www.elsevier.com / locate / bres
Interactive report
Effect of intrathecal galanin and its putative antagonist M35 on pain
1
behavior in a neuropathic pain model
*
¨
Hong-Xiang Liu, Tomas Hokfelt
Department of Neuroscience, Karolinska Institutet, S-171 77 Stockholm, Sweden Accepted 8 August 2000
Abstract
There is currently some debate over a possible role of galanin in pain processing. It was recently reported that the levels of galanin in dorsal root ganglia (DRGs) seem related to development of allodynia after unilateral sciatic nerve constriction injury. In our present study, we aimed at characterizing the effect of exogenous and endogenous galanin on pain behavior in allodynic and non-allodynic rats in which the levels of galanin in DRG neurons are low and high, respectively [28]. The results show that in allodynic rats, the mechanical threshold increases dose-dependently after intrathecal (i.t.) injection of galanin, while no significant changes were observed in groups treated with the putative galanin antagonist M35 or saline. In non-allodynic rats i.t. injection of M35 induced a significant mechanical allodynic state, which did not occur after injection of galanin, bradykinin, the bradykinin fragment(2–9) or saline. The results suggest that in the present experimental paradigm exogenous galanin has an anti-allodynic effect in the allodynic rats, and that endogenous galanin has a tonic inhibitory effect in the non-allodynic group. 2000 Elsevier Science B.V. All rights reserved.
Theme: Sensory systems
Topic: Pain modulation: pharmacology
Keywords: Allodynia; Dorsal root ganglion; Nerve injury; Neuropeptide; Spinal cord
1. Introduction like immunoreactivity (LI) [3,29], galanin mRNA [36] and galanin receptor mRNAs are expressed in dorsal root
Neuropeptides are widely distributed in the nervous ganglion (DRG) neurons, and also in dorsal horn
inter-system, virtually always coexisting with one or more neurons express galanin [3,7,21,23,25,27,30,37,45,49].
classic transmitters (see [11]). Their exact functional role Galanin expression is strongly upregulated in DRG
neu-has often not been defined, but they may both act as rons [10,36], and its basal release is increased in the dorsal
trophic molecules (see [26,32]) and / or as slow, mostly horn after peripheral nerve injury [4]. Many studies have
extrasynaptically released (see [34]) messengers (see focussed on a possible functional role of galanin in sensory
[17,19]), especially in situations when neurons are exposed processing in the dorsal horn of the spinal cord showing
to, in the broadest sense, ‘stressful’ stimuli (see [11]). In that the effects of galanin on nociception are complex, and
the present study we focus on one such situation, neuro- both facilitory, inhibitory and bi-phasic responses have
pathic pain, and the possible involvement of the 29- been reported (see [41,44]).
aminoacid peptide galanin [33] as a putative endogenous Several models of partial nerve injury have been
de-pain antagonist (see [41,44]). veloped, which can be used for the assessment of the
Immunohistochemical studies have shown that galanin- effects of analgesics on abnormal pain-like behaviors
(allodynia and hyperalgesia). One example is loose chronic constriction nerve injury (CCI) [2]. In this so-called
1
Published on the World Wide Web on 13 September 2000. Bennett model allodynia is induced only in some rats,
Abbreviations: ANOVA, analysis of variance; CCI, chronic constriction
which allows comparison between the two groups treated
injury; DRG, dorsal root ganglion; i.t., intrathecal; i.p., intraperitoneal
in an apparently identical way, that is allodynic versus
*Corresponding author. Tel.:146-8-728-7070; fax:146-8-331-692. ¨
E-mail address: [email protected] (T. Hokfelt). non-allodynic rats. Recently Shi et al. [28] found that
following sciatic nerve CCI galanin expression in DRG manner from below at the center of the plantar surface of
neurons is more strongly upregulated in non-allodynic rats hindpaw ipsi-lateral to the nerve injury. Each filament was
when compared to the allodynic group. In the present study delivered three times with approximately 2-s intervals. The
we used the same model to examine the effect of i.t. lowest force at which each of the three applications of the
galanin and a putative galanin antagonist, M35 [1], in both filament elicited a paw withdrawal was taken as the
allodynic and non-allodynic rats. mechanical response threshold.
