Brain Research 880 (2000) 183–186
www.elsevier.com / locate / bres
Short communication
The role of activity blockade on glutamate receptor subunit expression
in the spinal cord
a a,b ,
*
Stacie D. Grossman , Jean R. Wrathall
a
Department of Cell Biology, Georgetown University Medical Center, Washington, DC 20007, USA
b
Department of Neuroscience, Georgetown University Medical Center, Washington, DC 20007, USA
Accepted 2 August 2000
Abstract
Spinal cord injury (SCI) impairs descending glutamatergic neurotransmission reaching ventral motor neurons (VMN). Previously we reported up-regulation of NMDA receptor subunits NR1 and NR2A mRNAs in VMN caudal to the lesion site 24 h after SCI. The absence of effect rostral to the injury site suggested that injury-induced loss of descending activity might be involved. To test this hypothesis, we blocked axonal conduction by focal injection of tetrodotoxin into the spinal cord. We found increased NR1 and NR2A mRNAs in VMN similar in extent to that seen after SCI. Thus, the increase in NMDA subunit mRNAs may be ‘inactivity dependent’ and associated with reorganization of the spinal cord in response to loss of descending innervation. 2000 Elsevier Science B.V. All rights reserved.
Theme: Neurotransmitters, modulators, transporters, and receptors
Topic: Receptor modulation, up- and down-regulation
Keywords: NMDA; Glutamate; Ventral motor neuron; Tetrodotoxin; Spinal cord injury
NMDA receptors mediate activity-dependent synaptic level of injury [20]. We hypothesized that loss of descend-plasticity in the central nervous system [2,5]. High levels ing innervation regulates NMDA subunit expression and of NR1, the obligatory subunit of all functional NMDA changes seen after SCI are ‘inactivity dependent’. To channels [10,14], are correlated to periods of axonal examine this hypothesis, we administered a voltage-gated growth, synaptic plasticity, and stages in development sodium channel blocker, tetrodotoxin (TTX), via microin-when the capacity for anatomic and physiologic synaptic jection into the thoracic spinal cords of laminectomized plasticity is high [3]. Manipulation of sensory experience rats in a manner that blocks axonal conduction for 24–36 h and synaptic activity [4,19] alters NMDA subunit expres- [18,21]. If loss of activity was responsible for NMDA sion [15]. receptor subunit changes after SCI, TTX treatment should
SCI interrupts descending supraspinal neurotransmission produce the changes seen after SCI.
[12] from glutamatergic brain and brainstem motor-control Female, Spague–Dawley rats (225–250 g) were anes-centers reaching spinal ventral motor neurons (VMN), and thetized with 4% chloral hydrate (360 mg / kg, i.p.) and a causes paralysis distal to the injury site. Previously we laminectomy was performed at T8, exposing a circle of have shown up-regulation of NMDA receptor subunit NR1 dura. Allis clamps were attached at the seventh and ninth and NR2A mRNAs after SCI in VMN below, but not spinous processes to stabilize the animal. A Hamilton above, the level of a standardized contusion lesion pro- micro-syringe connected to an Alzet mini-pump was used duced with a weight-drop device [8]. This is consistent to administer either TTX (0.15 nmol) or vehicle (VEH) with the hypothesis that reorganization after SCI involves focally into the spinal cord, as previously described [21]. increases in excitatory glutamatergic synapses below the Rats were tested blindly for functional deficits 24 h after injection using a battery of tests for reflexes and co-ordinated motor function [6]. Overall hind limb impair-*Corresponding author. Tel.: 11-202-687-1196; fax: 1
1-202-687-ment was estimated with a Combined Behavioral Score 0617.
E-mail address: [email protected] (J.R. Wrathall). (CBS) that ranges from 0 in a normal rat to 100 in a rat
184 S.D. Grossman, J.R. Wrathall / Brain Research 880 (2000) 183 –186
jection itself did not cause injury to the tissue. A needle track was barely visible in only two of the rats, while the remainder had no detectable needle tracks. Sections ap-proximately 4 mm distal to the injection site were used for analysis to replicate the protocol used previously in SCI where VMN 4 mm rostral and caudal to the injury site were analyzed.
We counted VMN in each TTX and VEH rat to ensure that TTX administration did not cause cell loss at64 mm from the injection site. The average number of thoracic VMN per horn in TTX animals was 1061.2 (S.E.) and was Fig. 1. TTX completely blocks hindlimb function. (A) Results of
not statistically different from the number of VMN in VEH behavioral testing of rats with the CBS score [6] and (B) BBB scale [1].
controls, which was 9.360.9. Further, these numbers were Both measures of hindlimb function demonstrate profound deficit at 24 h
for TTX-injected rats. Data points indicate mean6S.E.M. for groups of not different from the number of VMN present in control rats (n55 each) after TTX injection (white bars) or VEH (black bars). rats with no injection at all, as determined in a previous *significantly different, P#0.05 (Student’s t-test).
study [8].
