www.elsevier.com / locate / bres
Short communication
Stimulation of the MLR inhibits the discharge of dorsal horn neurons
responsive to muscular contraction
*
Alexandr M. Degtyarenko , Marc P. Kaufman
Division of Cardiovascular Medicine, Departments of Internal Medicine and Human Physiology, TB 172, University of California, Davis,
CA95616, USA
Accepted 18 July 2000
Abstract
We found that electrical stimulation of the mesencephalic locomotor region (MLR) inhibited the discharge of deep dorsal horn neurons receiving group III afferent input from the triceps surae muscles. In contrast, contraction of these muscles induced by electrical stimulation of the tibial nerve activated these dorsal horn neurons. Our findings show that descending central motor commands can inhibit dorsal horn interneurons receiving input from group III afferents during exercise. 2000 Elsevier Science B.V. All rights reserved.
Keywords: Exercise; Group III muscle afferent; Cat; Cardiovascular control
Static and moderately intense dynamic exercise is well magnitude greater than those evoked by the algebraic sum known to increase cardiovascular and ventilatory function. of central command and the reflex evoked separately. These increases are widely believed to be caused by two Little is known about how the central nervous system neural mechanisms, namely central command [17] and the integrates the two mechanisms. The dorsal horn of the exercise pressor reflex [11]. The first mechanism, central spinal cord is a central site where central command and the command is defined as the parallel activation of exercise pressor reflex might interact. For example, the somatomotor, autonomic and ventilatory circuits at the dorsal horn is the site of termination of group III and IV onset of exercise. The second mechanism, the exercise muscle afferents [4,14], whose stimulation by contraction pressor reflex, arises from the contraction-induced stimula- is responsible for the exercise pressor reflex [1,13]. More-tion of group III and IV afferent endings in skeletal muscle. over, the activity of dorsal horn neurons receiving nocicep-Substantial evidence has been presented in humans and tive input has been shown to be inhibited by input from animals showing that both mechanisms play significant brainstem structures whose stimulation caused analgesia roles in evoking the cardiovascular and ventilatory re- [2,3]. In addition, a central motor command has been sponses to exercise. Both mechanisms, moreover, are shown to depress inhibitory motor reflexes arising from likely to be engaged simultaneously. This likelihood group II and III muscle afferents as well as from thin fiber prompted two prior studies in which central command and cutaneous afferents [8].
the exercise pressor reflex were evoked separately as well Despite these findings, little is known about how central as simultaneously in anesthetized cats [16,18]. These command interacts with group III and IV afferent input studies found that the two mechanisms evoked simul- onto dorsal horn neurons. In decerebrated cats we ex-taneously caused cardiovascular increases that were less amined the effect of central command on the discharge of than the algebraic sum of the two mechanisms evoked dorsal horn cells responsive to muscular contraction. Each separately. This finding was not attributable to a ceiling of these neurons, whose activity was recorded extracellu-effect because the cats were shown to be able to generate larly, was shown to receive group III muscle afferent input. pressor, cardioaccelerator and ventilatory responses of a We found that contraction-induced input onto cells located in laminae V and VI of the dorsal horn was inhibited by central command.
Cats (2.2–3.5 kg) were anesthetized with a mixture of *Corresponding author. Fax:11-530-752-3264.
