Recent Contributions from Brain Imaging Studies
Diana Martinez, Allegra Broft, and Marc Laruelle
Preclinical studies suggest that augmentation of selective serotonin (5-HT) reuptake inhibitors by the 5-HT1A
recep-tor agent pindolol might reduce the delay between initia-tion of treatment and antidepressant response, an effect largely mediated by blockade of 5-HT1A autoreceptors in
the dorsal raphe nuclei. Although some controlled clinical trials suggest that pindolol might reduce latency to selec-tive serotonin reuptake inhibitor response in acute depres-sive episodes, the effect is moderate and highly variable. Recent positron emission tomography studies investigat-ing the occupancy of 5-HT1A receptors in humans by
pindolol have shown that at the dose used most often in clinical trials the occupancy is low and variable, which might explain the inconsistent clinical results. Positron emission tomography studies also suggest that pindolol might be more potent at blocking 5-HT1A autoreceptors
than at blocking postsynaptic receptors, a property that may be useful in this pharmacologic strategy. Thus, the positron emission tomography data support the potential of pindolol to augment the antidepressant response of selective serotonin reuptake inhibitors, but also imply that this potential has not been fully evaluated. Here we review the clinical trials, the positron emission tomography studies, and the possible mechanisms of pindolol augmen-tation. It is also suggested that positron emission tomog-raphy may be used to define therapeutic dosing early on in the process of clinical evaluation of new treatment strategies. Biol Psychiatry 2000;48:844 – 853 © 2000 Society of Biological Psychiatry
Key Words: Serotonin, pindolol, major depression, PET,
[11C]WAY 100635
Introduction
T
he antidepressant effect of the selective serotonin reuptake inhibitors (SSRIs) is thought to be mediated by enhanced serotonin (5-HT) transmission; however, initial exposure to SSRIs is associated with no change or even a decrease in extracellular 5-HT levels in the terminalfields of the forebrain (Chaput et al 1986; Gartside et al 1999; Invernizzi et al 1992; Romero et al 1996), due to stimulation of somatodendritic 5-HT1A autoreceptors in
the dorsal raphe nuclei (DRN), the major source of serotonin projection fibers to the corticolimbic brain re-gions (for a review, see Artigas et al 1996). Electrophysi-ologic rodent studies have demonstrated that sustained administration of SSRIs or 5-HT1Aagonists is associated
with desensitization of 5-HT1A autoreceptors (Blier and
De Montigny 1983; Blier and de Montigny 1985, 1987; Chaput et al 1986; Godbout et al 1991; Jolas et al 1994; Schechter et al 1990). Desensitization of 5-HT1A
autore-ceptors has been shown to preferentially affect the soma-todendritic autoreceptor over the postsynaptic receptor (Blier and de Montigny 1987; Chaput et al 1986; Godbout et al 1991; Jolas et al 1994). Although desensitization has been demonstrated as early as 3 days following treatment (Le Poul et al 1995), the percentage of desensitized neurons reaches 60 – 80% at around 21 days, a time frame that approximates the time of onset of the antidepressant effect (for reviews, see Artigas et al 1996; Blier and de Montigny 1994).
The above observations led to the hypothesis that blockade of the somatodendritic 5-HT1A autoreceptor
might decrease the latency to improvement (Artigas et al 1996; Blier and de Montigny 1990). Pindolol, a
b-blocker with activity at the 5-HT1A receptor (Hoyer
and Schoeffter 1991), was therefore proposed as an augmentation strategy in SSRI treatment, to accelerate the clinical response. This strategy has been evaluated in several clinical trials, with inconsistent results. It is noteworthy that the dose of pindolol used in these clinical trials (7.5 mg/day) was selected based on the expectation that this dose would be too low to induce significant cardiovascular side effects. Yet, the extent to which 5-HT1Areceptors are blocked in humans
follow-ing this dose of pindolol has only recently been char-acterized, using positron emission tomography (PET)
and the selective 5-HT1A receptor radiotracer
[11C]WAY 100635 (Andree et al 1999; Martinez et al, in press; Rabiner et al 2000). These studies indicate that the occupancy of 5-HT1Areceptors achieved in clinical
trials has been low and variable, and that the strategy of
From the Department of Psychiatry and Radiology, Columbia University College of Physicians and Surgeons, New York State Psychiatric Institute, New York. Address reprint requests to Diana Martinez, New York State Psychiatric Institute,
1051 Riverside Drive, Box #42, New York NY 10032. Received May 2, 2000; revised July 11, 2000; accepted July 19, 2000.
blocking 5-HT1A autoreceptors as an adjunctive
treat-ment of depression has not yet been fully tested. In this review, we will briefly discuss the preclinical evidence supporting the potential usefulness of pindolol in augmenting 5-HT system response to SSRIs. Results of clinical trials are reviewed next, followed by a summary of the recent PET studies evaluating pindolol occupancy of 5-HT1Areceptors in humans. The implications of the brain
imaging studies for further development and validation of this augmentation strategy are then discussed.
Preclinical Evidence Supporting the
Rationale for Pindolol Augmentation
The pindolol augmentation strategy stems from the ratio-nale that pindolol should potentiate the increase in 5-HT transmission induced by SSRIs in cortical and limbic areas. To produce this effect, pindolol should preferen-tially block the 5-HT1Aautoreceptors in the DRN relative
to the postsynaptic 5-HT1A receptors in corticolimbic
areas. Although the augmentation of the SSRI effect on 5-HT levels by pindolol is well supported by preclinical studies, the potential selectivity of pindolol for DRN autoreceptors remains controversial.
The coadministration of pindolol with an SSRI has been shown to either prevent the immediate reduction in 5-HT transmission or augment 5-HT levels in the terminal fields of the brain, including the dorsal striatum (Romero et al 1996), hippocampus (Hjorth 1996; Hjorth and Auerbach 1994, 1996), and hypothalamus (Dreshfield et al 1996). In the prefrontal cortex, pindolol was shown to enhance 5-HT transmission in conjunction with fluoxetine, duloxetine, and imipramine (Dawson and Nguyen 2000; Gobert and Millan 1999; Maione et al 1997), although this was not seen in a study with paroxetine (Gartside et al 1999). Pindolol has also been shown to attenuate the SSRI-induced reduction in the firing rate of the 5-HT neurons in some studies (Artigas et al 1996; Romero et al 1996) but not in others (Fornal et al 1999b). Pindolol also attenuates the decrease in 5-HT release induced by 5-HT1Aagonists
(Bosker et al 1994; Gobert and Millan 1999; Sharp et al 1989, 1993; Sharp and Hjorth, 1990).
