cells. Reverse transcription–polymerase chain reaction
(RT–PCR) was used to demonstrate HO-1 mRNA. Rat C6 glioblastoma cell lines were obtained from the
American Type Culture Collection (ATCC, Manassas, USA) and raised in RPMI 1640 medium with Glutamax II
2. Materials and methods (Gibco BRL, Paisley, UK) containing 10% fetal calf serum (FCS, Gibco BRL, Paisley, UK) and 1.2% penicillin /
2.1. Oligodendroglioma patients streptomycin (Fluka, Buchs, Switzerland) at 378C and 5%
CO .2
All oligodendrogliomas were resected at the Department ¨
of Neurosurgery in Tubingen or at the Department of 2.4. Intracranial transplantation and rat control brains
Neurosurgery of the Asklepios Klinik Schildautal in
Seesen (Table 1). Resection was documented by the At near confluency, C6 glioblastoma cells were
har-surgeons as incomplete or macroscopically complete. We vested using a cell scraper and 5ml were injected into the
studied 42 oligodendroglioma tissue samples, 26 primary basal ganglia region of male Sprague–Dawley rats at a
5
WHO (World Health Organisation) grade II oligodendro- concentration of 4310 /ml [27]. After 2 weeks, rats were
gliomas and 16 primary WHO grade III anaplastic oligo- sacrificed, perfused with 4% paraformaldehyde and brains
dendrogliomas. Nineteen grade II tumors progressed, 10 were prepared as described. Control brains of healthy rats
were again grade II oligodendrogliomas and nine had were prepared as described above (Table 2).
progressed to higher grade lesions. Eight anaplastic
oligo-dendrogliomas progressed, five were again WHO grade III 2.5. Single labeling immunohistochemistry
tumors, and three had progressed to glioblastoma
mul-tiforme. Histological diagnosis was performed by routine Five micrometer sections were deparaffinized and
rehy-neuropathology according to the WHO classification sys- drated. For antigen retrieval, the sections were immersed in
tem [9]. WHO grade II oligodendrogliomas were character- 0.01 M citrate buffer and irradiated in a microwave oven at
ized by enlarged rounded cells with a well-defined cell 750 W, five cycles of 5 min. Endogenous peroxidase was
membrane and clear cytoplasm around a central spherical blocked with 1% H O in methanol and the slices were2 2
nucleus, uniformly round nuclei, high chromatin density, consequently incubated with nonspecific porcine serum.
low mitotic activity, microcalcifications and branching Rabbit polyclonal antibodies directed against recombinant
capillary network. WHO grade III oligodendrogliomas rat HO-1 (StressGen, Victoria, Canada) were diluted 1:200
were characterized by nuclear atypia, mitotic activity, high in 1% BSA (bovine serum albumin) TBS (Tris-balanced
proliferation rate, rounded hyperchromatic nuclei, perinu- salt solution, pH 7.5, containing 0.025 M Tris, 0.15 M
clear swelling, few cellular processes and again mi- NaCl). Secondary antibody biotinylated anti-rabbit IgG
crocalcifications and branching capillary network. WHO (Dako, Hamburg, Germany) was diluted at 1:400 in BSA /
grade IV glioblastoma multiforme were characterized by a TBS and applied to the slices for 30 min. Streptavidin–
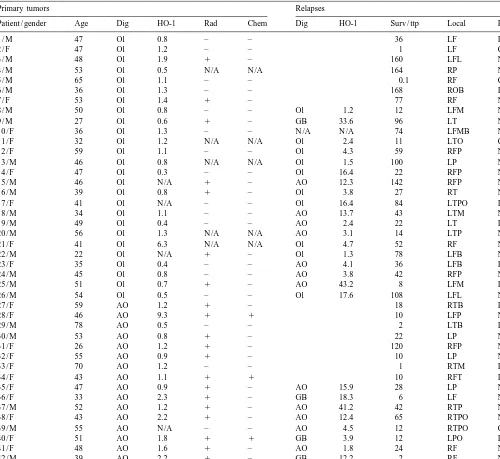
Table 1
a
Oligodendroglioma patients and HO-1 immunoreactivity
Primary tumors Relapses
Patient / gender Age Dig HO-1 Rad Chem Dig HO-1 Surv / ttp Local Res
1 / M 47 Ol 0.8 – – 36 LF I
Labeled cells are indicated as per cent of all counterstained nuclei. AO, anaplastic oligodendroglioma; B, basal; C, complete; Chem, chemotherapy; Dig, diagnosis; F, frontal; GB, glioblastoma multiforme; I, incomplete; L, left; Local, localization; M, medial; N /A, not available; O, occipital; Ol, oligodendroglioma; P, parietal; R, right; Rad, Radiotherapy; Res, resection; Surv, survival (months); T, temporal; ttp, time to progression (months).