2.4. Experimental design
2. Materials and methods
The measurement of the mechanical threshold started 14
Adult male Sprague–Dawley rats (240–260 g; ALAB, days after the nerve injury. The basal threshold was usually
Stockholm, Sweden) were used. The rats were housed in between 2.85 and 13.5 g (or higher), and then the rats were
cages at room temperature (20–258C) under a 12 / 12 h divided into two groups according to the threshold,
non-light / dark cycle with free access to food and water. The allodynic ($8.8 g) and allodynic (#5.2 g) rats.
experiments were conducted according to the Ethical In the first experiment, galanin was administered i.t. in
Guidelines for Investigation of Experimental Pain in four allodynic rats in a cumulative dose regime of 1, 9, and
Conscious Animals [50] and were approved by the local 20 mg, totally 30 mg. In non-allodynic rats, M35 was
ethics committee for animal research. administered in a similar manner in doses of 0.1, 0.2, and
0.4 mg, totally 0.7 mg. The same volume of saline was
2.1. Unilateral sciatic nerve injury injected in two allodynic and two non-allodynic rats.
To further confirm the effects of galanin in allodynic,
Unilateral sciatic nerve injury was produced under deep and of M35 in non-allodynic rats, a second series of
anesthesia with intraperitoneal (i.p.) injection of sodium experiments was performed blindly (six allodynic rats
pentobarbital (Mebumal ; 60 mg / kg), as described by received galanin, and six saline; six non-allodynic rats
Bennett and Xie [2]. The common sciatic nerve was received M35 and five saline). All testing procedures were
exposed and freed for about 10 mm at mid thigh level. the same as in the first series, except that the dose of M35
Four ligatures (Ethicon ; 4.0 plain gut) were tied loosely of the last injection was increased to 0.7mg, to give a total
around the nerve with about 1-mm spacing. Great care was cumulative dose of 1 mg.
taken to tie the ligatures such that the nerve was barely Additional control tests were carried out to check the
constricted. The incision was closed in layers with 3-0 silk effect of galanin (1, 9, 20mg), bradykinin (0.047, 0.095,
sutures. 0.33 mg), bradykinin fragment(2–9) (0.040, 0.080, 0.28
mg) in non-allodynic rats, and a high dose M35 (equimolar
2.2. Intrathecal catheterization and injection to the galanin, that is 0.7, 6.34, 14.1mg) in allodynic rats
in an alternate non-blind design. The experiments with
Seven days after sciatic nerve loose ligation, a chronic bradykinin and the bradykinin fragment were applied,
i.t. catheter was implanted according to a modification since bradykinin(2–9) represents the C-terminal part of
described by Storkson et al. [31]. After anesthesia with i.p. M35 [1].
sodium pentobarbital, a catheter (PE 10, o.d. 0.61 mm) was
inserted into the subarachnoid space through a guide- 2.5. Drugs
cannula (Sterican ; 20 G, 0.90340 mm) at the level of the
gap between the L5 and L6 vertebrae. It was then carefully Rat galanin was purchased from Peninsula (Belmont,
implanted rostrally with its tip at the lumbar enlargement. CA, USA) and M35 [galanin(1–13)-bradykinin(2–9)
I.t. injection was given 7 days later. The proper location of amide] from Bachem (Bubendorf, Switzerland).
Brady-the caBrady-theter was tested 24 h before Brady-the pharmacological kinin and bradykinin fragment (2–9) were purchased from
experiments by assessing sensory and motor blockade after ICN Biomedicals, Aurora, OH, USA. All the peptides were
¨
i.t. injection of 7 ml lidocaine (50 mg / ml; Astra, Soder- dissolved in distilled water and diluted with sterile saline
talje, Sweden). and stored in aliquots at 2208C until use.
2.3. Measurement of mechanical allodynia 2.6. Statistics
Behavioral testing was performed during daytime (9.00– The results were presented as median6median-derived
18.00) 14 days after sciatic nerve constriction. Rats were absolute deviation (MAD). Friedman ANOVA (one-way
placed in transparent plastic domes (838318 cm) on a repeated measures ANOVA on ranks) was used to analyze
metal mesh floor with a hole size of 333 mm. After 15 the data in each group with the time course. Kruskal–
min of adaptation, a series of von Frey filaments (0.5, 0.88, Wallis ANOVA test (one-way ANOVA on ranks) was used
point. A P value less than 0.05 was chosen as the significant level.