For pindolol to be maximally effective, it would be important for pindolol to have, relative to the postsynaptic receptor, a preferential effect at the presynaptic receptor. In these conditions, selective blockade of the presynaptic 5-HT1Areceptor would result in maximized 5-HT release
in the terminal fields, without interfering with 5-HT transmission at the postsynaptic 5-HT1Areceptor, as these
receptors may also be involved in the antidepressant effect of increased 5-HT transmission (Lopez et al 1998; Martin et al 1990). Some preclinical studies have demonstrated a greater effect of pindolol at the 5-HT1A autoreceptor
relative to the postsynaptic receptor. Romero et al (1996) found that pindolol failed to prevent 5-HT1A agonist–
induced inhibition in the hippocampus, and Tada et al (1999) demonstrated that pindolol itself did not affect neuronal activity of hippocampal cells, nor did it attenuate the inhibition of neuronal activity induced by the agonist buspirone. Other studies, however, have demonstrated that pindolol does have a pharmacologic effect at the postsyn-aptic receptor (Hadrava et al 1995; Millan et al 1993), and Corradetti et al (1998) demonstrated that pindolol antag-onizes the effects of a 5-HT1Areceptor agonist equally in
both the DRN and the hippocampus.
In vivo binding studies in rodents have also reported contradictory data. Hirani et al (2000) reported preferential inhibition of binding of the selective 5-HT1A receptor
antagonist [11C]WAY 100635 by various doses of (2 )pin-dolol (ranging from 0.001 to 3 mg kg21intravenously) in the DRN in rats studied with a small animal PET scanner. The median effective dose (ED50) of (2)pindolol was
significantly lower in the DRN (ED5050.2660.05 mg
kg21) than in the hippocampus (0.4860.12 mg kg21) and the frontal cortex (0.44 6 0.13 mg kg21); however, Corradetti et al (1998) reported blocking studies of [3H]WAY 100635 binding by (2)pindolol (15 mg kg21 intraoperitoneally [IP]) and showed an almost complete and equivalent binding inhibition in DRN (276%), CA1 (269%), CA3 (279%), and the parietal cortex (282%).
Finally, data from human postmortem autoradiographic binding studies are also contradictory. One study showed similar potencies of (2)pindolol to displace [3H]WAY 100635 in DRN and the hippocampus [Ki(2)pindolol was
17.065.9 nmol/L in the DRN and 17.363.7 nmol/L in the hippocampus; Raurich et al 1999]; however, Castro et al (1999) reported a significantly higher affinity of pindo-lol for the 5-HT1Areceptors in the DRN (Ki58.962.2
nmol/L) than for the receptors in the hippocampus (Ki5
14.4 6 2.9 nmol/L in CA1, p , .05), using the same ligand ([3H]WAY 100635) and same number of human subjects (n 5 4). Thus, some, but not all, preclinical pharmacologic and human postmortem data support the hypothesis that pindolol might act preferentially at the autoreceptor.
Clinical Trials
Open Clinical Trials
(Blier and Bergeron 1995; Blier et al 1997; Vinar et al 1996). Pindolol was also shown to function as an augmen-tation strategy with buspirone (Blier et al 1997) and nefazodone (Bakish et al 1997). On the other hand, the combination of pindolol with tricyclic antidepressant drugs devoid of effect on the 5-HT reuptake process (desipramine or trimipramine) did not appear to have an effect (Blier et al 1997). Of the open-label studies, only one reported no improvement in treatment-refractory pa-tients (Dinan and Scott 1996).
Double-Blind Controlled Trials
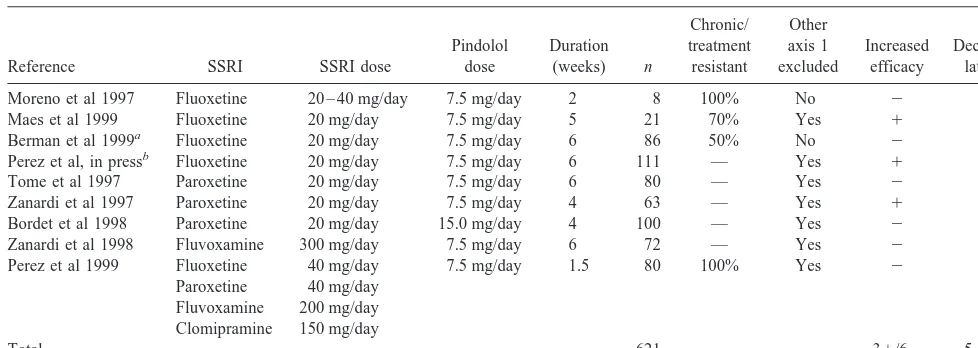
While initial open-label clinical trials suggested that this regimen of pindolol might accelerate and/or enhance clinical response, subsequent double-blind placebo-con-trolled studies provided mixed results. Table 1 lists pub-lished double-blind placebo-controlled studies of pindolol augmentation of antidepressant treatment with SSRIs. When results from the same data sets were included in more than one article, only the latest article with the largest number of subjects is listed. Studies are sorted by type of SSRI and publication year; published studies include studies of pindolol versus placebo augmentation of fluoxetine (n5 4, Berman et al 1999; Maes et al 1999; Moreno et al 1997; Perez et al, in press), paroxetine (n 5
3, Bordet et al 1998; Tome et al 1997; Zanardi et al 1997), fluvoxamine (n 5 1, Zanardi et al 1998), and various antidepressant drugs (Perez et al 1999). The pindolol regimen was typically 7.5 mg/day, with one study using higher doses (15 mg/day, Bordet et al 1998). Duration of the trials varied from 10 days to 6 weeks. The number of subjects per study varied from 8 to 111; a total of 621 subjects were included in these studies. All studies
in-cluded patients who met criteria for a major depressive episode, and inclusion criteria generally included a score greater than 18 on the Hamilton Depression Scale (HAM-D; Hamilton 1960). Other than these criteria, a considerable heterogeneity in clinical presentation is noted. Some studies excluded comorbid axis I diagnoses, whereas others did not. The degree to which each study included patients whose current episode showed resistance to previous treatment also varied (two studies were spe-cifically targeted at treatment-resistant patients: Moreno et al 1997; Perez et al 1999).
Two types of clinical outcomes were evaluated. First, increased efficacy of pindolol versus placebo augmenta-tion was tested by comparing the reducaugmenta-tion in HAM-D or the response rate at the end point. Increased efficacy was demonstrated in three studies and was not demonstrated in six studies. Second, decreased latency was evaluated as a difference in the time to reach clinical response between groups. Decreased latency was demonstrated in five stud-ies but not in four studstud-ies. Studstud-ies that reported decreased latency mostly included patients with recent onset of the current episode (Bordet et al 1998; Perez et al, in press; Tome et al 1997; Zanardi et al 1997, 1998). The two studies restricted to treatment-resistant patients (Moreno et al 1997; Perez et al 1999) failed to report superiority of pindolol augmentation versus placebo augmentation.