Germany) diluted 1:400 was subsequently applied for 30 Oxford, UK) to rat tissues and GFAP, CD68 and HLA-DR,
min. Labeled antigen was visualized with standard -DP, -DQ (macrophages, Dako, Denmark) to human slices.
diaminobenzidine techniques (Sigma, Deisenhofen, Ger- Visualization was achieved by adding rabbit anti-mouse
many). All sections were counterstained with hematoxylin. IgG diluted at 1:20 in TBS for 30 min and then APAAP
complex at a dilution of 1:100 in TBS for 30 min.
2.6. Double labeling experiments Consecutively, we developed with Fast Blue BB salt
(Fluka, Buchs, Switzerland) yielding a blue reaction
Slices were pretreated as described above. Then the product. To avoid antibody crossreactivity in double
differentiating mouse monoclonal antibodies were added to labeling experiments, slices were once more irradiated in a
the slices all at a dilution of 1:100 in TBS / BSA. We added microwave for 20 min in citrate buffer [12]. Complete
anti-GFAP (glial fibrillary acidic protein) (Boehringer, inhibition of alkaline phosphatase function was achieved as
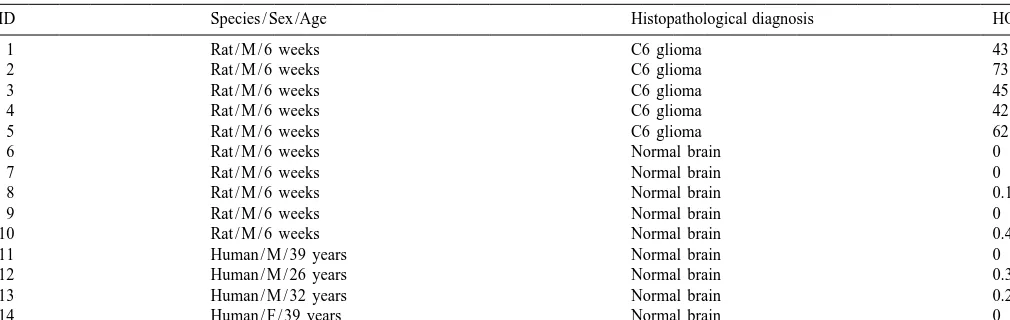
im-Table 2
a
HO-1 immunoreactivity in rat C6 gliomas and in rat and human control brains
ID Species / Sex /Age Histopathological diagnosis HO-1
1 Rat / M / 6 weeks C6 glioma 43.8
Labeled cells are indicated as per cent of all counterstained nuclei. F, female; M, male.
munolabeled as described above. No counterstain was mg / mg tissue. cDNA was prepared using random
hexa-
applied on double-labeled slices. mers, RNAsin ribonuclease inhibitor and Moloney
Mu-rine Leukemia Virus Reverse Transcriptase (Promega,
Madison, WI). Samples were incubated 30 min at 378C and
2.7. Evaluation
5 min at 958C. Polymerase chain reaction was then
performed using Thermus aquaticus (Taq) DNA Poly-Positive cells were counted in five regions of solid
merase Mini Kit (Promega) according to the manufactur-tumor growth in WHO II and WHO III
oligodendro-er’s instructions. The forward and reverse primers for
gliomas at 4003 magnification and compared to the total
HO-1 were: 59-TGCCCAGCTCCTGGCCCGCCGCTT-39
number of counterstained nuclei in that area. In WHO IV
and 59-GTGCATCAACACAGGCGCCTCTTC-39,
respec-glioblastomas and C6 gliomas of the rat, five regions in the
tively, and gave a single band corresponding to a fragment immediate vicinity of focal necrosis were evaluated to
in human HO-1 cDNA [24]. RNA integrity of the ex-demonstrate focal accumulation of HO-1 expressing cells.