3. Results
The data for galanin and saline in allodynic rats, and saline in non-allodynic rats were combined from the blind and non-blind series, since no differences were noted. The data for M35 in non-allodynic rats were blind. The rest of the data was non-blind.
3.1. Anti-allodynic effect of galanin in allodynic rats
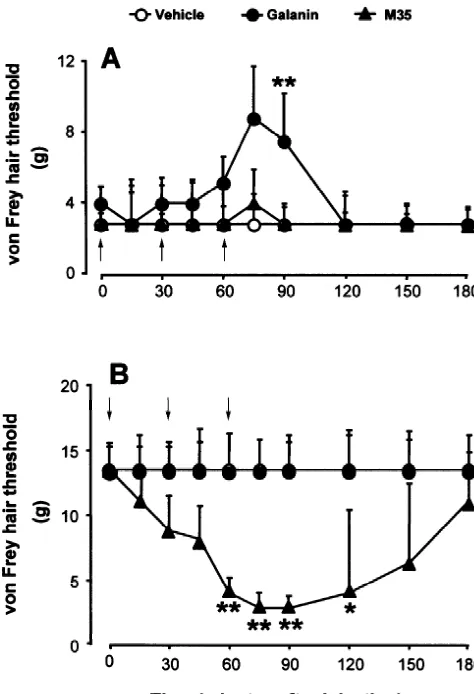
The effect of i.t. galanin or M35 on the behavioral thresholds for withdrawal of the hindpaw ipsilateral to the nerve injury was determined in allodynic rats. The results are shown as Fig. 1A and Table 1. Neither vehicle, nor M35, nor high dose M35 treatment had any significant effect on the mechanical threshold compared with baseline
(Friedman ANOVA, P.0.05), whereas the threshold
in-creased dose-dependently after i.t. injection of galanin (P,0.001). Galanin-treated rats had a significant increase in mechanical thresholds at 30 min after the last injection compared with saline and M35 groups (Kruskal–Wallis
ANOVA, P,0.01).
3.2. M35-induced allodynia in non-allodynic model rats
In non-allodynic rats with the same nerve injury
opera-Fig. 1. Effects of galanin and M35 on mechanical threshold in allodynic
tion, M35, galanin, bradykinin or its fragment
(A) and non-allodynic (B) rats. Galanin or M35 was given in a
bradykinin(2–9) were applied. M35 induced a dramatic
cumulative regime of 1, 9, 20mg, totally 30 mg and 0.1, 0.2, 0.7mg,
dose-dependent drop of the mechanical threshold
(Fried-totally 1 mg, respectively. In allodynic rats galanin dose-dependently
induced a significant increase of mechanical threshold (repeated measures man ANOVA, P,0.0001), while no significant changes ANOVA on ranks, P,0.001), neither M35 nor saline had any effect were observed for galanin, bradykinin, bradykinin(2–9) or (repeated measures ANOVA on ranks, P50.80, P50.18 respectively). In
saline (P.0.05) (Fig. 1B; Table 1). The mechanical
non-allodynic rats, M35 induced a dramatic drop of the mechanical
threshold was lower than in the other groups at 30 min
threshold (P,0.0001) which did not change after injection of galanin or
after 0.2 mg M35 and at 15 min, 30 min, 60 min after 0.7
saline (P50.22, P50.57, respectively). *,** indicate P,0.05, P,0.01
compared among groups at the same time point (one-way ANOVA on mg M35 injection (Kruskal–Wallis ANOVA, P,0.001, ranks). The arrows indicate that i.t. injection was given immediately after P,0.001, P,0.01, P,0.05 respectively).
the measurement of the value at the time point.