Finally, a common finding in these clinical studies is a high degree of variability in response. For example, even in the largest of the positive studies with fluoxetine the proportion of patients demonstrating a sustained response (greater than 19 days) was 38/55 in the pindolol group and 27/56 in the placebo group (Perez et al, in press). It is of note that the one study conducted with 15 mg/day of Table 1. Double-Blind Placebo-Controlled Clinical Trials of Pindolol Augmentation of Antidepressant Treatment
Reference SSRI SSRI dose
Pindolol dose
Duration (weeks) n
Chronic/ treatment resistant
Other axis 1 excluded
Increased efficacy
Decreased latency
Moreno et al 1997 Fluoxetine 20 – 40 mg/day 7.5 mg/day 2 8 100% No 2 2 Maes et al 1999 Fluoxetine 20 mg/day 7.5 mg/day 5 21 70% Yes 1 2 Berman et al 1999a Fluoxetine 20 mg/day 7.5 mg/day 6 86 50% No 2 2 Perez et al, in pressb Fluoxetine 20 mg/day 7.5 mg/day 6 111 — Yes 1 1 Tome et al 1997 Paroxetine 20 mg/day 7.5 mg/day 6 80 — Yes 2 1 Zanardi et al 1997 Paroxetine 20 mg/day 7.5 mg/day 4 63 — Yes 1 1 Bordet et al 1998 Paroxetine 20 mg/day 15.0 mg/day 4 100 — Yes 2 1 Zanardi et al 1998 Fluvoxamine 300 mg/day 7.5 mg/day 6 72 — Yes 2 1 Perez et al 1999 Fluoxetine 40 mg/day 7.5 mg/day 1.5 80 100% Yes 2 2
Paroxetine 40 mg/day Fluvoxamine 200 mg/day Clomipramine 150 mg/day
Total 621 31/62 51/42
SSRI, selective serotonin reuptake inhibitor.
pindolol showed both increased SSRI efficacy and de-creased latency period (Bordet et al 1998). With two exceptions (Perez et al 1997, in press), studies did not report pindolol plasma levels.
PET Studies
WAY-100635 [N-(2-(4-(2-methoxyphenyl)-1-piperazin-yl)ethyl)-N-(2-pyridinyl) cyclohexane carboxamide] is a potent and selective 5-HT1A antagonist (Kd 5 0.1– 0.4
nmol/L) (Fletcher et al 1993; Forster et al 1995; Gozlan et al 1995). Labeled with carbon 11 in the carbonyl position, this ligand allows reliable quantification of 5-HT1A
recep-tor availability in the human brain using PET (Farde et al 1998; Gunn et al 1998; Parsey et al, in press; Pike et al 1995). Three studies have measured the occupancy of the 5-HT1Areceptor achieved in humans by pindolol (Andree
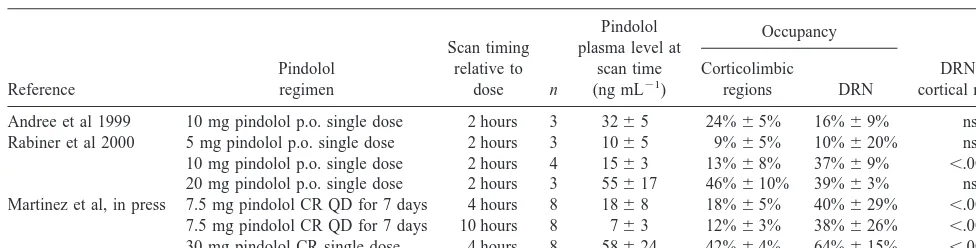
et al 1999; Martinez et al, in press; Rabiner et al 2000). Findings from these PET studies are summarized in Table 2.
Andree et al (1999) studied inhibition of [11C]WAY 100635 binding in three male volunteers, 2 hours follow-ing the oral administration of 10 mg of pindolol. Pindolol occupancy of 5-HT1Areceptors in the DRN (16%6 9%)
was not significantly different from the occupancy in temporal and frontal cortices (24% 6 5%), at a mean plasma pindolol level of 32 ng mL21.
Rabiner et al (in press) reported occupancy of 5-HT1A
receptors by pindolol following oral administration of three different doses of pindolol (5 mg, 10 mg, and 20 mg), given as a single dose 2 hours before scanning. At the 5-mg dose (plasma level 1065 ng mL21), no significant occupancy was detected in either the DRN or temporal regions, whereas occupancy was seen in all regions examined (DRN, insula, and medial temporal lobe) at 10-mg and 20-mg doses. Following the 10-mg dose (plasma level 15 6 3 ng mL21), a significantly larger occupancy was measured in the DRN (37%69%) than in the cortical regions (13%6 8%, p , .001). Following
the 20-mg dose (plasma level 55.1616.8 ng mL21), the DRN occupancy (39% 63%) was similar to the cortical occupancy (46%610%, p 5 .24). Rabiner et al (2000) also evaluated the 5-HT1A receptor occupancy of two
other beta blockers, penbutolol and tertatolol, which have also been shown to exhibit 5-HT1Aactivity and to enhance
the effect of paroxetine on 5-HT in the frontal cortex (Gartside et al 1999). Penbutolol achieved significant occupancy of 5-HT1A receptors at both doses tested (40
mg and 80 mg), with no differences between DRN and corticolimbic 5-HT1Areceptors. Tertatolol did not
signif-icantly affect [11C]WAY 100635 binding at the doses tested (5 mg and 10 mg).
Martinez et al (in press) studied eight male subjects under four conditions: at baseline (scan 1), then following 5 days of 7.5-mg controlled release pindolol (scan 2 at 4 hours after dose and scan 3 at 10 hours after dose), and 4 hours following an increase of the oral dose to 30 mg (scan 4). Pindolol occupancy of all brain regions examined (dorsolateral, medial, orbitofrontal, and subgenual pre-frontal cortices; anterior cingulate; parietal, occipital, in-sular, and medial temporal cortices; and DRN) was sig-nificant (20%68% at scan 2, 14%68% at scan 3, and 44%68% at scan 4 at plasma pindolol concentrations of 1868 ng mL21, 763 ng mL21, and 58624 ng mL21, respectively). Occupancy in the DRN was significantly higher than occupancy in all other regions at each dose. Dorsal raphe nuclei occupancy was 40%629% on scan
2, 38% 6 26% on scan 3, and 64% 6 15% on scan 4
compared to an average occupancy in all other regions of 18%65% on scan 2, 12%63% on scan 3, and 42%6
4% on scan 4. Analysis of the relationship between pindolol plasma level and 5-HT1A occupancy in scans 2
and 4 revealed maximal effect values (6SE) of 95% 6
23% in the DRN and 53%617% in postsynaptic regions and concentration at the half maximal response values of 24615 ng mL21for the DRN and 21618 ng mL21in postsynaptic regions. This result suggests that the sites that display high affinity for pindolol corresponded to the Table 2. Occupancy of Corticolimbic and DRN 5-HT1AReceptors by Pindolol in Humans: PET Studies
Reference
Pindolol regimen
Scan timing relative to
dose n
Pindolol plasma level at
scan time (ng mL21)
Occupancy
DRN vs. cortical regions Corticolimbic
regions DRN
Andree et al 1999 10 mg pindolol p.o. single dose 2 hours 3 3265 24%65% 16%69% ns Rabiner et al 2000 5 mg pindolol p.o. single dose 2 hours 3 1065 9%65% 10%620% ns 10 mg pindolol p.o. single dose 2 hours 4 1563 13%68% 37%69% ,.001 20 mg pindolol p.o. single dose 2 hours 3 55617 46%610% 39%63% ns Martinez et al, in press 7.5 mg pindolol CR QD for 7 days 4 hours 8 1868 18%65% 40%629% ,.001
7.5 mg pindolol CR QD for 7 days 10 hours 8 763 12%63% 38%626% ,.001 30 mg pindolol CR single dose 4 hours 8 58624 42%64% 64%615% ,.001
whole population of 5-HT1A receptors in the DRN and
only about half of the 5-HT1Areceptors in the postsynaptic
areas.
