*Corresponding author. Tel.: 01189-318168; fax: 01189-753676. E-mail address:[email protected] (C.A. Williams)
Geographical variation in the surface
#
avonoids
of
Pulicaria dysenterica
Christine A. Williams
*
, Je
!
rey B. Harborne, Jenny Greenham
Department of Botany, School of Plant Sciences, The University of Reading, Whiteknights, P.O. Box 221,Reading, RG6 6AS, UK
Received 11 May 1999; accepted 8 August 1999
Abstract
Four chemical races were detected inPulicaria dysenterica, when sampled within Europe, on the basis of the surface #avonoids present. One race uniquely contained quercetagetin 3,7-dimethyl ether and another 6-hydroxykaempferol 3,4@-dimethyl ether. A third race was
based on plants having 6-hydroxykaempferol 3,7-dimethyl ether together with quercetagetin 3,7,3@-trimethyl ether. The fourth race contained the above two compounds, as well as
quer-cetagetin 3,7,3@,4@-tetramethyl ether and 6-hydroxykaempferol 3,7,4@-trimethyl ether. These
lipophilic constituents were variously present on the surfaces of leaf, ray#oret, disc#oret and fruit. By contrast, the vacuolar#avonoid of all tissues and all races was uniformly quercetin 3-glucuronide. The kaempferol 3-glucoside previously reported in#owers was not detected. Of the lipophilic #avonoids newly reported from this plant, one 6-hydroxykaempferol 3,7,4@
-trimethyl ether is new to nature. ( 2000 Elsevier Science Ltd. All rights reserved.
Keywords:Compositae; Inuleae;Pulicaria dysenterica; Surface#avonoids; Methylated 6-hydroxy#avonols; Geography
1. Introduction
Pulicaria dysenterica(L.) Bernh. Is a well-known medicinal plant of the past, which was used for treating dysentery. However, the English name,#eabane, refers to its use
in mediaeval times when the plant itself or smoke from the burnt plant was used to drive away#eas and other insects (Grieve, 1980).
Considering its long historic use, chemical information for this plant is limited. Previous reports include two acetylenic compounds, thymol methyl ether, thymohyd-roquinone dimethyl ether and*8(9)-dehydrothymohydroquinone dimethyl ether from the root tissue and¸-inositol from the leaf (Schulte et al., 1968). In a later study of the aerial parts, Bohlmann and Zdero (1981) identi"ed nine new caryophyllene deriva-tives and a hydroxyisocomene in addition to two further thymol constituents. The thymol derivatives are probably responsible for the insect repellent properties of the plant since thymol itself has been shown to be insecticidal against house#ies (Dev and Koul, 1997).
There have been two previous investigations ofP. dysenterica#avonoids but these di!er in some details. Thus, Schulte et al. (1968) working at the University of MuKnster, Germany, reported a #avonol aglycone, quercetagetin 3,7,4@-trimethyl ether (oxy-ayanin B) and a#avonol glycoside, kaempferol 3-glucoside from#owers of this plant. In a later investigation Pares et al. (1981) found that the leaf pro"le of a plant collected in Istanbul (Turkey) was completely di!erent. They identi"ed: quercetagetin and 6-hydroxykaempferol 3,7-dimethyl ethers, 6-hydroxykaempferol 3,6,7-trimethyl ether (penduletin), scutellarein, 6-hydroxykaemperol 3-methyl ether 6-glucoside and aes-culetin from the leaves.
Since no attempt appears to have been made to explain the early medicinal use of this plant, we decided to reinvestigate the lipophilic#avonoids to see whether they had any useful activity. In fact, we have analysed a further seven leaf samples for both lipophilic and polar#avonoid constituents from a number of di!erent geographical localities to test for infraspeci"c variation. Furthermore, we have examined two of these samples for#avonoid variation between the di!erent plant parts, i.e. leaf, disc #oret, ray#oret and fruit.
2. Materials and methods
2.1. Plant material
Seven samples (1}7) ofP. dysentericawere collected from various sources, both fresh and herbarium, details of which are given in Table 1. Samples 1}3 were veri"ed by Mr. R. Rutherford, School of Plant Sciences, The University of Reading and voucher specimens have been deposited in RNG.
2.2. Flavonoid analysis
2.2.1. Lipophilicyavonoids
Lipophilic#avonoids were removed from leaves of samples 1}7 and additionally from the disc#orets, ray#orets and fruits of samples 1 and 2, by brie#y dipping in Me
Table 1
Source data forPullicaria dysentericasamples 1}7 Sample number Source
1 Plants grown from seed provided by Conservatoire et Jardin Botaniques Gene`ve and grown in the walled garden in the Harris garden at the School of Plant Sciences, The University of Reading.