In view of a bradykinin fragment being part of the M35
Table 1
a
Effects of high dose M35 on mechanical threshold in allodynic and of bradykinin, and bradykinin fragment(2–9) in non-allodynic rats
Group Drug Time (minutes after i.t. injection) Friedman
ANOVA
0↓ 15 30↓ 45 60↓ 75 90 120 150 180
Allodynic rats High dose
M35 2.8560.78 2.8560.78 2.8560.39 2.8561.04 2.8561.43 2.8561.43 2.8560.39 2.8560.26 2.8560.78 2.8560.0 P50.55
Non-allodynic rats Bradykinin 13.560.0 13.562.38 13.561.0 13.560.0 13.560.78 13.561.0 13.561.38 13.561.78 13.561.78 11.1562.78 P50.46 Bradykinin(2–9) 13.560.78 13.561.38 13.561.38 13.561.78 13.561.0 13.562.78 13.561.78 13.560.78 13.562.78 13.561.0 P50.52
a
molecule, a high dose (10 mg) of bradykinin or its ng–1 mg) increased reflex excitability. It has also been
fragment was injected in non-allodynic rats. All five rats reported that in normal rats a single i.t. injection of galanin
receiving this dose of bradykinin started to struggle in the (0.1 and 1 nmol) decreases the nociceptive threshold for
domes within 1 min and this behavior lasted for about 1 mechanical stimulation [18]. Recently Kerr et al. [15]
min. No behavioral response was observed in the four rats found that following full sciatic transection or partial nerve
that received 10mg of bradykinin(2–9). injury, spontaneous and evoked neuropathic pain behaviors
are largely eliminated or severely compromised in galanin null mutant mice. Furthermore, chronic intrathecal delivery
4. Discussion of low dose (25 ng / h, 14 d) exogenous galanin to nerve-intact adult rats, which should mimic nerve injury-induced
The function of galanin in pain processing at the spinal upregulation of galanin, induces persistent mechanical
level has been subject of many studies since its discovery hypersensitivity. These data suggest that the upregulation
in 1983 by Tatemoto and collaborators [33], and complex of galanin is associated with the development of
neuro-functional patterns have emerged, whereby the endogenous pathic pain after the peripheral nerve injury. However, the
galanin levels, alternatively the dose of exogenously putative galanin receptor antagonist M35 given i.t. did not
applied galanin appear to be important factors. significantly alter pain behavior after photochemically
Under normal circumstances, galanin can only be de- induced ischaemic peripheral nerve injury [8]. In
agree-tected in less than 5% of sensory neurons in adult dorsal ment, our present study shows no significant effect of i.t.
root ganglia [3,29], but may be synthesized at a low rate in M35 on the mechanical threshold in allodynic rats with
a considerably larger number of neurons [16]. These unilateral CCI of sciatic nerve in a wide range of doses. It
galanin neurons give predominantly rise to small diameter is possible that M35 blocked both inhibitory and facilitory
fibers, presumably nociceptors with unmyelinated, slowly effects of galanin.
conducting axons that also coexpress the neuropeptide There is evidence that high levels / doses of galanin have
calcitonin gene-related peptide [12,48]. Using the micro- antinociceptive effects. Thus, i.t. administration of high
probe technique, originally described by Duggan et al. [6], doses (.1mg) of galanin inhibits the nocifensive reflex in
basal release of galanin-LI has been demonstrated in the spinalized rats [39,40] and normal rats [5,43], in contrast to
dorsal horn [9], although it is not clear if this galanin the facilitory effect of low doses mentioned above.
More-originates from primary afferents, or local dorsal horn over, very high doses of exogenous galanin alleviate the
neurons, or both. Functional studies have been carried out neuropathic pain behaviors following peripheral nerve
to characterize the role of galanin in normal animals, that injury [8,47]. The question therefore arises to what extent
is animals with low galanin DRG neuron levels. Wiesenfel- high levels of endogenous galanin really can act as an
d-Hallin and colleagues [42] found that i.t. injection of the analgesic factor in the spinal cord. In our present study, we
putative galanin receptor antagonist M35 potentiated the examined the effect of exogenous and endogenous galanin
facilitation of the flexor reflex by conditioning stimulation on pain behavior in non-allodynic and allodynic Bennett
of cutaneous unmyelinated afferents in rats with intact model rats, in which the levels of galanin in DRG have
nerves. In accordance with this, Kerr et al. [15] recently been reported to be different, that is 43% of neuron profiles
reported that the sensitivity to noxious stimuli is sig- expressed galanin in non-allodynic rats versus 23% in the
nificantly higher in galanin null mutant mice than wild- allodynic group [28]. We found that i.t. galanin induced an
type controls. These results indicate that endogenous anti-allodynic effect in allodynic rats which is identical
galanin plays a tonic inhibitory role in nociceptive process- with other reports [8,47]. Moreover, in non-allodynic rats
ing in the spinal cord under normal conditions. the putative galanin receptor antagonist M35, when given
In all animal models based on peripheral nerve injury i.t., dose-dependently induced a long-lasting allodynic
known to be associated with neuropathic pain behaviors state, indicating that high-level endogenous galanin exerts
there is upregulation of galanin in DRG neurons. This is a tonic inhibition of pain processing in the spinal cord
true for complete axotomy [10,13,36], nerve crush [36], following nerve injury. It should, however, be remembered
CCI [20,22,28], local treatment with mitosis inhibitor [13] that the presently available galanin ‘antagonists’, including
and partial nerve ligation [20,28]. In agreement enhanced M35, have been shown to exert agonist activity in many
immunoreactive galanin release was found in the superfi- models, at least at high doses (see e.g. [14,24,46] and
cial dorsal horn ipsi-lateral to the sciatic nerve injury [4]. below).