amined samples was confirmed using b-actin forward and
Then the percentage of HO-1 expressing cells was
calcu-reverse primers: 59-TCACCCTGAAGTACCCCATCGAG
lated. Statistical analysis was performed using the Mann–
-39 and 59-TTGGCCTTGGGGTTCAGGGGGG -39,
re-Whitney U-test.
spectively, yielding a fragment in human beta-actin cDNA
[20]. Reaction conditions were: denaturation at 948C for 2
2.8. Controls and blocking experiments min followed by 30 cycles of denaturation at 948C for 1
min, annealing at 608C, 558C (HO-1), 558C (b-actin),
Adjacent sections were stained omitting addition of elongation at 728C for 1 min. Finally, the reaction was
HO-1 antibody. HO-1 immunoreactivity was abolished incubated at 728C for 10 min. The resulting reaction
following overnight incubation of antibody with blocking products were analyzed on agarose gels stained with 0.5
peptide (StressGen) at 48C according to the manufacturer’s mg / ml ethidium bromide.
instructions. No cross-reactivity is observed with HO-2.
2.9. RNA extraction and reverse transcriptase–
polymerase chain reaction (RT–PCR) 3. Results
Oligodendroglioma tissue of three primary WHO grade 3.1. Neuropathologically unaltered brains
II oligodendrogliomas, one WHO grade III anaplastic
oligodendroglioma and four WHO grade IV glioblastoma In rat and human control brains without
neuropathologi-relapse patients were collected immediately after resection cal alterations, HO-1 was expressed by singular astrocytes,
and frozen in liquid nitrogen. Total RNA extraction was neurons and macrophages situated in the cortex of the
performed using the RNeasy Mini Kit protocol as sug- forebrain, diencephalon, cerebellum, and brainstem
re-gested by the manufacturer (Qiagen GmbH, Hilden, Ger- gions. The number of HO-1 expressing cells was
com-many). The RNA yields of all patients did not differ parably low in rat (Mean50.1, S.E.M.50.078) and human
3.2. Oligodendroglioma patients expressing macrophages were found in subendothelial localizations of the vasculature.
In human oligodendrogliomas (Table 1), most
promi-nently, HO-1 expression was observed in macrophages / 3.4. Double labeling experiments
microglial cells. In both, primary WHO grade II
oligo-dendrogliomas (Mean51.157, S.E.M.50.2477) and WHO Double labeling experiments revealed the nature of
HO-grade III anaplastic oligodendrogliomas (Mean51.893, 1 expressing cells. In perinecrotic areas of rat C6 gliomas,
S.E.M.50.5476), only singular HO-1 expressing macro- the majority of HO-1 expressing macrophages / microglial
phages / microglial cells were observed. However, signifi- cells (brown color) coexpressed ED1 (blue color) (Fig.
cantly (P50.0292) fewer HO-1 expressing cells were 1F). In contrary, the majority of HO-1 expressing cells did
observed in primary WHO grade II oligodendrogliomas not coexpress GFAP (Fig. 1G). In human
oligodendro-(Fig. 1A) than in primary anaplastic oligodendrogliomas. glioma specimens, predominant colocalization of HO-1
Surprisingly, in areas of infiltrative oligodendroglioma and CD68 (Fig. 1H) and HLA-DR, -DP, -DQ was
ob-growth, higher numbers of HO-1 expressing cells were served. Occasionally, double labeling of GFAP (blue color)
observed than in areas of solid tumor growth. Here, HO-1 and HO-1 (brown color) was detected (Fig. 1I).