E-mail address: [email protected] (A.M. Degtyarenko). halothane (3–4%) and oxygen. A jugular vein, a common
carotid artery and the trachea were cannulated. The lungs fictive locomotion. The paralytic agents in the doses used, were then ventilated with the anesthetic / oxygen mixture were short lasting (i.e. no more than 30 min), and had through the trachea cannula. The remaining carotid artery graded effects on the neuromuscular junction. This allowed was ligated, and the cat was placed in a Kopf stereotaxic us to record the discharge of dorsal horn neurons during and spinal unit. The right tibial nerve was dissected free muscular contraction while the MLR was stimulated. We and placed on a stimulating electrode. The right triceps advanced the recording microelectrode through the dorsal surae muscles were exposed and the right calcaneal horn while stimulating the tibial nerve at 2–3 times motor (Achilles) tendon was cut. The L4–S2 spinal segments threshold (1 Hz; pulse duration: 0.025 ms). In this manner were exposed, after which a pre-collicular post-mammil- we were able to search for dorsal horn neurons responsive lary decerebration was performed. All neural tissue rostral to repetitive muscle twitch. In some cases we searched for to the section was removed, and the cranial vault was filled neurons responsive to static contraction. To accomplish with warm (378C) agar. After the decerebration was this, the tibial nerve was still stimulated at 2–3 times completed, the lungs were ventilated with a mixture of motor threshold but the frequency was increased from 1 to room air and oxygen. Arterial blood gases were measured 40 Hz (pulse duration: 0.025 ms). Static contractions and maintained at normal limits either by adjusting ventila- usually lasted 5–30 s. In some instances, the L7 ventral tion or by injecting sodium bicarbonate (8.5%; i.v.). root was identified, cut and placed on a stimulating A stainless steel monopolar electrode (Kopf, tip impe- electrode. Both twitch and static contractions were evoked dance at 1000 Hz of 10–60 kV) was placed over the by stimulating the ventral root (pulse duration: 0.1 ms) inferior colliculus and was advanced into the mesence- instead of the tibial nerve.
phalic locomotor region (MLR; coordinates: P2, L4, HC1) We determined if the neurons whose impulse activity for the delivery of monophasic square wave electrical was recorded in the dorsal horn received group III afferent pulses (25–50 Hz; 0.1–0.5 ms; 80–120 mA). The MLR input from the tibial nerve. While the cats were paralyzed, electrode was judged to be positioned correctly when we stimulated (1 Hz; 0.1–0.5 ms) the tibial nerve at stimulation evoked either locomotion in unparalyzed cats multiples of motor threshold to determine the current or efferent activity in the peroneal and / or posterior biceps threshold needed to activate the neuron. Only neurons semitendinosus (PBST) nerves in paralyzed cats [6]. The having a latency of at least 7 ms, which was evoked by a intensity of the current applied to the tibial nerve was current intensity of at least 10 times motor threshold, were expressed, as multiples of the threshold current needed to classified as having group III afferent input; those having evoke a muscle twitch. Extracellular impulses from dorsal shorter onset latencies were discarded. While the cat was horn neurons were recorded with tungsten microelectrodes paralyzed we recorded the neuron’s response to both (FHC; tip impedance at 1000 Hz of 4–12 MV) from either stretching the calcaneal tendon and pinching in a noxious the L7 or S1 spinal segments. Arterial blood pressure was manner the triceps surae muscles. All values are reported measured from a carotid arterial catheter, which in turn as means6standard errors. When appropriate, paired t-tests was connected, to a Statham transducer (P23XL). All were performed to determine statistical significance. The signals were written on a chart recorder (Gould) and were criterion level was set at P,0.05.
also recorded on videotape after being digitized (Vetter). We recorded the impulse activity of 31 dorsal horn The impulse activity of dorsal horn neurons was displayed neurons, each of which received input from group III on a storage oscilloscope. The latency of response of the afferents in the tibial nerves. The current intensity of the dorsal horn neurons to electrical stimulation of the tibial electrical pulse applied to the tibial nerve that was required nerve was measured from stimulus onset. to activate these neurons when the cats were paralyzed Next, with the cat paralyzed with either vecuronium averaged 18.162.8 times motor threshold (range: 10–30); bromide (0.01 mg / kg; i.v.) or rocuronium bromide (0.01– their onset latency averaged 9.961.3 ms (range: 7–33 ms). 0.1 mg / kg; i.v.), we determined the effect of MLR The depth from the surface of the spinal cord for the 31 stimulation (i.e., central command) on the discharge of the neurons averaged 1.8660.07 mm. Each of the neurons dorsal horn neuron if it was spontaneous active. The MLR responded either to stretching the calcaneal tendon or to was stimulated for 5–30 s. If an effect on the discharge of pinching the triceps surae muscles. Specifically, fourteen the dorsal horn neuron was observed, the stimulus was of the neurons responded to tendon stretch, ten responded turned off and then repeated. This sequence was repeated to pinching, and seven responded to both tendon stretch 2–4 times until a clear pattern of response emerged. If the and to pinching.