2 Fresh material collected from Bin"eld Heath nr. Reading, Berks, for which a Herbar-ium specimen has been lodged in RNG.
3 Fresh material collected from the top end of the Whiteknights Lake, The University of Reading for which a herbarium specimen has been lodged in RNG.
4 Herbarium specimen (RNG) from Calabria, Italy. Optima Iter VIII, 1577; 15-6-97. 5 Herbarium specimen (RNG) from Caorle, prov. Venezia, Italy.; 11-9-93.
6 Herbarium specimen (RNG) from Gi!aumont-Champaubert, deHp. Marne, France. No. 16592; 30-8-93.
7 Herbarium specimen (RNG) from nr. Santo Stafano, Italy. R.E.Longton 5106; 21-8-97.
H
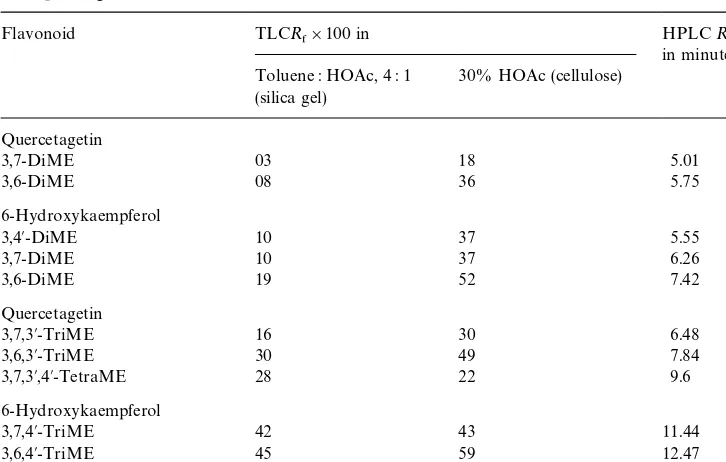
2O. The following constituents: quercetagetin 3,7-dimethyl ether (1), 6-hy-droxykaempferol 3,4@-dimethyl ether (2) and 3,7-dimethyl ether (3), quercetagetin 3,7,3@-trimethyl ether (4) and 3,7,3@,4@-tetramethyl ether (5) and 6-hydroxykaempferol 3,7,4@-trimethyl ether (6), were standardised as far as possible by UV spectral analysis, MS and HPLCR
5 and TLCR& comparison with marker compounds available from previous studies of feverfew and from our marker collection (Table 2).
2.2.2. Vacuolaryavonoids
The water soluble vacuolar#avonoids were extracted with hot 100% MeOH (fresh material) or 80% MeOH (dried material), from the remaining tissues after removal of the lipophilic #avonoids by dipping in Me
2CO. The concd MeOH extracts were separated by PPC in BAW (n-BuOH : HOAc : H
2O,4 : 1 : 5, top layer) and the result-ing#avonoid bands eluted and puri"ed by PPC in 15% HOAc, H
2O and/or BEW (n-BuOH : EtOH : H
2O, 6 : 1 : 2.2). The identity of the only#avonoid constituent in disc, leaf and ray tissues, quercetin 3-glucuronide, was con"rmed by UV spectral analysis, by mobility during paper electrophoresis at pH 4.4 using an acetate bu!er, by PC co-chromatography with an authentic sample in three solvents (BAW, BEW and H
2O) and by acid hydrolysis to give quercetin and glucuronic acid.
3. Results
3.1. Leaf surfaceyavonoids
Table 2
R
& and HPLC R5 data for the lipophilic #avonoids from Pulicaria dysenterica compared with the corresponding isomers from feverfew
18*phenyl reverse phase column at 253C using a linear gradient of 40% A:60% B over 20 min,#ow rate 1 ml min~1. A"2% HOAc, B"MeOH}HOAc}H
2O, 18 : 1 : 1. UV detection at 260 and 350 nm. Abbreviations:HOAc"acetic acid;ME"methyl ether.
