A combination of classical and powerful new combinatorial genetic techniques allows the redesign of enzyme activities and creation of proteins that are tailored to have specific properties. These technologies have far-reaching
consequences for the future design of crop plants and the storage compounds within them.
Addresses
Department of Biology, Building 463, 50 Bell Avenue, Brookhaven National Laboratory, Upton, New York 11786, USA;
e-mail: [email protected]
Current Opinion in Plant Biology2000, 3:243–248
1369-5266/00/$ — see front matter. Published by Elsevier Science Ltd.
Abbreviations
ACP acyl-carrier protein
DIBOA 2,4-dihydroxy-1,4-benzoxazin-3-one
PCR polymerase chain reaction
Introduction
Gene transfer into all common crop species can now be achieved routinely. This progress raises the issue of which genes should be introduced to convey desired traits. Genomic sequencing efforts have provided a rich resource of genetic material from which genes encoding valuable traits can be selected [1]. Nevertheless, even with this ever-increasing resource, genes with ideal properties may not be found. For instance, transferred genes may not perform in the transgenic plant as they did in the plant from which they were taken, and there-fore the phenotype of interest may not be conferred to the transgenic plant. Such was the case when the 16:0-∆4-desaturase gene from coriander was transferred to tobacco. The accumulation of this unusual fatty acid was much smaller in tobacco than in coriander [2]. For nam-ing fatty acids, X:Y indicates that a fatty acid contains X carbons atoms and Y number of double bonds. ∆z indi-cates that a double bond is located at the z carbon atom relative to the carboxyl terminus of the fatty acid. A sec-ond problem might be that the available genes, though exhibiting functional diversity, might not include a gene that has ideal properties. An alternative approach to transferring naturally occurring genes into crops is to extract their information content and to use this to redesign desired properties, or to use them as starting points for combinatorial genetic manipulation to evolve a property of interest.
The intent of this review is to bring to the attention of plant biologists examples of approaches that have proven successful in protein redesign. Because of the broad scope of this review, readers are directed to a number of excellent reviews on specific topics.
Evolution of enzymatic plasticity
In the order of 103 individual protein folds (the core arrangement of secondary structural elements) are thought to occur in nature, far fewer than the number of individual proteins (which is closer to 105) [3]. This is because many proteins share common folds. Many proteins that have the same fold are presumed to have arisen from a common ancestor [4]; hence enzymes with the same fold perform a variety of reactions. An illustration of gene duplication and functional specialization was presented by Gierl and col-leagues [5,6] who reported on the biosynthetic pathway of the plant defense compound 2,4-dihydroxy-1,4-benzox-azin-3-one (DIBOA). They found that genes Bx2 through Bx5 encode cytochrome P450-dependent monooxygenas-es (P450s) that catalyze four consecutive hydroxylations and one ring expansion to form DIBOA. These P450s are thought to have arisen though gene duplication and the accumulation of mutations that led to a change in both substrate- and regio-specificity (i.e., the position on the substrate molecule that is modified). In another example from the family of membrane-bound di-iron enzymes [7,8•,9•], mutations affecting the desaturase reaction have given rise to at least four distinct enzymatic activities: hydroxylase, epoxidase, acetylenase and conjugase. New catalytic activities are now widely thought to evolve from pre-existing enzymes when a partial reaction, which is cat-alyzed by a progenitor enzyme, is retained but the architecture of the active-site of the enzyme is modified so as to allow the intermediate generated to be directed into the synthesis of different end-products [10].
Comparative structure–function analysis
Gene fusions can be used to identify domains within enzymes that control properties of interest. Once such a region is identified, site-directed mutants can be constructed and used to evaluate the contribution of individual amino-acid residues. Examples of the successful application of this approach include changing an acyl-ACP (acyl-carrier protein)Exploring the possibilities presented by protein engineering
John Shanklin
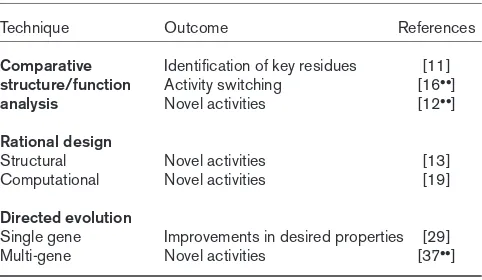
Table 1
Techniques for improving enzymes and the resulting information.
Technique Outcome References
Comparative Identification of key residues [11]
structure/function Activity switching [16••]
analysis Novel activities [12••]
Rational design
Structural Novel activities [13]
Computational Novel activities [19]
Directed evolution
Single gene Improvements in desired properties [29]
thioesterase with 12:0 specificity into one specific for 14:0 by substituting three amino acids [11], or increasing the 18:0-substrate preference of an acyl-ACP thioesterase up to 13-fold [12••] by site-specific mutagenesis. In a more com-plex example, both the chain length- and the regio-specificity of a 16:0-∆6-ACP desaturase was converted into predominantly an 18:0-∆9-ACP desaturase by substitut-ing five amino acids [13].