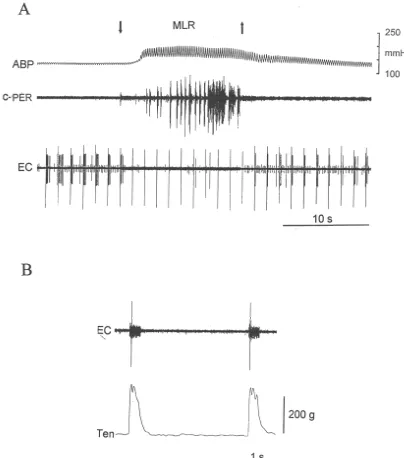
Fig. 1. Electrical stimulation of the mesencephalic locomotor region (MLR) inhibits the discharge of a dorsal horn neuron in a paralyzed cat. (A) The tibial nerve was electrically stimulated at 20 times motor threshold (|0.8 Hz; 0.5 ms) to activate a previously silent neuron. Note that MLR stimulation, initiated
at downwards pointing arrow, inhibited the response of the neuron to tibial nerve stimulation. Also, note that this response returned when the MLR stimulation ended (upwards pointing arrow). (B) Thirty minutes after the end of A, response of dorsal horn neuron to stimulation of L7 ventral root (0.1 ms; 2 times motor threshold) after the effects of the paralyzing agent had partly worn off. Note that the ventral root was stimulated with a short train (40 Hz) of impulses, lasting about 50 ms. Note also that amplifier gain was increased in B over that in A. ABP, arterial blood pressure; c-Per, activity recorded from cut central end of contralateral peroneal nerve; EC, extracellular impulse activity from dorsal horn neuron.
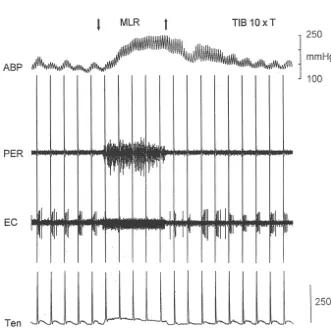
surae muscles (Fig. 2). The discharge of six was recorded was the same as one of the 21 tested with repetitive twitch during MLR stimulation while the cat was paralyzed; five contractions and by MLR stimulation during paralysis (see were inhibited and one was not affected. above).
Fig. 2. MLR stimulation inhibited the response of a dorsal horn neuron to static contraction of the triceps surae muscles. Note that neuron responded to contraction (middle) only when the MLR was not stimulated. Downward and upward arrows represent onset and offset of MLR and tibial nerve stimulation (2 times motor threshold, respectively). Also, note that stimulation of the tibial nerve at 2 times threshold did not activate the dorsal horn neuron when the cat was paralyzed. EC, extracellular activity recorded from dorsal horn neuron; Ten, tension recorded from triceps surae muscles; TIB 23T, tibial nerve stimulation at 2 times motor threshold.
[3] E. Carstens, D. Klumpp, M. Zimmermann, Differential inhibitory impulses recorded from these neurons were at depths
effects of medial and lateral midbrain stimulation on spinal neuronal suggesting that they were located in laminae V and VI of
discharges to noxious skin heating in the cat, J. Neurophysiol. 43 the dorsal horn. (1980) 332–342.
We have no evidence that the dorsal horn neurons [4] A.D. Craig, S. Mense, The distribution of afferent fibers from the described in our experiments received monosynaptic input gastrocnemius-soleus muscle in the dorsal horn of the cat as revealed by the transport of horseradish peroxidase, Neurosci. Lett. from thin fiber afferents innervating the triceps surae
41 (1983) 233–238. muscles. Nevertheless, the location of the neurons was
[5] A.M. Degtyarenko, M.P. Kaufman, Fictive locomotion and scratch-consistent with existing knowledge about the termination ing inhibits dorsal horn neurons receiving thin fiber afferent input, of group III muscle afferents in the lumbar dorsal horn. Am. J. Physiol. 279 (2000) R394–R403.