In summary, PET studies demonstrate a significant occupancy of 5-HT1Areceptors in humans by pindolol but
also suggest that the occupancy resulting from the regi-mens used in clinical trials (2.5 mg thrice daily) is low and highly variable between subjects. Higher occupancy and lower variability are observed at large doses.
Differences in DRN versus corticolimbic occupancy have not been consistently reported by PET studies (Table 2). These differences might be related to differences in experimental design and imaging protocol. Martinez et al (in press) measured occupancy following sustained admin-istration of a controlled release formulation of pindolol, rather than following a single dose of pindolol (Andree et al 1999; Rabiner et al 2000). The duration of the scans varied and data were analyzed using different methods; however, none of these differences are suspected a priori to bias the comparison of the DRN versus cortical occu-pancy. More likely, the failure to observe DRN selectivity in the study of Andree et al (1999) and in the high dose used by Rabiner et al (2000) might be due to the noise associated with the measurement of DRN [11C]WAY 100635 binding potential (BP) and the small number of subjects (three to four) included in these two studies.
Lastly, it is important to note that the partial volume effect will influence [11C]WAY 100635 BP measurements more in the DRN than in corticolimbic regions, given the difference in the respective sizes of these regions. Yet, the magnitude of the partial volume effect is dependent only on the volume of the structure and not on the level of activity (Kessler et al 1984). Therefore, the partial volume effect would be expected to affect both the baseline and pindolol occupancy scans equally, and would not explain the greater pindolol-induced decrease in DRN binding relative to corticolimbic regions.
Potential Mechanisms Underlying Pindolol
DRN Selectivity
The apparent relative selectivity of pindolol for DRN 5-HT1A autoreceptors might result from the fact that
pindolol is a weak agonist rather than a pure antagonist. Pindolol was initially characterized as an antagonist at the 5-HT1Areceptor, given that it was shown to have no effect
on 5-HT levels (Hjorth and Sharp 1990, 1993; Romero et al 1996; Sharp et al 1989) or was reported to increase extracellular 5-HT in the prefrontal cortex (Maione et al 1997), hippocampus (Assie and Koek 1996; Bosker et al 1994; Gur et al 1997; Matos et al 1996), and caudate (Fornal et al 1999a), as well as the DRN itself (Maione et al 1997; Matos et al 1996), via blockade of the 5-HT1A
autoreceptor. More recent studies, however, have indi-cated that pindolol acts as a partial agonist at the 5-HT1A
autoreceptor. Pindolol alone has been shown to inhibit the cells of the DRN (Clifford et al 1998; Fornal et al 1999c,c; Haddjeri et al 1999), decrease 5-HT in the frontal cortex (Gobert and Millan 1999), and decrease 5-HT synthesis and metabolism (Hjorth and Carlsson 1986). Newman-Tancredi et al (1998) demonstrated that pindolol produces an increase in guanosine-59-O-(3-[35S]thio)-triphosphate binding in cells transfected with the human 5-HT1A
receptor, but that the increase is only 20% that of induced by 5-HT, consistent with weak partial agonist activity. Taking all of these findings together, it appears that the effect of pindolol as an antagonist or weak partial agonist at the 5-HT1A receptor may ultimately depend upon the
local concentration of endogenous 5-HT (Gobert and Millan 1999).
The agonist nature of pindolol at the 5-HT1Areceptor
might account for the regional differences in binding observed in the PET studies. The binding of agonists to the 5-HT1A receptor is modulated by G protein coupling
(Harrington and Peroutka 1990); the addition of GTP or its analogue guanylyl imidodiphosphate results in a signifi-cant reduction in the percentage of 5-HT1A receptors
configured in a state of high affinity for agonists (Mon-geau et al 1992; Nenonene et al 1994). Thus, addition of GTP results in reduced binding of 5-HT1A agonists
(Emerit et al 1990; Hall et al 1986; Mongeau et al 1992), whereas the binding of pure antagonists such as [3H]WAY 100635 is unaffected (Emerit et al 1990; Gozlan et al 1995; Khawaja et al 1995). As a partial agonist, the binding of pindolol is expected to be affected by G protein coupling and affinity state. Therefore, the preferential occupancy by pindolol of the DRN 5-HT1A receptors
relative to corticolimbic 5-HT1A receptors might stem
from the presence of a larger proportion of DRN 5-HT1A
receptors being configured in the high-affinity state. This hypothesis is supported by preclinical data. The number of receptor sites labeled by [3H]WAY 100635 in
corticolim-bic areas is 36 – 60% higher than those labeled with agonist [3H]8-OH-DPAT (Fletcher et al 1993; Gozlan et al 1995; Khawaja 1995; Khawaja et al 1995), suggesting that only a subset of 5-HT1Areceptors is in the high-affinity
state in these regions. In contrast, Khawaja et al (1995) reported similar Bmax for [
3
H]WAY 100635 and [3 H]8-OH-DPAT in the DRN, suggesting that most of the DRN 5-HT1A receptors are configured in the high agonist
affinity state.
in the hippocampus and DRN, a finding also reported in a more recent study by Meller et al (2000). Although both receptors have been shown to be linked to pertussis toxin–sensitive Gi/Goregulatory proteins, activation of the
somatodendritic autoreceptor results in membrane hyper-polarization secondary to G protein– coupled K1 conduc-tance, which inhibits firing and results in a reduction of 5-HT synthesis and release in the forebrain (Blier et al 1993; Hjorth and Magnusson 1988; Meller et al 2000); however, unlike the postsynaptic receptor, the presynaptic receptor does not appear to affect adenylyl cyclase activity (Clarke et al 1996; Johnson et al 1997). Furthermore, the postsynaptic receptor appears to have two populations of receptors, one that produces hyperpolarization via in-creased K1conductance and another that inhibits forsko-lin-mediated stimulation of adenylyl cyclase activity (Meller et al 2000). The relevance of these differences between pre- and postsynaptic 5-HT1Afor the binding of
pindolol remains to be elucidated.