expressing cells were characterized by morphological
characteristics of neurons, astrocytes and macrophages / 3.5. Blocking experiments
microglial cells. In oligodendroglioma (Mean510.32,
S.E.M.52.787, P,0.0001) and anaplastic oligodendro- Antibody specificity was confirmed by blocking
experi-glioma relapses (Mean513.78, S.E.M.54.449, P5 ments with recombinant rat HO-1 peptide. The previously
0.0006), we observed significantly higher numbers of HO- observed labeling pattern was completely abrogated (data
1 expressing macrophages / microglial cells than in the not shown).
primary tumors (Fig. 1B). The most striking accumulation
of HO-1 expressing macrophages / microglial cells was 3.6. Reverse transcriptase–polymerase chain reaction
observed adjacent to areas of focal necrosis in glioblas- (RT–PCR)
toma relapses (Fig. 1C). Furthermore, in areas of necrosis,
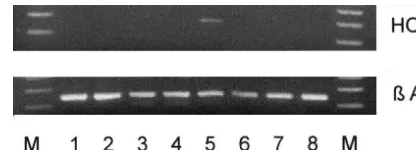
single disseminated macrophages / microglial cells express- HO-1 mRNA was not observed in WHO grade II
ing HO-1 were readily detected. Prominent infiltration of oligodendrogliomas (lanes 1–3) (Fig. 2), WHO grade III
HO-1 expressing macrophages was frequently observed in anaplastic oligodendroglioma (lanes 4) or three WHO
the walls of the tumor vasculature of high grade oligo- grade IV glioblastoma (lanes 6–8). In one glioblastoma
dendroglioma relapses and glioblastoma multiforme re- multiforme relapse (lane 5), however, HO-1 mRNA was
lapses. detected.
3.3. Rat C6 gliomas 4. Discussion
In order to verify accumulation of HO-1 expressing Three heme oxygenase isoforms (HO-1, HO-2, and
macrophages / microglial cells adjacent to areas of focal HO-3) have been identified to date. HO-1 is ubiquitous and
necrosis, we used the rat C6 glioblastoma model (Table 2). its mRNA and activity can be increased several-fold by
C6 glioblastomas are characterized by a high proliferation heme, other metalloporphyrins, transition metals, and
rate, highly invasive growth and formation of areas of stimuli that induce cellular stress. In contrast, HO-2 is
zonal necrosis and are therefore a widely used model for present chiefly in the brain and testes and is virtually
human malignant glioma. Accordingly, we observed ac- uninducible. HO-3 has very low activity; its physiological
cumulation of HO-1 expressing macrophages / microglial function probably involves heme binding [3]. These
pro-cells most prominently in areas of solid tumor growth teins, which are different gene products, have little in
adjacent to areas of focal necrosis (Fig. 1D). In areas of common in primary structure, regulation, or tissue
dis-infiltrative tumor growth, the number of HO-1 expressing tribution.
macrophages / microglial cells decreased with the distance In this report, we report the accumulation of HO-1
from the tumor (Fig. 1E). As in human glioblastoma expressing macrophages / microglial cells during
oligo-multiforme relapses, we observed significant accumulation dendroglioma progression adjacent to areas of focal
necro-of HO-1 expressing macrophages / microglial cells in areas sis. Previous reports have demonstrated that HO-1
over-adjacent to focal necrosis (Mean553.58, S.E.M.56.124, expression is cytoprotective by attenuating nitric oxide
P50.0079) compared to normal controls. In the tumor mediated proinflammatory reaction cascades and HO-1
parenchyma, accumulation of HO-1 expressing macro- overexpression has consequently been suggested to
consti-phages / microglial cells was occasionally observed in tute a novel therapeutic approach to disrupt inflammatory
Detailed analyses, however, revealed cell-type specific differences of HO-1 bioactivity. In endothelial cells, NO was identified as a determinant in the modulation of the activity of heme oxygenase leading to a major resistance of the endothelium to oxidative stress [18]. In macrophages, HO-1 is a physiological inhibitor of nitrite formation by decreasing heme availability for NOS2 synthesis [31].