The association of galanin expression with the develop- Taken together, galanin’s role in pain processing in the
ment of neuropathic pain behaviors following peripheral spinal cord seems to include inhibition and excitation. The
nerve damage suggests that galanin may modulate the mechanisms underlying varying response properties to
sensory transmission in the spinal cord, especially after either endogenous or exogenous galanin are unknown. It
nerve injury [35,39,40,42]; (see [41,44]). Wiesenfeld-Hal- has been reported that all the three galanin receptor
lin et al. [38,39] examined the effect of i.t. galanin on the subtype mRNAs are expressed within DRGs and spinal
nocifensive flexor reflex in decerebrate, spinalized, un- cord [7,23,25,27,30,37,45], where their distribution and
endogen-[2] G.J. Bennett, Y.K. Xie, A peripheral mononeuropathy in rat that
ous and exogenous galanin may have pre- and
post-synap-produces disorders of pain sensation like those seen in man, Pain 33
tic actions in the spinal cord. Maybe the differential
(1988) 87–107.
distribution and regulation of galanin receptors underlie the [3] J.L.C. Ch’ng, N.D. Christofides, P. Anand, S.J. Gibson, Y.S. Allen,
wide range of behavioral and electrophysiological re- H.C. Su, K. Tatemoto, J.F.B. Morrison, J.M. Polak, S.R. Bloom,
sponses. Distribution of galanin immunoreactivity in the central nervous
system and responses of galanin-containing neuronal pathways to
The chimeric peptide M35
[galanin(1–13)-bradykin-injury, Neuroscience 16 (1985) 343–354.
in(2–9) amide] is a putative high-affinity galanin receptor
[4] L.A. Colvin, M.A. Mark, A.W. Duggan, The effect of a peripheral
antagonist acting in various central and peripheral tissues
mononeuropathy on immunoreactive (ir)-galanin release in the
¨
[1]. For example, the studies of Ogren’s group indicate that spinal cord of the rat, Brain Res. 766 (1997) 259–261.
a low dose of M35 (1 nmol) acts as a galanin receptor [5] R.A. Cridland, J.L. Henry, Effects of intrathecal administration of
antagonist, whereas higher doses behave as a mixed neuropeptides on a spinal nociceptive reflex in the rat: VIP, galanin,
CGRP, TRH, somatostatin and angiotensin. II, Neuropeptides 11
agonist–antagonist in vivo in the rat striatum (see [24]).
(1988) 23–32.
M35 also has a dual effect on the galanin-mediated
[6] A.W. Duggan, I.A. Hendry, J.L. Green, C.R. Morton, W.D.