For in situ hybridization, adjacent tissue sections were with no hindlimb function [6]. Open-field locomotion was postfixed, acetylated, dehydrated and hybridized with
35
also analyzed using the Basso, Beattie, Bresnahan (BBB) solution containing S-labeled antisense oligoprobes to locomotor rating scale, that ranges from 0 to 21, where 0 GluR2 [11], NR1, NR2A or NR2B [13], as described reflects no locomotory function and 21 reflects a normal previously [7,8]. Grain counting was done using a com-performance [1]. At 24 h after injection each rat in the puterized system (Scion Image, Frederick, MD), which
2 TTX treatment group (n55) displayed complete hind limb quantified the area of the VMN cell body (mm ), and paralysis, as assessed with the CBS (Fig. 1A) and the BBB counted the grains overlying it. The average density of
2
(Fig. 1B) scales. Average scores were 95 for CBS and 0.7 grains (grains /mm ), corrected for background, was de-for BBB, indicating loss of hind limb locomotion. Con- termined for 45–60 VMN per rat for each of the 5 rats per versely, rats injected with VEH (n55) displayed normal group.
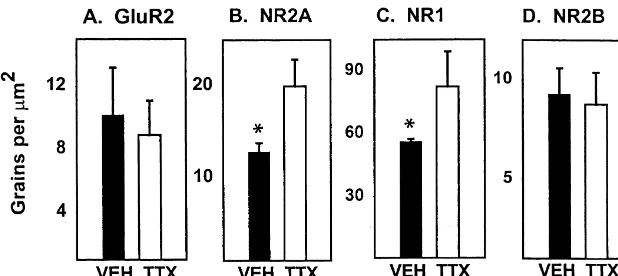
hind limb function, with CBS scores of 0 and BBB scores The expression of mRNAs for the AMPA subunit GluR2 of 21. and NMDA subunits NR1 and NR2A were analyzed, based After behavioral evaluation rats were re-anesthetized on previous data showing preferential changes of these and the spinal cords removed. Tissue from VEH and TTX subunits in VMN 4 mm distal to the spinal cord injury site rats were blocked together and serial 20 mm frozen [7,8]. After TTX administration we found no difference in sections through the injury site were prepared. Slides GluR2 mRNA expression in VMN 4 mm from the in-representing each 1 mm length of cord were stained with jection site, compared to controls (Figs. 2A, B and 3A). Eriochrome C and counterstained with Hematoxylin and However, NR2A mRNA in VMN was significantly up-Eosin [16]. Examination of the tissue showed little or no regulated in rats injected with TTX to 70% greater than in evidence of tissue disruption, indicating that the microin- the VEH group (Figs. 2C, D and 3B). NR1 mRNA was
S.D. Grossman, J.R. Wrathall / Brain Research 880 (2000) 183 –186 185
Fig. 3. NR1 and NR2A mRNAs are up-regulated in VMN adjacent to the TTX injection site at 24 h. Graphs represent grain counts for individual VMN 4 mm rostral and caudal to T8. (A) TTX injection has no effect on GluR2 mRNA. (B) There is a significant increase in NR2A mRNA grain density in neurons from TTX rats, compared to control neurons from the same slide. (C) There is a significant increase in NR1 mRNA in TTX rats, compared to VEH. (D) There is no change in NR2B expression. White bars indicate TTX rats (n55) and black bars indicate VEH rats (n55). Bars represent means6S.E.M. A total of 45–60 VMN counted per rat. *significantly different, P#0.05 (Student’s t-test).
also up-regulated in VMN approximately 45% above sible for changing GluR2 expression after SCI remains to control values (Figs. 2E, F and 3C). Similar up-regulation be investigated.
was present rostral and caudal to the injection site, In summary, we conclude that increased NMDA re-presumably due to diffusion of TTX in both directions. ceptor subunit mRNA expression seen after SCI is ‘inac-NR2B mRNA, which is not altered after SCI, was un- tivity dependent’ and likely associated with plasticity and changed after TTX injection (Fig. 3D). reorganization of the distal spinal cord in response to the
Thus, TTX, at the dose used, caused hind limb paralysis loss of descending innervation. in rats that was indistinguishable from that seen at 24 h
after SCI. TTX also produced up-regulation of NR1 and
NR2A mRNA in VMN surrounding the injection site, with Acknowledgements no effect on NR2B. TTX closely mimicked the effects on
NMDA subunit mRNA expression seen in VMN caudal to Special thanks to Lisa Rosenberg for assisting with a SCI that interrupts their descending innervation from microinjections and Stefano Vicini for critical review of supraspinal pathways. While SCI only affects NMDA the manuscript. NIH NRSA F31 MH 12038 (S.D.G.) and subunit mRNA below the level of the lesion, TTX induced R01 NS 37733 (J.R.W.) supported this work.
similar changes rostral and caudal to the injection site. This is probably because drug diffusion blocks activity in VMN on both sides of the injection site. Although SCI causes the
References
symmetrical release of several factors that could affect synaptic activity, only VMN caudal to the injury site show
[1] D.M. Basso, M.S. Beattie, J.C. Bresnahan, A sensitive and reliable decreased spontaneous cord potentials due to reduced locomotor rating scale for open field testing in rats, J. Neurotrauma supraspinal regulation and temporary conduction blockade 12 (1) (1995) 1–21.