Key amino acids are most easily identified by this gene-fusion approach when the residues that contribute to the interesting properties reside in a linear domain within the sequence. Within the crystal structure of the 18:0-∆9-ACP desaturase [14], the determinants of acyl-chain length
(substrate) specificity and regiospecificity can be classified into two types: those amino acids that directly affect the substrate binding interface and those occupying positions remote from the binding cavity that must exert their effects indirectly [13]. The logical gene-fusion approach is better suited to structure–function studies than to efforts to redesign proteins. This is because the outcome of gene-fusion experiments is usually to define the positions of key residues that affect the property of interest rather than to facilitate the design of novel activities (Table 1). It is possi-ble, however, that information generated from gene-fusion experiments could subsequently be used, either alone [12••] or in conjunction with a combinatorial method [15], to generate novel activities.
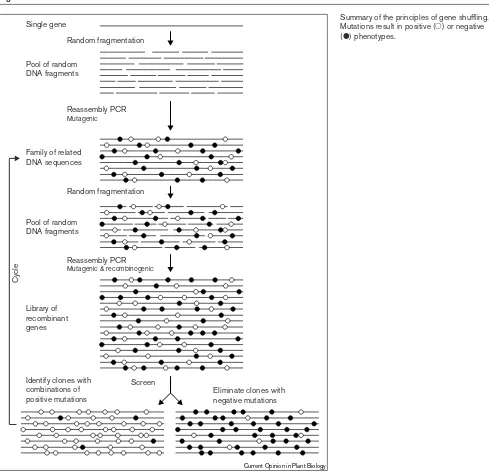
Figure 1
Summary of the principles of gene shuffling. Mutations result in positive (䊊) or negative
(䊉) phenotypes.
Eliminate clones with negative mutations Screen
Identify clones with combinations of positive mutations Library of recombinant genes
Reassembly PCR
Mutagenic & recombinogenic
Pool of random DNA fragments
Random fragmentation Family of related
DNA sequences
Reassembly PCR
Mutagenic
Pool of random DNA fragments
Random fragmentation Single gene
Cycle
A second logical approach is available when several homolo-gous amino acid sequences are associated with two distinct enzyme activities (paralogs). For example, Broun et al.[16••] compared the sequences of five related oleate desaturases with those of two independently-evolved oleate hydroxy-lases. Seven conserved positions within the five desaturases were identified at which the residues differed from those at equivalent positions in the two hydroxylases. Reciprocal changes between pairs composed of one of the desaturases and one of the hydroxylases were made at the seven sites, resulting in dramatic shifts in the ratio of desaturase to hydroxylase function, that is, away from the original enzyme activity and towards that of the alternate activity. An advan-tage of this logic-based approach is that the number, as well as the relative position, of key residues is unimportant.
Rational design
The application of protein crystallography to enzymes brought with it the expectation that enzymes could be ratio-nally designed to have desired properties. Although there are examples of successful rational design, such as the engi-neering of a novel 16:0-∆9-ACP desaturase [13], and the design of a thermolysin-like protease from Bacillus stearother-mophilus that has improved thermostability [17•], this method has, so far, generally failed to meet expectations. There are many reasons for this, one being that changes in the residues of the active-site often produce the desired results in terms of specificity, but at a large cost to catalytic rate. In addition, many residues that control key enzyme properties occupy positions that are distant from the active site and that are not readily identified by the examination of a crystal structure. Consequently, there has been a steady shift in the emphasis of enzyme redesign studies from ‘ratio-nal’ to ‘irratio‘ratio-nal’ (i.e., combinatorial) genetic approaches [18]. Despite this general trend, there is also renewed inter-est in a specific method of rational design, pioneered by Mayo and colleagues [19]. This method involves cycling between new computational methods and experiments [20]. Their computational approach identifies probable function-al solutions using sophisticated function-algorithms that eliminate dead-end design solutions [21].
Gene shuffling: directed evolution of single
genes
It is clear that many enzymes with very different proper-ties have evolved one from another in nature. Researchers have therefore explored the possibility of ‘directing evolu-tion’ or molecular breeding in the laboratory [22]. For instance, the Arnold laboratory at Caltech [23•] and the Stemmer group at the company Maxygen [24•] have devel-oped powerful methodologies for mimicking the process of natural evolution using isolated genes in the test tube. Evolution that would take eons in nature can now occur within days or weeks in the laboratory.
The strategy for evolving enzymes involves two steps (Figure 1). In the first step, random mutations are introduced into the target gene either by fragmentation followed by
reassembly or by error-prone PCR (polymerase chain reac-tion) [25,26]. Improved genes are then identified and isolated and, in a second step, are genetically recombined (i.e., shuffled), resulting in the generation of a new gene library that contains combinations of the mutations isolated in the first stage. Improved genes are again identified and isolated. The cycle, of in vitrorecombination followed by the identification of improved activity is typically repeated between five and seven times to evolve a final product. The rationale for performing iterative cycles is that mutations are rare (~1%), mutations that enhance the enzyme property of interest are therefore very rare, and combinations of benefi-cial mutations are consequently extremely rare. The cyclical process allows the sequential accumulation of positive muta-tions at the expense of negative or neutral mutamuta-tions.