Overexpression of HO-1 resulted in the inhibition of Fig. 2. HO-1 mRNA was observed in one WHO grade IV glioblastoma
multiforme relapse (lane 5), but not in oligodendrogliomas (lanes 1–3),
several immune effector functions and thus provides an
anaplastic oligodendroglioma (lane 4) or three other glioblastomas (lanes
explanation for stress-induced immunosuppression [35]. In
6–8).
the brain, HO-1 overexpression in macrophages / microglial cells has been suggested to be cytoprotective in the
contused spinal cord of the rat [17], following pre- and 39-UTRs of the mRNAs [3]. Accumulation of HO-1
postganglionic axotomy [13], hyperosmotic opening of the expressing macrophages / microglial cells in the immediate
blood–brain barrier [25], hypoxia-ischemia [1,23,28] and vicinity of necroses during the progression of
oligodendro-trauma [5]. In human gliomas, expression of HO-1 mRNA gliomas therefore might indicate a prominent mechanism
and protein was correlated with macrophage infiltration to confirm neoangiogenesis in hypoxic areas. These data
and vascular density [22]. In this context, however, are supported by the finding that HO-1 mRNA was only
detrimental HO-1 biological activity has been described. detectable in one glioblastoma multiforme tissue specimen.
HO-1 is responsible for the physiological breakdown of Although we do not have histological evidence, we
heme into equimolar amounts of biliverdin, carbon monox- speculate that HO-1 is only expressed in distinct areas of
ide, and iron and there is convincing evidence that tumor growth, most likely in perinecrotic regions.
accumulation of HO-1 expressing macrophages in the Our data provide convincing evidence for the
accumula-immediate vicinity of necroses in high grade gliomas is a tion of HO-1 expressing macrophages / microglial cells in
rather detrimental mechanism that contributes to neoplastic areas of focal necrosis. However, further studies are of
outgrowth and tissue damage. HO-1 expression is induced need to resolve whether this phenomenon contributes to
following multiple pathological stimuli including reactive overall neoplastic outgrowth and cytoprotection or
pro-oxygen species, cytokines, hyperthermia and radiation longed cytotoxicity.
[11,14,16,30]. Therefore, accumulation of HO-1 express-ing macrophages / microglial cells in oligodendroglioma
relapses may at least in part be induced by irradiation and Acknowledgements
chemotherapy administered to the patients (Table 1). In
neoplasia, this is of note, because tumor cells induce Supported by a grant from the fortune program (¨ [
638-reactive oxygen species in monocytes and consequently 0-0) of the University of Tuebingen. We thank Thai Dung
contribute to spontaneous monocyte cytotoxicity and pro- Nguyen for expert technical assistance.
longed tissue damage [19]. Moreover, activation of HO-1 is a major stimulus for the synthesis and release of pro-inflammatory cytokines in macrophages [29]. It has
there-References
fore been suggested that this pathway may play a central role in pathological situations in which local tissue
hypo-[1] M. Bergeron, D.M. Ferriero, H.J. Vreman, D.K. Stevenson, F.R.
xia / oxygenation triggers a systemic inflammatory
re-Sharp, Hypoxia-ischemia, but not hypoxia alone, induces the
sponse. Furthermore, HO-mediated heme degradation is expression of heme oxygenase-1 (HSP32) in newborn rat brain, J.
the primary mechanism for cellular CO production, and Cerebr. Blood Flow Metab. 17 (1997) 647–658.
reactive oxygen intermediates including CO are capable to [2] M.H. Deininger, R. Meyermann, Multiple epitope labeling by the
exclusive use of alkaline phosphatase conjugates in
immunohisto-induce angiogenesis and thus promote neoplastic
out-chemistry, Histochem. Cell Biol. 110 (1998) 425–430.
growth [10]. Iron increases oxidative stress and regulates
[3] K.K. Elbirt, H.L. Bonkovsky, Heme oxygenase: recent advances in
the expression of proinflammatory mRNAs by affecting understanding its regulation and role, Proc. Assoc. Am. Physicians
the conformation of iron regulatory protein (IRP)-1 and its 111 (1999) 438–447.
binding to iron regulatory elements (IREs) in the 59- or [4] J.F. Ewing, S.N. Haber, M.D. Maines, Normal and heat-induced
patterns of expression of heme oxygenase-1 (HSP32) in rat brain: structure of the human cytoplasmic beta-actin gene: interspecies hyperthermia causes rapid induction of mRNA and protein, J. homology of sequences in the introns, Proc. Natl. Acad. Sci. USA Neurochem. 58 (1992) 1140–1149. 82 (1985) 6133–6137.