were:- 6-hydroxykaempferol 3,7-dimethyl ether (3) and quercetagetin 3,7,3@-trimethyl ether (4). In a previous analysis of a Turkish leaf specimen Pares et al. (1981) also identi"ed 6-hydroxykaemperol 3,7-dimethyl ether but the only quercetagetin deriva-tive they recorded was the 3,7-dimethyl ether. However, Schulte et al. (1968) have reported the 3,7,4@-isomer from#ower tissue of their German specimen. Mass spectral data for4:- [M] 360 and ions at 359 (M-1), 345 (M-15), 317 (M-43 characteristic of
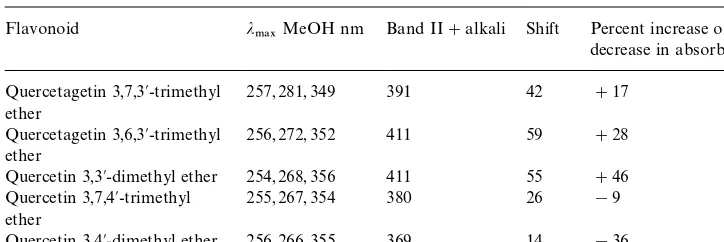
Table 3
UV spectral comparison of the alkaline shifts in#avonols with 3@and/4@substitution
Flavonoid j
.!9MeOH nm Band II#alkali Shift Percent increase ordecrease in absorbance !
Quercetin 3,3@-dimethyl ether 254, 268, 356 411 55 #46 Quercetin 3,7,4@-trimethyl
ether
255, 267, 354 380 26 !9
Quercetin 3,4@-dimethyl ether 256, 266, 355 369 14 !36
!Approximate values based on adding 3 drops of 2N aqueous NaOH to compound in MeOH.
compared with the smaller shifts and decrease in absorbance shown by the two 4@-substituted#avonols.
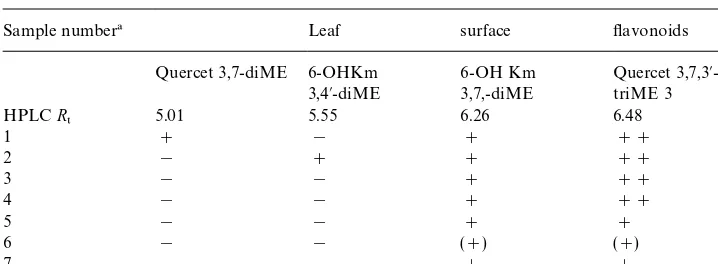
In sample 7 from Santo Stephano in Italy and sample 6 from France, no other lipophilic#avonoids were detected. However, the presence of additional minor components in the other accessions suggested the existence of three further chemotypes (Table 4). Thus, sample 1, which was grown from seed obtained from Geneva, di!ered from all the other samples in producing quercetagetin 3,7-dimethyl ether. Similarly, sample 2 from Bin"eld Heath, nr. Reading was distinguished by the presence of a second 6-hydroxykaempferol dimethyl ether (2), whch had an HPLC R
5 of 5.55 compared with 6.26 for the 3,7-dimethyl ether. Compound 2 was identi"ed as the 3,4@-rather than the 7,4@-isomer on the basis of UV spectral properties including the diagnostic low wavelength of band II, i.e.j
.!9 MeOH 280, 340 nm.
Two further 6-hydroxy#avonols : quercetagetin 3,7,3@,4@-tetramethyl ether (5) and 6-hydroxykaempferol 3,7,4@-trimethyl ether (6), a new compound, were identi"ed in the Bin"eld Heath sample (No. 2) and also in the remaining accessions, i.e. No. 3 from Whiteknights Lake, The University of Reading and samples 4 and 5 from two di!erent localities in Italy (Table 1). The latter three samples (3}5) form the fourth chemotype. 6-Hydroxykaempferol 3,7,4@-trimethyl ether (6) was characterised by HPLCR
5and TLC comparison with the 3,6,4@- and 3,6,7-isomers and from the lack of sodium acetate and boric acid shifts in the UV spectral analysis (UVj
.!9MeOH : 280, 331;#NaOAc 282, 330 and#H
3BO3282,330. The band I at 280 also indicated that the 6-hydroxyl was free. It is clearly di!erent from the previously reported (Schulte et al., 1968) penduletin, 6-hydroxykaempferol 3,6,7-trimethyl ether which has j
.!9 MeOH 271,343 and HPLCR5 10.16 (see Table 2) compared with anR5 of 11.44 for the present compound and from the 3,6,4@-isomer, santin (j
.!9MeOH 273,337 and
HPLC R
-Table 4
Leaf surface#avonoid variation amongst di!erent accessions ofPulicaria dysenterica
Sample number! Leaf surface #avonoids
Quercet 3,7-diME 6-OHKm 3,4@-diME
6-OH Km 3,7,-diME
Quercet 3,7,3@ -triME 3 HPLCR
5 5.01 5.55 6.26 6.48
1 # ! # ##
2 ! # # ##
3 ! ! # ##
4 ! ! # ##
5 ! ! # #
6 ! ! (#) (#)
7 ! ! # #
!For key to sample numbers Table 1. Abbreviations:Quercet"quercetagetin; 6-OHKm" 6-hy-droxykaempferol; ME"methyl ether.
trimethyl ether (santin) (Williams et al., 1999). This is therefore the"rst report of the 3,7,4@-trimethyl ether in nature.