Clinical Implications of PET Data
Estimation of Occupancy Achieved in Clinical Trials
To our knowledge, only two clinical studies reported plasma levels of pindolol. Perez et al (1999) measured pindolol plasma levels of 9.96 5.1 (SD) ng mL21in a sample of 40 subjects treated with a combination of pindolol (2.5 mg thrice daily) and SSRIs (fluoxetine, paroxetine, fluvoxamine, or clomipramine). Slightly lower pindolol plasma values (6 –7 ng mL21) were reported in a group of 55 patients under a pindolol–fluoxetine combi-nation (Perez et al, in press). These data indicate that the pindolol plasma levels reached in clinical studies are in the 6 –10 ng mL21range. Using pharmacokinetic parameters derived from Martinez et al (in press), a plasma level of 10 ng mL21 is predicted to be associated with low 5-HT1A
occupancy (28% and 17% in the DRN and corticolimbic regions, respectively). Plasma levels of 6 ng mL21 are predicted to be associated with only 19% and 12% occupancy in the DRN and corticolimbic regions, respec-tively. The average DRN 5-HT1A receptor occupancy is
thus in the 25% range in these clinical studies.
It is unclear if 25% occupancy of DRN 5-HT1A
recep-tors is enough to achieve the desired clinical effect. The minimal occupancy of DRN 5-HT1Areceptors needed to
achieve blockade of the SSRI-induced activation of these receptors has not been well documented. In the classic study of Romero et al (1996), a (2)pindolol dose of at least 10 mg/kg IP was required to significantly block the decrease in striatal 5-HT induced by direct application of citalopram (50mM) in the DRN. To our knowledge, the occupancy of DRN 5-HT1A receptors achieved by 10
mg/kg IP pindolol in the rat has not been reported. Yet, a dose of 15 mg/kg IP (2)pindolol has been reported to occupy 76% of DRN 5-HT1Areceptors (Corradetti et al
1998), and it is likely that the occupancy following 10 mg/kg IP is in the range of 50% (i.e., higher than the 25% achieved in clinical studies). Studies combining occu-pancy and electrophysiologic or microdialysis measure-ments are needed to clarify this question.
Between-Subject Variability
The second practical implication of the PET studies is the large between-subject variability in both pindolol plasma levels and occupancy, particularly at the lower dose. For example, the occupancy of DRN 5-HT1Areceptors
follow-ing 1 week of pindolol 7.5 mg/day varied from no detectable occupancy to 48% occupancy in the group studied by Martinez et al (in press). Rabiner et al (2000) also reported a greater degree of variability between subjects at the lower dose. This variability might account for some of the variability in response observed in the clinical studies. In the study by Martinez et al (in press) the variability in DRN occupancy was partially accounted for by between-subject differences in pindolol plasma levels, indicating that plasma level monitoring would be useful in clinical studies to adjust the dose to a level compatible with appropriate occupancy.
Therapeutic Window
The selective DRN occupancy of pindolol observed in PET studies implies the existence of a therapeutic window of pindolol plasma concentrations. The therapeutic win-dow includes the range of pindolol concentrations at which a marked difference is reached between DRN occupancy (which should be high to potentiate SSRI effects on 5-HT transmission) and cortical occupancy (which should be low to maintain activation of postsyn-aptic 5-HT1A receptors). Positron emission tomography
studies predict that a pindolol regimen of 6.25 mg thrice daily (18.75 mg/day) would be necessary to achieve 50% occupancy of DRN 5-HT1Areceptors. At this dose,
occu-pancy in corticolimbic regions would be only 32%, and the difference in occupancy between the two regions would be 18%. The difference in occupancy achieved at this dose is close to the maximal difference in occupancy, which is predicted to occur at a plasma pindolol level of 63 ng/mL (DRN occupancy of 73% and corticolimbic occupancy of 52%, difference of 21%).
Profile of the Ideal Compound for Augmentation of SSRI Antidepressant Effects
Assuming that selective blockade of 5-HT1A
SSRI, the PET data presented here provide some clues about the desired properties of compounds selected for this application. The data suggest that pindolol might not be the ideal compound, since the side effects associated with its b-adrenergic blocking properties might preclude its use in routine clinical practice at doses (15–25 mg/day) required to block 50% of the DRN 5-HT1A receptors. It is currently assumed that
silent 5-HT1A antagonists would be the optimal
phar-macologic agents for this application: WAY 100635 or other silent antagonists provide superior potentiation of SSRIs’ acute effects on 5-HT transmission compared with pindolol, a difference attributed to the fact that pindolol is a partial agonist (Clifford et al 1998; Gartside et al 1999; Sharp et al 1993); however, if DRN selectivity is a unique feature of partial agonists and if it is important to minimize blockade of postsynaptic 5-HT1A receptors, a weak and DRN-selective partial
agonist might still be the drug of choice for this application. Positron emission tomography studies of other 5-HT1Apartial agonists and silent antagonists are
warranted to evaluate the relationship between pharma-cologic profile and DRN selectivity. The higher the DRN selectivity of the candidate drug, the larger the therapeutic window within which DRN autoreceptors would be inhibited without interfering with 5-HT trans-mission at the postsynaptic receptors. Finally, given that the goal of this pharmacologic strategy is to hasten therapeutic response, appropriate plasma levels of the candidate drug should be reached rapidly, maybe using an initial loading dose. Again, PET imaging provides a unique tool to define the relationship between pharma-cokinetic and therapeutic windows.
Conclusion
Recent PET imaging studies revealed that the occupancy of DRN 5-HT1A receptors achieved during clinical trials
aimed at hastening or augmenting the antidepressant effects of SSRIs might have been suboptimal for achieving the desired potentiation of 5-HT transmission. This factor, as well as the important between-subject variability in occupancy, might account for the mixed results reported in double-blind, placebo-controlled studies. On the other hand, pindolol appeared to be associated with significant in vivo selectivity for DRN 5-HT1Aautoreceptors, relative
to corticolimbic postsynaptic receptors. This DRN selec-tivity is presumably desirable for potentiation of 5-HT transmission and represents an important proof of concept for the development of new 5-HT1A agents for this
application. Early evaluation of new drugs with PET imaging will enable rapid screening of compounds based
on DRN selectivity, and more rigorous determination of doses to be tested in clinical trials.
This work was supported by the U.S. Public Health Service (National Institute of Mental Health Grants Nos. K02 MH01603-0 and T32-MH 18870).
References
Andree B, Thorberg SO, Halldin C, Farde L (1999): Pindolol binding to 5-HT1A receptors in the human brain confirmed with positron emission tomography. Psychopharmacology
(Berl) 144:303–305.
Artigas F, Perez V, Alvarez E (1994): Pindolol induces a rapid improvement of depressed patients treated with serotonin reuptake inhibitors. Arch Gen Psychiatry 51:248 –251. Artigas F, Romero L, de Montigny C, Blier P (1996):
Acceler-ation of the effect of selected antidepressant drugs in major depression by 5-HT1A antagonists. Trends Neurosci 19:378 – 383.
Assie MB, Koek W (1996): (2)-Pindolol and (6)-tertatolol affect rat hippocampal 5-HT levels through mechanisms involving not only 5-HT1A, but also 5-HT1B receptors.
Neuropharmacology 35:213–222.