[5] K. Fukuda, S.S. Panter, F.R. Sharp, L.J. Noble, Induction of heme [21] T. Nimura, P.R. Weinstein, S.M. Massa, S. Panter, F.R. Sharp, Heme oxygenase-1 (HO-1) after traumatic brain injury in the rat, Neurosci. oxygenase-1 (HO-1) protein induction in rat brain following focal Lett. 199 (1995) 127–130. ischemia, Mol. Brain Res. 37 (1996) 201–208.
[6] E. Hara, K. Takahashi, T. Tominaga, T. Kumabe, T. Kayama, H. [22] A. Nishie, M. Ono, T. Shono, J. Fukushi, M. Otsubo, H. Onoue, Y. Suzuki, H. Fujita, T. Yoshimoto, K. Shirato, S. Shibahara, Expres- Ito, T. Inamura, K. Ikezaki, M. Fukui, T. Iwaki, M. Kuwano, sion of heme oxygenase and inducible nitric oxide synthase mRNA Macrophage infiltration and heme oxygenase-1 expression correlate in human brain tumors, Biochem. Biophys. Res. Commun. 224 with angiogenesis in human gliomas, Clin. Cancer Res. 5 (1999)
(1996) 153–158. 1107–1113.
[7] C.L. Hartsfield, J. Alam, J.L. Cook, A.M. Choi, Regulation of heme [23] N. Panahian, M. Yoshiura, M.D. Maines, Overexpression of heme oxygenase-1 gene expression in vascular smooth muscle cells by oxygenase-1 is neuroprotective in a model of permanent middle nitric oxide, Am. J. Physiol. 273 (1997) L980–L988. cerebral artery occlusion in transgenic mice, J. Neurochem. 72 [8] I. Horvath, L.E. Donnelly, A. Kiss, P. Paredi, S.A. Kharitonov, P.J. (1999) 1187–1203.
Barnes, Raised levels of exhaled carbon monoxide are associated [24] D.R. Premkumar, M.A. Smith, P.L. Richey, R.B. Petersen, R. with an increased expression of heme oxygenase-1 in airway Castellani, R.K. Kutty, B. Wiggert, G. Perry, R.N. Kalaria, Induction macrophages in asthma: a new marker of oxidative stress, Thorax 53 of heme oxygenase-1 mRNA and protein in neocortex and cerebral (1998) 668–672. vessels in Alzheimer’s disease, J. Neurochem. 65 (1995) 1399– [9] P. Kleihues, P.C. Burger, B.W. Scheithauer, The new classification of 1408.
brain tumors, Brain Pathol. 3 (1993) 255–268. [25] J.D. Richmon, K. Fukuda, N. Maida, M. Sato, M. Bergeron, F.R. [10] M. Kuroki, E.E. Voest, S. Amano, L.V. Beerepoot, S. Takashima, M. Sharp, S.S. Panter, L.J. Noble, Induction of heme oxygenase-1 after Tolentino, R.Y. Kim, R.M. Rohan, K.A. Colby, K.T. Yeo, A.P. hyperosmotic opening of the blood–brain barrier, Brain Res. 780 Adamis, Reactive oxygen intermediates increase vascular endotheli- (1998) 108–118.
al growth factor expression in vitro and in vivo, J. Clin. Invest. 98 [26] H.M. Schipper, S. Cisse, E.G. Stopa, Expression of heme (1996) 1667–1675. oxygenase-1 in the senescent and Alzheimer-diseased brain, Ann. [11] R.K. Kutty, G. Kutty, B. Wiggert, G.J. Chader, R.M. Darrow, D.T. Neurol. 37 (1995) 758–768.