Hut-inhibition of forskolin-stimulated cAMP production in the
chison, The preparation and use of antibody microprobes, J.
rat pancreatic beta-cell line Rin m 5F: at low concen- Neurosci. Meth. 23 (1988) 241–247.
trations M35 antagonizes the effect of galanin, while at [7] E.L. Gustafsson, K.E. Smith, M.M. Durkin, C. Gerald, T.A.
concentrations above 10 nM M35 acts as a galanin Branchek, Distribution of a rat galanin receptor mRNA in rat brain,
Neuroreport 7 (1996) 953–957.
receptor agonist [14]. In the locus coeruleus it also exhibits
¨ [8] J.X. Hao, T.J. Shi, I.S. Xu, T. Kaupilla, X.J. Xu, T. Hokfelt, T.
agonist action [46]. In our present study, no similarity in
Bartfai, Z. Wiesenfeld-Hallin, Intrathecal galanin alleviates
al-the effects of M35 and galanin was found after i.t.
lodynia-like behavior in rats after partial peripheral nerve injury,
administration, neither in allodynic nor in non-allodynic Eur. J. Neurosci. 11 (1999) 427–432.
rats at a cumulative dose of 1 mg (even over 20 mg in [9] P.J. Hope, C.W. Lang, B.D. Grubb, A.W. Duggan, Release of
non-allodynic rats). The results suggest that in our model immunoreactive galanin in the spinal cord of rats with ankle
inflammation: studies with antibody microprobes, Neuroscience 60
and at the doses used M35 had no agonistic effect in the
(1994) 801–807.
spinal cord. This is in agreement with earlier results by
¨
[10] T. Hokfelt, Z. Wiesenfeld-Hallin, M. Villar, T. Melander, Increase of
Hao et al. [8].
galanin-like immunoreactivity in rat dorsal root ganglion cells after
In this study we also paid attention to the possible peripheral axotomy, Neurosci. Lett. 83 (1987) 217–220.
activity of the bradykinin fragment included in the M35 [11] T. Hokfelt, C. Broberger, Z.Q. Xu, V. Sergeyev, R. Ubink, M. Diez,¨
molecule. Bradykinin is a well-known potent algesic Neuropeptides — an overview, Neuropharmacology 39 (2000)
1337–1356.
factor. To exclude bradykinin-like algesic activity of its
¨
[12] G. Ju, T. Hokfelt, E. Brodin, J. Fahrenkrug, J.A. Fischer, P. Frey,
fragment (2–9) in non-allodynic rats, we used the same
R.P. Elde, J.C. Brown, Primary sensory neurons of the rat showing
molecular doses of bradykinin and bradykinin(2–9) as
calcitonin gene-related peptide immunoreactivity and their relation
control, but no significant changes of the mechanical to substance P-, somatostatin-, galanin-, vasoactive intestinal poly-threshold were observed. This indicates that the allodynic peptide- and cholecystokinin-immunoreactive ganglion cells, Cell
effect induced by M35 in non-allodynic rats represents a Tissue Res. 247 (1987) 417–431.
[13] H. Kashiba, E. Senba, Y. Kawai, Y. Ueda, M. Tohyama, Axonal
galanin receptor-related action.
blockade induces the expression of vasoactive intestinal polypeptide
In summary, the results in our study suggest that
and galanin in rat dorsal root ganglion neurons, Brain Res. 577
exogenous galanin can play an anti-allodynic role in (1992) 19–28.
allodynic rats with peripheral nerve injury. Moreover, M35 [14] K. Kask, M. Berthold, J. Bourne, S. Andell, U. Langel, T. Bartfai,
dose-dependently changed non-allodynic rats into an al- Binding and agonist / antagonist actions of M35,
galanin(1–13)-bradykinin(2–9) amide chimeric peptide, in Rin m 5F insulinoma
lodynic state, indicating that high endogenous galanin
cells, Regul. Pept. 59 (1995) 341–348.
levels in non-allodynic model rats play a tonic inhibitory
[15] B.J. Kerr, W.B. Cafferty, Y.K. Gupta, A. Bacon, D. Wynick, S.B.
role in the neuropathic pain processing in the spinal cord.
McMahon, S.W. Thompson, Galanin knockout mice reveal nocicep-tive deficits following peripheral nerve injury, Eur. J. Neurosci. 12 (2000) 793–802.
[16] C.M. Klein, K.N. Westlund, R.E. Coggeshall, Percentages of dorsal
Acknowledgements
root axons immunoreactive for galanin are higher than those immunoreactive for calcitonin gene-related peptide in the rat, Brain
This study was supported by an Unrestricted
Bristol-Res. 519 (1990) 97–101.
Myers Squibb Neuroscience Grant, Marianne and Marcus [17] I. Kupfermann, Functional studies of cotransmission, Physiol. Rev.