3.2. Lipophilicyavonoidvariation between tissues
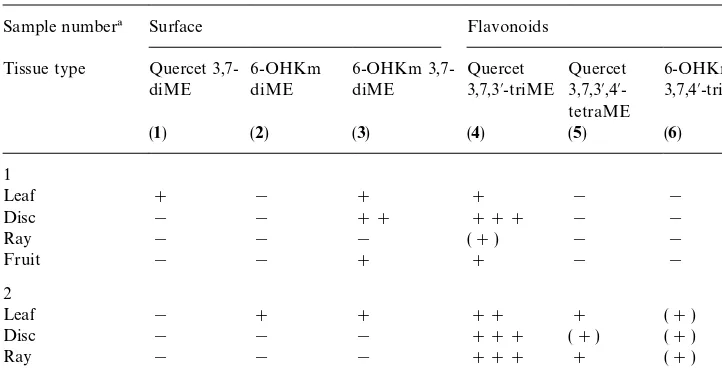
The lipophilic#avonoids of the leaf, disc#orets, ray#orets and fruits were separate-ly anaseparate-lysed in samples 1 and 2 (Table 1) and the results are summarized in Table 5. In sample 1 each tissue can be distinguished either qualitatively or quantitatively by its #avonoid pro"le. Thus, the leaf alone produced quercetagetin 3,7-dimethyl ether, while the disc#orets were the richest source of3and4. The ray tissue of this sample gave only a trace of one compound,4. This is in contrast to sample 2 where4is the major constituent of ray and disc. Here disc, ray and fruit have the same qualitative pro"le and only the leaf is distinguished by the presence of the two 6-hydroxykaem-pferol dimethyl ethers,2and3.
3.3. Vacuolaryavonoids
The only vacuolar#avonoid constituent in all the leaf samples of#eabane and in the fruits, ray and disc #orets of samples 1 and 2 was identi"ed as quercetin 3-glucuronide. A previous report of kaempferol 3-glucoside from a German sample (Schulte et al.) could not be substantiated. This represents the"rst record of quercetin 3-glucuronide inP. dysenterica.
4. Discussion
Table 5
The distribution of surface#avonoids in the di!erent tissues of two samples ofPulicaria dysenterica
Sample number! Surface Flavonoids
exception of kaempferol and quercetagetin 3,7-dimethyl ethers we found none of their reported compounds (6-hydroxykaempferol 3,6,7-trimethyl ether, scutellarein, 6-hy-droxykaempferol 3-methyl ether 6-glucoside, quercetagetin 3,7,4@-trimethyl ether and kaempferol 3-glucoside) in our samples. Both previous research teams failed to look at more than one plant sample or plant part and failed to separate the external from the internal#avonoids. How they failed to "nd the major leaf and #ower constituent, quercetagetin 3,7,3@-trimethyl ether, is surprising unless of course their plant material was yet another chemical race. Similarly, how could they mistake kaempferol 3-glucoside for the only vacuolar constituent, quercetin 3-glucuronide? This emphasizes the need to look at as many veri"ed specimens from di!erent localities as possible and to analyse separately the external and internal#avonoid constituents of the di!erent plant parts.
It was not surprising to "nd quantitative di!erences in the lipophilic #avonoid pro"les of the di!erent leaf samples as this has been observed in another temperate medicinal composite, feverfew, Tanacetum parthenium (Williams et al., unpublished results), where the amounts of the sesquiterpene lactone,parthenolide, also vary from one accession to another. However, the latter species has been much cultivated and is far more catholic in its choice of habitat than P. dysenterica, which grows only in damp places. On the other hand, the qualitative di!erences in the lipophilic#avonoids between samples and between plant parts were unexpected. There could be some ecological explanation, e.g. a protective role against the damaging e!ects of UV light. However, an increase in UV B irradiation has been shown to equally enhance both external and epidermal cell vacuolar#avonoids (Cuadra et al., 1997).