Organisciak, Induction of heme oxygenase 1 in the retina by intense [27] H.J. Schluesener, K. Seid, J. Kretzschmar, R. Meyermann, Allograf-visible light: suppression by the antioxidant dimethylthiourea, Proc. t-inflammatory factor-1 in rat experimental autoimmune en-Natl. Acad. Sci. USA 92 (1995) 1177–1181. cephalomyelitis, neuritis and uveitis: expression by activated macro-[12] H.Y. Lan, W. Mu, Y.Y. Ng, D.J. Nikolic-Paterson, R.C. Atkins, A phages and microglial cells, Glia 24 (1998) 244–251.
simple, reliable, and sensitive method for nonradioactive in situ [28] S. Takizawa, H. Hirabayashi, K. Matsushima, K. Tokuoka, Y. hybridization: use of microwave heating to improve hybridization Shinohara, Induction of heme oxygenase protein protects neurons in efficiency and preserve tissue morphology, J. Histochem. Cytochem. cortex and striatum, but not in hippocampus, against transient 44 (1996) 281–287. forebrain ischemia, J. Cerebr. Blood Flow Metab. 18 (1998) 559– [13] S. Magnusson, M. Kanje, Differential macrophage responses follow- 569.
ing pre- and postganglionic axotomy, NeuroReport 9 (1998) 841– [29] F. Tamion, V. Richard, S. Lyoumi, M. Hiron, G. Bonmarchand, J.
846. Leroy, M. Daveau, C. Thuillez, J.P. Lebreton, Induction of haem
[14] M.D. Maines, B.C. Eke, C.M. Weber, J.F. Ewing, Corticosterone has oxygenase contributes to the synthesis of pro-inflammatory cyto-a permissive effect on expression of heme oxygencyto-ase-1 in CA1– kines in re-oxygenated rat macrophages: role of cGMP, Cytokine 11 CA3 neurons of hippocampus in thermal-stressed rats, J. Neuro- (1999) 326–333.
chem. 64 (1995) 1769–1779. [30] C.M. Terry, J.A. Clikeman, J.R. Hoidal, K.S. Callahan, TNF-alpha [15] M.D. Maines, The heme oxygenase system: a regulator of second and IL-1alpha induce heme oxygenase-1 via protein kinase C, messenger gases, Annu. Rev. Pharmacol. Toxicol. 37 (1997) 517– Ca21, and phospholipase A2 in endothelial cells, Am. J. Physiol.
554. 276 (1999) H1493–H1501.
[16] Y. Matsuoka, Y. Kitamura, J. Kakimura, T. Taniguchi, Expression of [31] V. Turcanu, M. Dhouib, P. Poindron, Heme oxygenase inhibits nitric heme oxygenase-1 mediated by non-NMDA and metabotropic oxide synthase by degrading heme: a negative feedback regulation receptors in glial cells: possible involvement of reactive oxygen mechanism for nitric oxide production, Transplant Proc. 30 (1998) species production and protein kinase C activation, Neurophar- 4184–4185.
macology 38 (1999) 825–834. [32] S.R. Vincent, S. Das, M.D. Maines, Brain heme oxygenase iso-[17] A.E. Mautes, D.H. Kim, F.R. Sharp, S. Panter, M. Sato, N. Maida, enzymes and nitric oxide synthase are co-localized in select neurons,
M. Bergeron, K. Guenther, L.J. Noble, Induction of heme Neuroscience 63 (1994) 223–231.
oxygenase-1 (HO-1) in the contused spinal cord of the rat, Brain [33] L.J. Wang, T.S. Lee, F.Y. Lee, R.C. Pai, L.Y. Chau, Expression of Res. 795 (1998) 17–24. heme oxygenase-1 in atherosclerotic lesions, Am. J. Pathol. 152 [18] R. Motterlini, R. Foresti, M. Intaglietta, R.M. Winslow, NO-me- (1998) 711–720.
diated activation of heme oxygenase: endogenous cytoprotection [34] D. Willis, A.R. Moore, R. Frederick, D.A. Willoughby, Heme against oxidative stress to endothelium, Am. J. Physiol. 270 (1996) oxygenase: a novel target for the modulation of the inflammatory
107–114. response, Nature Med. 2 (1996) 87–97.