Wallenberg’s Foundation and the Swedish MRC (04X- 71 (1991) 683–732.
2887). [18] Y. Kuraishi, M. Kawamura, T. Yamaguchi, T. Houtani, S. Kawabata,
S. Futaki, N. Fujii, M. Satoh, Intrathecal injections of galanin and its antiserum affect nociceptive response of rat to mechanical, but not thermal, stimuli, Pain 44 (1991) 321–324.
References [19] J.M. Lundberg, Pharmacology of cotransmission in the autonomic
nervous system: integrative aspects on amines, neuropeptides, [1] T. Bartfai, G. Fisone, U. Langel, Galanin and galanin antagonists: adenosine triphosphate, amino acids and nitric oxide, Pharmacol.
molecular and biochemical perspectives, Trends Pharmacol. Sci. 13 Rev. 48 (1996) 113–178.
immuno-reactivities in the primary sensory neurons following partial and in the rat after sciatic nerve section: demonstrated by chronic complete sciatic nerve injuries, Neuroscience 79 (1997) 1183–1195. intrathecal infusion of a high affinity galanin receptor antagonist,
¨ ¨
[21] T. Melander, T. Hokfelt, A. Rokaeus, Distribution of galanin-like Neurosci. Lett. 149 (1993) 193–197.
immunoreactivity in the rat central nervous system, J. Comp. [36] M.J. Villar, R. Cortes, E. Theodorsson, Z. Wiesenfeld-Hallin, M. ¨
Neurol. 248 (1986) 475–517. Schalling, J. Fahrenkrug, P.C. Emson, T. Hokfelt, Neuropeptide [22] R.L. Nahin, K.R. De Marino Leon, M. Ruda, Primary sensory expression in rat dorsal root ganglion cells and spinal cord after neurons exhibit altered gene expression in a rat model of neuro- peripheral nerve injury with special reference to galanin, Neuro-pathic pain, Pain 58 (1994) 95–108. science 33 (1989) 587–604.
[23] D. O’Donnell, S. Ahmad, C. Wahlestedt, P. Walker, Expression of [37] S.M. Waters, J.E. Krause, Distribution of galanin-1, -2 and -3 the novel galanin receptor subtype GALR-2 in the adult rat CNS. receptor messenger RNAs in central and peripheral rat tissues, Distinct distribution from GALR-1, J. Comp. Neurol. 409 (1999) Neuroscience 95 (2000) 265–271.
¨
469–481. [38] Z. Wiesenfeld-Hallin, M.J. Villar, T. Hokfelt, Intrathecal galanin at
¨ ¨
[24] S.O. Ogren, P.A. Schott, J. Kehr, T. Yoshitake, I. Misane, P. low doses increases spinal reflex excitability in rats more to thermal ¨
Mannstrom, J. Sandin, Modulation of acetylcholine and serotonin than mechanical stimuli, Exp. Brain Res. 71 (1988) 663–666. ¨
transmission by galanin. Relationship to spatial and aversive learn- [39] Z. Wiesenfeld-Hallin, M.J. Villar, T. Hokfelt, The effects of in-ing, Ann. NY Acad. Sci. 863 (1998) 342–363. trathecal galanin and C-fiber stimulation on the flexor reflex in the [25] E.M. Parker, D.G. Izzarelli, H.P. Nowak, C.D. Mahle, J. Wang, M.E. rat, Brain Res. 486 (1989) 205–213.
¨
Goldstein, Cloning and characterization of the rat GAL R1 galanin [40] Z. Wiesenfeld-Hallin, X.J. Xu, M.J. Villar, T. Hokfelt, The effect of receptor from Rin14B insulinoma cells, Mol. Brain Res. 34 (1995) intrathecal galanin on the flexor reflex in rat: increased depression 179–189. after sciatic nerve section, Neurosci. Lett. 105 (1989) 149–154.
¨
[26] J. Schwartz, Neurotransmitters as neurotrophic factors: a new set of [41] Z. Wiesenfeld-Hallin, T. Bartfai, T. Hokfelt, Galanin in sensory functions, Int. Rev. Neurobiol. 34 (1990) 1–23. neurons in the spinal cord, Front. Neuroendocrinol. 13 (1992)
¨
[27] T.J.S. Shi, X. Zhang, K. Holmberg, Z.Q.D. Xu, T. Hokfelt, 319–343.