Bakish D, Hooper CL, Thornton MD, Wiens A, Miller CA, Thibaudeau CA (1997): Fast onset: An open study of the treatment of major depressive disorder with nefazodone and pindolol combination therapy. Int Clin Psychopharmacol 12:91–97.
Berman RM, Anand A, Cappiello A, Miller HL, Hu XS, Oren DA, Charney DS (1999): The use of pindolol with fluoxetine in the treatment of major depression: Final results from a double-blind, placebo-controlled trial. Biol Psychiatry 45: 1170 –1117.
Berman RM, Darnell AM, Miller HL, Anand A, Charney DS (1997): Effect of pindolol in hastening response to fluoxetine in the treatment of major depression: A double-blind, place-bo-controlled trial. Am J Psychiatry 154:37– 43.
Blier P, Bergeron R (1995): Effectiveness of pindolol with selected antidepressant drugs in the treatment of major depression. J Clin Psychopharmacol 15:217–222.
Blier P, Bergeron R, de Montigny C (1997): Selective activation of postsynaptic 5-HT1A receptors induces rapid antidepres-sant response. Neuropsychopharmacology 16:333–338. Blier P, De Montigny C (1983): Electrophysiological
investiga-tions on the effect of repeated zimelidine administration on serotonergic neurotransmission in the rat. J Neurosci 3:1270 – 1278.
Blier P, de Montigny C (1985): Serotoninergic but not noradren-ergic neurons in rat central nervous system adapt to long-term treatment with monoamine oxidase inhibitors. Neuroscience 16:949 –955.
Blier P, de Montigny C (1987): Modification of 5-HT neuron properties by sustained administration of the 5-HT1A agonist gepirone: Electrophysiological studies in the rat brain.
Syn-apse 1:470 – 480.
presynaptic and postsynaptic serotonin receptors: Single-cell recording studies. J Clin Psychopharmacol 10:13S–20S. Blier P, de Montigny C (1994): Current advances and trends in
the treatment of depression. Trends Pharmacol Sci 15:220 – 226.
Blier P, Lista A, De Montigny C (1993): Differential properties of pre- and postsynaptic 5-hydroxytryptamine1A receptors in the dorsal raphe and hippocampus: II. Effect of pertussis and cholera toxins. J Pharmacol Exp Ther 265:16 –23.
Bordet R, Thomas P, Dupuis B (1998): Effect of pindolol on onset of action of paroxetine in the treatment of major depression: Intermediate analysis of a double-blind, placebo-controlled trial. Reseau de Recherche et d’Experimentation Psychopharmacologique. Am J Psychiatry 155:1346 –1351. Bosker FJ, Donker MG, Klompmakers AA, Kurata K,
Westen-berg HG (1994): 5-Hydroxytryptamine release in dorsal hippocampus of freely moving rats: Modulation by pindolol.
Prog Neuropsychopharmacol Biol Psychiatry 18:765–778.
Castro ME, Harrison PJ, Pazos A, Sharp T (1999): Affinity of beta-adrenergic ligands for pre- and postsynaptic 5-HT1A recetors in human brain. Soc Neurosci Abstr 25:175. Chaput Y, de Montigny C, Blier P (1986): Effects of a selective
5-HT reuptake blocker, citalopram, on the sensitivity of 5-HT autoreceptors: Electrophysiological studies in the rat brain.
Naunyn Schmiedebergs Arch Pharmacol 333:342–348.
Clarke WP, Yocca FD, Maayani S (1996): Lack of 5-hydroxytryptamine1A-mediated inhibition of adenylyl cy-clase in dorsal raphe of male and female rats. J Pharmacol
Exp Ther 277:1259 –1266.
Clifford EM, Gartside SE, Umbers V, Cowen PJ, Hajos M, Sharp T (1998): Electrophysiological and neurochemical evidence that pindolol has agonist properties at the 5-HT1A autorecep-tor in vivo. Br J Pharmacol 124:206 –212.
Corradetti R, Laaris N, Hanoun N, Laporte AM, Le Poul E, Hamon M, Lanfumey L (1998): Antagonist properties of (2)-pindolol and WAY 100635 at somatodendritic and postsynaptic 5-HT1A receptors in the rat brain. Br J
Phar-macol 123:449 – 462.
Dawson LA, Nguyen HQ (2000): The role of 5-HT(1A) and 5-HT(1B/1D) receptors on the modulation of acute fluoxetine-induced changes in extracellular 5-HT: The mechanism of action of (6)pindolol. Neuropharmacology 39:1044 –1052.
Dinan TG, Scott LV (1996): Does pindolol induce a rapid improvement in depressed patients resistant to serotonin reuptake inhibitors? J Serotonin Res 3:119 –121.
Dreshfield LJ, Wong DT, Perry KW, Engleman EA (1996): Enhancement of fluoxetine-dependent increase of extracellu-lar serotonin (5-HT) levels by (2)-pindolol, an antagonist at 5-HT1A receptors. Neurochem Res 21:557–562.
Emerit MB, el Mestikawy S, Gozlan H, Rouot B, Hamon M (1990): Physical evidence of the coupling of solubilized 5-HT1A binding sites with G regulatory proteins. Biochem
Pharmacol 39:7–18.
Farde L, Ito H, Swahn CG, Pike VW, Halldin C (1998): Quantitative analyses of carbonyl-carbon-11-WAY-100635 binding to central 5-hydroxytryptamine-1A receptors in man.
J Nucl Med 39:1965–1971.
Fletcher A, Bill DJ, Bill SJ, Cliffe IA, Dover GM, Forster EA, et al (1993): WAY100135: A novel, selective antagonist at
presynaptic and postsynaptic 5-HT1A receptors. Eur J
Phar-macol 237:283–291.
Fornal CA, Martin FJ, Mendlin A, Metzler CW, Bjorvatn B, Jacobs BL (1999a): Pindolol increases extracellular 5-HT while inhibiting serotonergic neuronal activity. Eur J
Phar-macol 377:187–191.
Fornal CA, Martin FJ, Metzler CW, Jacobs BL (1999b): Pindo-lol, a putative 5-hydroxytryptamine(1A) antagonist, does not reverse the inhibition of serotonergic neuronal activity in-duced by fluoxetine in awake cats: comparison to WAY-100635. J Pharmacol Exp Ther 291:220 –228.
Fornal CA, Martin FJ, Metzler CW, Jacobs BL (1999c): Pindolol suppresses serotonergic neuronal activity and does not block the inhibition of serotonergic neurons produced by 8-hy-droxy-2-(di-n-propylamino)tetralin in awake cats. J
Pharma-col Exp Ther 291:229 –238.
Forster EA, Cliffe IA, Bill DJ, Dover GM, Jones D, Reilly Y, Fletcher A (1995): A pharmacological profile of the selective silent 5-HT1A receptor antagonist, WAY-100635. Eur
J Pharmacol 281:81– 88.