Specifically, electrophysiological evidence indicates that [6] F.L. Eldridge, D.E. Millhorn, T.G. Waldrop, Exercise hyperpnea and locomotion: Parallel activation from the hypothalamus, Science 211 group III afferents innervating the triceps surae muscles
(1981) 844–846. project both monosynaptically and polysynaptically to
[7] R.D. Foreman, R.F. Schmidt, W.D. Willis, Effects of mechanical and laminae I, IV and V [7,10,15]. Likewise, anatomical chemical stimulation of fine muscle afferents upon primate evidence indicates that group III afferents terminate in spinothalamic tract cells, J. Physiol. 286 (1979) 215–231. lamina V [9]. [8] S. Grillner, M.L. Shik, On the descending control of the lumbosacral
spinal cord from the ‘mesencephalic locomotor region’, Acta Previously, we found that stimulation of the MLR
Physiol. Scand. 87 (1973) 320–333. inhibited the discharge of lamina V neurons receiving thin
[9] U. Hoheisel, E. Lehmann-Willenbrock, S. Mense, Termination fiber input from the tibial nerve. In contrast, MLR stimula- patterns of identified group II and III afferent fibres from deep tion had no effect on the discharge of laminae II through tissues in the spinal cord of the cat, Neuroscience 28 (1989) IV neurons which also received this input [5]. The present 495–507.
[10] U. Hoheisel, S. Mense, Response behaviour of cat dorsal horn report demonstrates that these lamina V neurons are
neurons receiving input from skeletal muscle and other deep somatic stimulated by muscular contraction, a maneuver known to
tissues, J. Physiol. 426 (1990) 265–280.
activate group III muscle afferents [12]. In addition, the [11] M.P. Kaufman, H.V. Forster, Reflexes controlling circulatory, ven-present report demonstrates that MLR stimulation, a tilatory and airway responses to exercise, in: L.B. Rowell, J.T. maneuver that evoked central command, inhibited this Shepherd (Eds.), Exercise: Regulation and Integration of Multiple Systems. II. Control of Respiratory and Cardiovascular Systems, input while the cat was exercising. Finally, the present
Handbook of Physiology, Vol. Section 12, Oxford University Press, report may provide an electrophysiological basis for
New York, NY, 1996, pp. 381–447.
previous reports [16,18] showing that the cardiovascular [12] M.P. Kaufman, J.C. Longhurst, K.J. Rybicki, J.H. Wallach, J.H. and ventilatory responses to central command and the Mitchell, Effects of static muscular contraction on impulse activity exercise pressor reflex evoked simultaneously were less of groups III and IV afferents in cats, J. Appl. Physiol. 55 (1983)
105–112. than the algebraic sum of these responses evoked
in-[13] D.I. McCloskey, J.H. Mitchell, Reflex cardiovascular and respiratory dividually.
responses originating in exercising muscle, J. Physiol. 224 (1972) 173–186.
[14] S. Mense, A.D.I. Craig, Spinal and supraspinal terminations of
Acknowledgements primary afferent fibers from the gastrocnemius-soleus muscle in the
cat, Neuroscience 26 (1988) 1023–1035.
[15] B. Pomeranz, P.D. Wall, W.V. Weber, Cord cells responding to fine This work was supported by NIH Grant HL 30710. We
myelinated afferents from viscera, muscle, and skin, J. Physiol. 199 thank Eileen English for typing the manuscript. (1968) 511–532.
[16] K.J. Rybicki, R.W. Stremel, G.A. Iwamoto, J.H. Mitchell, M.P. Kaufman, Occlusion of pressor responses to posterior diencephalic stimulation and muscular contraction, Brain Res. Bull. 22 (1989) References
305–312.
[17] T.G. Waldrop, F.L. Eldridge, G.A. Iwamoto, J.H. Mitchell, Central [1] C.M. Adreani, J.M. Hill, M.P. Kaufman, Responses of group III and neural control of respiration and circulation during exercise, in: L.B. IV muscle afferents to dynamic exercise, J. Appl. Physiol. 86 (1997) Rowell, J.T. Shepherd (Eds.), Exercise: Regulation and Integration
1811–1817. of Multiple Systems, Handbook of Physiology, Vol. Section 12,
[2] E. Carstens, H. Gilly, H. Schrieber, M. Zimmerman, Effects of Oxford University Press, New York, NY, 1996, pp. 333–380. midbrain stimulation and iontophoretic application of serotonin, [18] T.G. Waldrop, D.C. Mullins, D.E. Millhorn, Control of respiration noradrenaline, morphine, and GABA on electrical thresholds of by the hypothalamus and by feedback from contracting muscles in afferent C- and A-fiber terminals in cat spinal cord, Neuroscience 21 cats, Respir. Physiol. 64 (1986) 317–328.