[2] M.F. Bear, NMDA-receptor-dependent synaptic plasticity in the from long, descending spinal pathways [12]. Our results
visual cortex, Prog. Brain Res. 108 (1996) 205–218. support the hypothesis that the acute changes in NMDA
[3] S.M. Catalano, C.K. Chang, C.J. Shatz, Activity-dependent regula-receptor subunit mRNAs produced by SCI are due to loss
tion of NMDAR1 immunoreactivity in the developing visual cortex, of descending activity, they are ‘inactivity dependent’. J. Neurosci. 17 (1997) 8376–8390.
Injection of TTX had no effect on the expression of the [4] E.A. Debski, H.T. Cline, M. Constantine-Paton, Activity-dependent tuning and the NMDA receptor, J. Neurobiol. 21 (1990) 18–32. GluR2 subunit of the AMPA receptor. Previously we have
[5] K. Fox, N. Daw, H. Sato, D. Czepita, The effect of visual experience shown VMN down-regulate GluR2 mRNA expression after
on development of NMDA receptor synaptic transmission in kitten SCI [7], and that the effect is similar both rostral and
visual cortex, J. Neurosci. 12 (1992) 2672–2684.
caudal to the injury site. Although TTX injection up- [6] K. Gale, H. Kerasidis, J.R. Wrathall, Spinal cord contusion in the rat: regulates NR1 and NR2A mRNAs similar to SCI, it does behavioral analysis of functional neurologic impairment, Exp. not induce the SCI-associated change in GluR2. Lack of Neurol. 88 (1985) 123–134.
[7] S. Grossman, B. Wolfe, R. Yasuda, J. Wrathall, Alterations in AMPA AMPA changes after TTX treatment is also seen in
receptor subunits following contusive spinal cord injury in the rat, J. cultured hippocampal neurons [17] that do increase NR2A
Neurosci. 19 (1999) 5711–5720.
186 S.D. Grossman, J.R. Wrathall / Brain Research 880 (2000) 183 –186
[9] M. Hofer, M. Constantine-Paton, Regulation of N-methyl-D-aspar- [16] A.G. Rabchevsky, I. Fugaccia, A. Fletcher-Turner, D.A. Blades, tate (NMDA) receptor function during the rearrangement of de- M.P. Mattson, S.W. Scheff, Basic fibroblast growth factor (bFGF) veloping neuronal connections, Prog. Brain Res. 102 (1994) 277– enhances tissue sparing and functional recovery following moderate
285. spinal cord injury, J. Neurotrauma 16 (1999) 817–830.
[10] M. Hollmann, S. Heinemann, Cloned glutamate receptors, Annu. [17] A. Rao, A.M. Craig, Activity regulates the synaptic localization of Rev. Neurosci. 17 (1994) 31–108. the NMDA receptor in hippocampal neurons, Neuron 19 (1997) [11] K. Keinanen, W. Wisden, B. Sommer, P. Werner, A. Herb, T.A. 801–812.
Verdoorn et al., A family of AMPA-selective glutamate receptors, [18] L.J. Rosenberg, Y.D. Teng, J.R. Wrathall, Effects of the sodium Science 249 (1990) 556–560. channel blocker tetrodotoxin on acute white matter pathology after [12] J.T. Molt, D.A. Poulos, R.S. Bourke, Evaluation of experimental experimental contusive spinal cord injury, J. Neurosci. 19 (1999)
spinal cord injury by measuring spontaneous spinal cord potentials, 6122–6133.
J. Neurosurg. 48 (1978) 985–992. [19] A.J. Scheetz, M. Constantine-Paton, Modulation of NMDA receptor [13] H. Monyer, N. Burnashev, D.J. Laurie, B. Sakmann, P.H. Seeburg, function: implications for vertebrate neural development, FASEB J.
Developmental and regional expression in the rat brain and func- 8 (1994) 745–752.
tional properties of four NMDA receptors, Neuron 12 (1994) 529– [20] S. Shapiro, Neurotransmission by neurons that use serotonin,
540. noradrenaline, glutamate, glycine, and gamma-aminobutyric acid in
[14] H. Monyer, R. Sprengel, R. Schoepfer, A. Herb, M. Higuchi, H. the normal and injured spinal cord, Neurosurgery 40 (1997) 168– Lomeli et al., Heteromeric NMDA receptors: molecular and func- 176.
![Fig. 1. TTX completely blocks hindlimb function. (A) Results ofbehavioral testing of rats with the CBS score [6] and (B) BBB scale [1].Both measures of hindlimb function demonstrate profound deficit at 24 hfor TTX-injected rats](https://thumb-ap.123doks.com/thumbv2/123dok/3140327.1382928/2.612.48.551.539.672/completely-ofbehavioral-measures-hindlimb-function-demonstrate-profound-decit.webp)