Gartside SE, Clifford EM, Cowen PJ, Sharp T (1999): Effects of (2)-tertatolol, (2)-penbutolol and (6)-pindolol in combina-tion with paroxetine on presynaptic 5-HT funccombina-tion: An in vivo microdialysis and electrophysiological study. Br J
Phar-macol 127:145–152.
Gobert A, Millan MJ (1999): Modulation of dialysate levels of dopamine, noradrenaline, and serotonin (5-HT) in the frontal cortex of freely-moving rats by (2)-pindolol alone and in association with 5-HT reuptake inhibitors: Comparative roles of beta-adrenergic, 5-HT1A, and 5-HT1B receptors.
Neuro-psychopharmacology 21:268 –284.
Godbout R, Chaput Y, Blier P, de Montigny C (1991): Tandospi-rone and its metabolite, 1-(2-pyrimidinyl)-piperazine—I. Ef-fects of acute and long-term administration of tandospirone on serotonin neurotransmission. Neuropharmacology
30:679 – 690.
Gozlan H, Thibault S, Laporte AM, Lima L, Hamon M (1995): The selective 5-HT1A antagonist radioligand [3H]WAY 100635 labels both G-protein-coupled and free 5-HT1A receptors in rat brain membranes. Eur J Pharmacol 288:173– 186.
Gunn RN, Sargent PA, Bench CJ, Rabiner EA, Osman S, Pike VW, et al (1998): Tracer kinetic modeling of the 5-HT1A receptor ligand [carbonyl-11C]WAY-100635 for PET.
Neu-roimage 8:426 – 440.
Gur E, Lerer B, Newman ME (1997): Chronic electroconvulsive shock and 5-HT autoreceptor activity in rat brain: An in vivo microdialysis study. J Neural Transm 104:795– 804. Haddjeri N, de Montigny C, Blier P (1999): Modulation of the
firing activity of rat serotonin and noradrenaline neurons by (6)pindolol. Biol Psychiatry 45:1163–1169.
Hadrava V, Blier P, Dennis T, Ortemann C, de Montigny C (1995): Characterization of 5-hydroxytryptamine1A proper-ties of flesinoxan: In vivo electrophysiology and hypothermia study. Neuropharmacology 34:1311–1326.
Hamilton M (1960): A rating scale for depression. J Neurol
Neurosurg Psychiatry 23:56 – 62.
Harrington MA, Peroutka SJ (1990): Modulation of 5-hydroxytryptamine1A receptor density by nonhydrolyzable GTP analogues. J Neurochem 54:294 –299.
Hirani E, Opacka-Juffry J, Gunn R, Khan I, Sharp T, Hume S (2000): Pindolol occupancy of 5HT1A receptors measured in vivo using small animal positron emission tomography with carbon-11 labelled WAY 100635. Synapse 36:330 –341. Hjorth S (1996): (2)-Pindolol, but not buspirone, potentiates the
citalopram-induced rise in extracellular 5-hydroxytryptamine.
Eur J Pharmacol 303:183–186.
Hjorth S, Auerbach SB (1994): Further evidence for the impor-tance of 5-HT1A autoreceptors in the action of selective serotonin reuptake inhibitors. Eur J Pharmacol 260:251–255. Hjorth S, Auerbach SB (1996): 5-HT1A autoreceptors and the mode of action of selective serotonin reuptake inhibitors (SSRI). Behav Brain Res 73:281–283.
Hjorth S, Carlsson A (1986): Is pindolol a mixed agonist-antagonist at central serotonin (5-HT) receptors? Eur J
Phar-macol 129:131–138.
Hjorth S, Magnusson T (1988): The 5-HT 1A receptor agonist, 8-OH-DPAT, preferentially activates cell body 5-HT autore-ceptors in rat brain in vivo. Naunyn Schmiedebergs Arch
Pharmacol 338:463– 471.
Hjorth S, Sharp T (1990): Mixed agonist/antagonist properties of NAN-190 at 5-HT1A receptors: Behavioural and in vivo brain microdialysis studies. Life Sci 46:955–963.
Hjorth S, Sharp T (1993): In vivo microdialysis evidence for central serotonin1A and serotonin1B autoreceptor blocking properties of the beta adrenoceptor antagonist (2)penbutolol.
J Pharmacol Exp Ther 265:707–712.
Hoyer D, Schoeffter P (1991): 5-HT receptors: Subtypes and second messengers. J Recept Res 11:197–214.
Invernizzi R, Belli S, Samanin R (1992): Citalopram’s ability to increase the extracellular concentrations of serotonin in the dorsal raphe prevents the drug’s effect in the frontal cortex.
Brain Res 584:322–324.
Johnson RG, Fiorella D, Winter JC, Rabin RA (1997): [3H]8-OH-DPAT labels a 5-HT site coupled to inhibition of phos-phoinositide hydrolysis in the dorsal raphe. Eur J Pharmacol 329:99 –106.
Jolas T, Haj-Dahmane S, Kidd EJ, Langlois X, Lanfumey L, Fattaccini CM, et al (1994): Central pre- and postsynaptic 5-HT1A receptors in rats treated chronically with a novel antidepressant, cericlamine. J Pharmacol Exp Ther 268: 1432–1443.
Kessler RM, Ellis J Jr, Eden M (1984): Analysis of emission tomographic scan data: Limitations imposed by resolution and background. J Comput Assist Tomogr 8:514 –522. Khawaja X (1995): Quantitative autoradiographic
characterisa-tion of the binding of [3H]WAY-100635, a selective 5-HT1A receptor antagonist. Brain Res 673:217–225.
Khawaja X, Evans N, Reilly Y, Ennis C, Minchin MC (1995): Characterisation of the binding of [3H]WAY-100635, a novel 5-hydroxytryptamine1A receptor antagonist, to rat brain.
J Neurochem 64:2716 –2726.
Le Poul E, Laaris N, Doucet E, Laporte AM, Hamon M, Lanfumey L (1995): Early desensitization of
somato-den-dritic 5-HT1A autoreceptors in rats treated with fluoxetine or paroxetine. Naunyn Schmiedebergs Arch Pharmacol 352: 141–148.
Lopez JF, Chalmers DT, Little KY, Watson SJ (1998): A.E. Bennett Research Award. Regulation of serotonin1A, glu-cocorticoid, and mineralocorticoid receptor in rat and human hippocampus: Implications for the neurobiology of depres-sion. Biol Psychiatry 43:547–573.
Maes M, Libbrecht I, van Hunsel F, Campens D, Meltzer HY (1999): Pindolol and mianserin augment the antidepressant activity of fluoxetine in hospitalized major depressed patients, including those with treatment resistance. J Clin
Psychophar-macol 19:177–182.
Maione S, Palazzo E, Pallotta M, Leyva J, Berrino L, Rossi F (1997): Effects of imipramine on raphe nuclei and prefrontal cortex extracellular serotonin levels in the rat.
Psychophar-macology (Berl) 134:401– 405.
Martin P, Beninger RJ, Hamon M, Puech AJ (1990): Antidepres-sant-like action of 8-OH-DPAT, a 5-HT1A agonist, in the learned helplessness paradigm: Evidence for a postsynaptic mechanism. Behav Brain Res 38:135–144.