¨ Expression and regulation of galanin-R2 receptors in rat primary [42] Z. Wiesenfeld-Hallin, X.J. Xu, U. Langel, K. Bedecs, T. Hokfelt, T. sensory neurons — Effect of axotomy and inflammation, Neurosci. Bartfai, Galanin-mediated control of pain: enhanced role after nerve Lett. 237 (1997) 57–60. injury, Proc. Natl. Acad. Sci. USA 89 (1992) 3334–3337.
¨ ¨
[28] T.J. Shi, J.G. Cui, B.A. Meyerson, B. Linderoth, T. Hokfelt, [43] Z. Wiesenfeld-Hallin, X.J. Xu, J.X. Hao, T. Hokfelt, The behavioral Regulation of galanin and neuropeptide Y in dorsal root ganglia and effects of intrathecal galanin on tests of thermal and mechanical dorsal horn in rat mononeuropathic models: possible relation to nociception in the rat, Acta Physiol. Scand. 147 (1993) 457–458.
¨
tactile hypersensitivity, Neuroscience 93 (1999) 741–757. [44] X.J. Xu, T. Hokfelt, T. Bartfai, Z. Wiesenfeld-Hallin, Galanin and [29] G. Skofitsch, D.M. Jacobowitz, Galanin-like immunoreactivity in spinal nociceptive mechanisms: recent advances and therapeutic
capsaicin sensitive sensory neurons and ganglia, Brain Res. Bull. 15 implications, Neuropeptides (2000), in press. ¨
(1985) 191–195. [45] Z.Q. Xu, T.J. Shi, M. Landry, T. Hokfelt, Evidence for galanin [30] K.E. Smith, M.W. Walker, R. Artymyshyn, J. Bard, B. Borowsky, receptors in primary sensory neurons and effect of axotomy and
J.A. Tamm, W.J. Yao, P.J. Vaysse, T.A. Branchek, C. Gerald, K.A. inflammation, Neuroreport 8 (1996) 237–242. ¨
Jones, Cloned human and rat galanin GALR3 receptors. Pharma- [46] Z.Q. Xu, T. Bartfai, U. Langel, T. Hokfelt, Effects of three galanin
1
cology and activation of G-protein inwardly rectifying K channels, analogs on the outward current evoked by galanin in locus J. Biol. Chem. 273 (1998) 23321–23326. coeruleus, Ann. NY Acad. Sci. 863 (1998) 459–465.
[31] R.V. Storkson, A. Kjorsvik, A. Tjolsen, K. Hole, Lumbar catheteriza- [47] L.C. Yu, S. Lundeberg, H. An, F.X. Wang, T. Lundeberg, Effects of tion of the spinal subarachnoid space in the rat, J. Neurosci. Meth. intrathecal galanin on nociceptive responses in rats with
mononeuro-65 (1996) 167–172. pathy, Life Sci. 64 (1999) 1145–1153.
¨
[32] F.L. Strand, K.J. Rose, L.A. Zuccarelli, J. Kume, S.E. Alves, F.J. [48] X. Zhang, A.P. Nicholas, T. Hokfelt, Ultrastructural studies on Antonawich, L.Y. Garrett, Neuropeptide hormones as neurotrophic peptides in the dorsal horn of the spinal cord — I. Co-existence of factors, Physiol. Rev. 71 (1991) 1017–1046. galanin with other peptides in primary afferents in normal rats,
¨ ¨
[33] K. Tatemoto, A. Rokaeus, H. Jornvall, T.J. McDonald, V. Mutt, Neuroscience 57 (1993) 365–384. ¨
Galanin — a novel biologically active peptide from porcine intes- [49] X. Zhang, A.P. Nicholas, T. Hokfelt, Ultrastructural studies on tine, FEBS Lett. 164 (1983) 124–128. peptides in the dorsal horn of the rat spinal cord — II. Co-existence
˚
[34] A. Thureson-Klein, R.L. Klein, Exocytosis from neuronal large of galanin with other peptides in local neurons, Neuroscience 64 dense-cored vesicles, Int. Rev. Cytol. 121 (1990) 67–126. (1995) 875–891.
¨