from P. arabica the highly methylated #avonols: quercetagetin 3,6,7,3@,4@-penta, 3,5,6,7,3@-penta and 3,5,6,7,3@,4@-hexamethyl ethers were isolated from the aerial parts (Melek et al., 1988). InP. incisa, kaempferol and quercetin 3-galactosides, kaempferol and quercetin 3-methyl ethers, quercetin 3,7-dimethyl ether and dihydroquercetin 7-methyl ether were characterised (Mansour et al., 1990). In the third species, P. undulata, kaempferol 3-methyl ether, quercetin 3,7-dimethyl ether and"ve dihyd-ro#avonols: dihydrokaempferol and its 7-methyl ether, dihydroquercetin and its 7-mono and 7,3@-dimethyl ethers, were identi"ed from the aerial parts (Abdel-Mogib et al., 1989).Pulicaria undulatais considered to be closely related to or synonymous withP. incisaand this is re#ected in their#avonoid chemistry. In a previous study of P. undulata(Metwally et al., 1986), a thymol derivative, 2-hydroxyacetylthymol, and two #avanoids: dihydrokaempferol 7-methyl ether and the #avanone, eriodictyol 7-methyl ether were reported, again from the aerial parts. This is the only report of a#avanone from aPulicariaspecies. Thymol derivatives on the other hand appear to be characteristic constituents of the genus. We would have analysed the only other British species,P. vulgaris, to compare withP. dysentericabut it is now a very rare plant and was not available to us. However, this species and all the otherPulicaria species, which grow in Europe are morphologically distinct from and can not there-fore be mistaken forP. dysenterica.
Having isolated the major lipophilic #avonoid constituents of P. dysenterica in reasonable quantity, we now intend to test them for their anti-in#ammatory activity. We have already tested some of the lipophilic#avonoids of feverfew and found that 6-hydroxykaempferol 3,6,4@-trimethyl ether (santin) inhibited both pathways of arachidonate metabolism (cyclo-oxygenase and 5-lipoxygenase) with the same high potency and that 6-hydroxykaempferol 3,6-dimethyl ether had an equivalent pro"le of enzyme activity but was much less potent. On the other hand, quercetagetin 3,6,3@ -trimethyl ether showed preferential activity against cyclo-oxygenase (Williams et al., 1999). Thus, it would be interesting to test the corresponding 7 methylated isomers from #eabane, initially:6-hydroxykaempferol 3,7-dimethyl ether and quercetagetin 3,7,3@-trimethyl ether, the major#avonoid constituents.
References
Abdel-Mogib, M., Dawidar, A.-M., Metwally, M.A., Abou-Elzahab, M., 1989. Flavonols ofPulicaria undulata. Pharmazie 44, 801.
Bohlmann, F., Zdero, C., 1981. Caryophyllene derivatives and a hydroxyisocomene fromPulicaria dysen-terica. Phytochemistry 20, 2529}2534.
Cuadra, P., Harborne, J.B., Waterman, P., 1997. Increases in the surface#avonols and photosynthetic pigments inGnaphalium luteo-albumin response to UV-B radiation. Phytochemistry 45, 1377}1383. Dev, S., Koul, O., 1997. Insecticides of Natural Origin. Harwood Academic Publishers, Amsterdam,
pp. 118.
Grieve, M., 1980. A Modern Herbal. Penguin Books Ltd., Harmondsworth, England, pp. 32.
Mansour, R.M.A., Ahmed, A.A., Melek, F.R., Saleh, N.A.M., 1990. The#avonoids ofPulicaria incisa. Fitoterapia 61, 186}187.
Metwally, R.M.A., Dawidar, A.A., Metwally, S., 1986. A new thymol derivative fromPulicaria undulata. Chem. Pharm. Bull. 34, 378}379.
Pares, J.O., Oksuz, S., Ulubelen, A., Mabry, T.J., 1981. 6-Hydroxy#avonoids fromPulicaria dysenterica.. Phytochemistry 20, 2057.
Schulte, K.E., RuKcker, G., MuKller, F., 1968. Einige Inhaltssto!e der BluKtenkoKpfchen vonPulicaria dysen-terica. Arch. Pharm. 301, 115}119.
Williams, C.A., Harborne, J.B., Geiger, H., Hoult, J.R.S., 1999. The#avonoids ofTanacetum partheniumand T.vulgareand their anti-in#ammatory properties. Phytochemistry 51, 417}423.
Williams, C.A., Hoult, J.R.S., Harborne, J.B., Greenham, J., Eagles, J., 1995. A biologically active lipophilic