Martin P, Tissier MH, Adrien J, Puech AJ (1991): Antidepres-sant-like effects of buspirone mediated by the 5-HT1A post-synaptic receptors in the learned helplessness paradigm.
Life Sci 48:2505–2511.
Martinez D, Hwang DR, Mawlawi O, Slifstein M, Kent J, Simpson N, et al (in press): Differential occupancy of somatodendritic and postsynaptic 5HT1Areceptors by
pindo-lol: A dose-occupancy study with [11
C]WAY 100635 and positron emission tomography in humans.
Neuropsychophar-macology.
Matos FF, Urban C, Yocca FD (1996): Serotonin (5-HT) release in the dorsal raphe and ventral hippocampus: Raphe control of somatodendritic and terminal 5-HT release. J Neural
Transm 103:173–190.
Meller E, Li H, Carr KD, Hiller JM (2000): 5-Hydroxy-tryptamine(1A) receptor-stimulated [(35)S]GTPgammaS bind-ing in rat brain: Absence of regional differences in couplbind-ing efficiency. J Pharmacol Exp Ther 292:684 – 691.
Millan MJ, Rivet JM, Canton H, Le Marouille-Girardon S, Gobert A (1993): Induction of hypothermia as a model of 5-hydroxytryptamine1A receptor-mediated activity in the rat: A pharmacological characterization of the actions of novel agonists and antagonists. J Pharmacol Exp Ther 264:1364 – 1376.
Mongeau R, Welner SA, Quirion R, Suranyi-Cadotte BE (1992): Further evidence for differential affinity states of the serotonin1A receptor in rat hippocampus. Brain Res 590: 229 –238.
Moreno FA, Gelenberg AJ, Bachar K, Delgado PL (1997): Pindolol augmentation of treatment-resistant depressed pa-tients. J Clin Psychiatry 58:437– 439.
Nenonene EK, Radja F, Carli M, Grondin L, Reader TA (1994): Heterogeneity of cortical and hippocampal 5-HT1A recep-tors: A reappraisal of homogenate binding with 8-[3H]hy-droxydipropylaminotetralin. J Neurochem 62:1822–1834. Newman-Tancredi A, Verriele L, Chaput C, Millan MJ (1998):
antagonists and inverse agonists. Naunyn Schmiedebergs
Arch Pharmacol 357:205–217.
Parsey RV, Slifstein M, Hwang DR, Abi-Dargham A, Simpson N, Mawlawi O, et al (in press): Validation and reproducibility of measurement of 5-HT1A receptors parameters with [carbonyl-11C]WAY-100635 in humans: Comparison of arterial and reference tissue input functions. J Cereb Blood Flow Metab. Perez V, Gilaberte I, Faries D, Alvarez E, Artigas F (1997):
Randomised, double-blind, placebo-controlled trial of pindo-lol in combination with fluoxetine antidepressant treatment.
Lancet 349:1594 –1597.
Perez V, Puigdemont D, Gilaberte I, Alvarez E, Artigas F (in press): Augmentation of fluoxetine antidepressant action by pindolol: Analysis of clinical pharmacokinetic, and method-ological factors. J Clin Psychopharmacol.
Perez V, Soler J, Puigdemont D, Alvarez E, Artigas F (1999): A double-blind, randomized, placebo-controlled trial of pindo-lol augmentation in depressive patients resistant to serotonin reuptake inhibitors. Grup de Recerca en Trastorns Afectius.
Arch Gen Psychiatry 56:375–379.
Pike VW, McCarron JA, Lammerstma AA, Hume SP, Poole K, Grasby PM, et al (1995): First delineation of 5-HT1A receptors in human brain with PET and [11C]WAY-100635.
Eur J Pharmacol 283:R1–R3.
Rabiner EA, Gunn RN, Castro E, Sargent PA, Cowen PJ, Koepp M, et al (2000): Beta-blocker binding to human 5HT1A receptors in vivo and in vitro: Implications for antidepressant therapy. Neuropsychopharmacology 23:285–293.
Radja F, Daval G, Hamon M, Verge D (1992): Pharmacological and physicochemical properties of pre-versus postsynaptic 5-hydroxytryptamine1A receptor binding sites in the rat brain: A quantitative autoradiographic study. J Neurochem 58:1338 –1346.
Raurich A, Mengod G, Artigas F, Cortes R (1999): Displacement of the binding of 5-HT(1A) receptor ligands to pre- and postsynaptic receptors by (2)pindolol. A comparative study in rodent, primate and human brain. Synapse 34:68 –76. Romero L, Bel N, Artigas F, de Montigny C, Blier P (1996):
Effect of pindolol on the function of pre- and postsynaptic 5-HT1A receptors: In vivo microdialysis and
electrophysio-logical studies in the rat brain. Neuropsychopharmacology 15:349 –360.
Schechter LE, Bolanos FJ, Gozlan H, Lanfumey L, Haj-Dah-mane S, Laporte AM, et al (1990): Alterations of central serotoninergic and dopaminergic neurotransmission in rats chronically treated with ipsapirone: Biochemical and electro-physiological studies. J Pharmacol Exp Ther 255:1335–1347. Sharp T, Bramwell SR, Hjorth S, Grahame-Smith DG (1989): Pharmacological characterization of 8-OH-DPAT-induced in-hibition of rat hippocampal 5-HT release in vivo as measured by microdialysis. Br J Pharmacol 98:989 –997.
Sharp T, Hjorth S (1990): Application of brain microdialysis to study the pharmacology of the 5-HT1A autoreceptor. J
Neu-rosci Methods 34:83–90.
Sharp T, McQuade R, Bramwell S, Hjorth S (1993): Effect of acute and repeated administration of 5-HT1A receptor ago-nists on 5-HT release in rat brain in vivo. Naunyn
Schmiede-bergs Arch Pharmacol 348:339 –346.
Tada K, Kasamo K, Ueda N, Suzuki T, Kojima T, Ishikawa K (1999): Anxiolytic 5-hydroxytryptamine1A agonists suppress firing activity of dorsal hippocampus CA1 pyramidal neurons through a postsynaptic mechanism: Single-unit study in unanesthetized, unrestrained rats. J Pharmacol Exp Ther 288:843– 848.
Tome MB, Isaac MT, Harte R, Holland C (1997): Paroxetine and pindolol: A randomized trial of serotonergic autoreceptor blockade in the reduction of antidepressant latency. Int Clin
Psychopharmacol 12:81– 89.
Vinar O, Vinarova E, Horacek J (1996): Pindolol accelerates the therapeutic action of selective serotonin reuptake inhibitors (SSRI) in depression. Homeostasis 37:93–95.
Zanardi R, Artigas F, Franchini L, Sforzini L, Gasperini M, Smeraldi E, Perez J (1997): How long should pindolol be associated with paroxetine to improve the antidepressant response? J Clin Psychopharmacol 17:446 – 450.