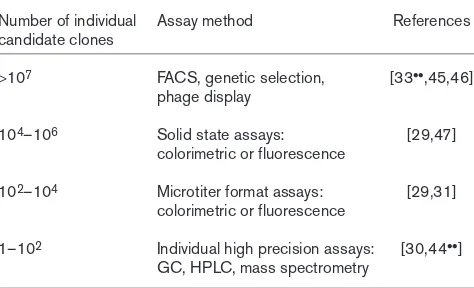
The process of molecular breeding of isolated genes has par-allels with conventional breeding at the organismal level. Indeed, there are molecular methods — such as backcross PCR, which mimics the process of backcrossing in conven-tional breeding — that can eliminate all but the positive amino-acid changes in much the same way as backcrossing removes detrimental traits during classical plant breeding [25]. One key to success in the use of single gene shuffling is the selection of a target gene that encodes a protein with properties as close as possible to the desired property so that the evolutionary distance to be traversed can be minimized [23•]. Another is to choose a selection or screening proce-dure that allows large numbers of recombinant genes to be evaluated. Perhaps the best way to achieve these goals is to create a tiered screening system that comprises a series of assays that are successively more accurate and time con-suming and that might reduce a population from 106or more to 104to 102candidates until the optimal solution is finally identified (Table 2).
This area of technology is still in its infancy and many of its applications are focused at present on enzymes that are
Table 2
Appropriate methods for tiered-screen selection of the best performing individual clones from large combinatorial populations.
Number of individual Assay method References candidate clones
>107 FACS, genetic selection, [33••,45,46] phage display
104–106 Solid state assays: [29,47]
colorimetric or fluorescence
102–104 Microtiter format assays: [29,31]
colorimetric or fluorescence
1–102 Individual high precision assays: [30,44••] GC, HPLC, mass spectrometry
used in industry rather than in plant biotechnology; how-ever, the principles that apply to altering the properties of enzymes are for the most part generic. I will therefore dis-cuss first several examples of the current application of this technology, and then, examples of its potential application to plant biotechnology.
Properties such as substrate- and regio-selectivity make enzymes desirable catalysts for the industrial synthesis of complex chemicals. Nevertheless, enzymes are not widely used in industrial processes because they are relatively unstable at elevated temperatures and in organic solvents. Directed evolution experiments are ideally suited to solve such ‘global property’ problems because selection pres-sures for the desired properties can be easily controlled. For instance, the Arnold group has used the protease sub-tilisin E as a model enzyme for the directed evolution of both solvent stability (in up to 60% dimethylformamide) [22,27] and thermal stability [28]. Great advances have also been made in the evolution of specific properties of enzymes and proteins. In experiments focused on sub-strate specificity, a fucosidase was evolved from a galactosidase [29], and with respect to stereochemical dis-crimination between substrate isomers, lipase enantioselectivity was improved from 2%–81% in favor of the S configuration at the expense of the R isomer [30]. These experiments focused on individual proteins that do not have complex cofactor requirements. In contrast, enzymes such as cytochrome P450s are components of complex electron transport chains, and therefore present a greater technical challenge to the protein engineer who would like to exploit their ability to perform the regiospe-cific insertion of oxygen into complex substrates.
Recently, Arnold and colleagues [31] devised an ingenious hydrogen-peroxide-mediated screen for the directed evolu-tion of the Pseudomonas putidaP450camenzyme. First, they were able to select a mutant P450 that had 20-times greater activity than the wild-type enzyme when using peroxide as an oxygen source, obviating the need for the complex redox chain. Second, coexpression of this mutant P450cam along with the enzyme horseradish peroxidase resulted in a screen that was used to isolate a variety of novel regiospecifically hydroxylated products [32••]. Experiments of this type will undoubtedly lead to the evolution of monooxygenases and dioxygenases capable of specifically hydroxylating a wide variety of aromatic compounds.
Multi-gene shuffling: accessing a larger
portion of sequence space
Although single gene shuffling is useful for evolving a partic-ular property by iteratively recombining point mutations, a logical extension of this procedure, in which two or more homologous genes are shuffled, has proven to be even more powerful [24•,33••]. In ‘family shuffling’, genes encoding enzymes that share the same fold, each representing a ‘suc-cessful’ but different evolutionary pathway, replace a single gene as the starting material. The procedure can be regarded
as ‘sexual PCR’ in that related genes are amplified under conditions that favor crossover or intergenic recombination [33••,34–36]. As the related enzymes share a common fold, chimeric polypeptides are likely to be functional because they can fold appropriately. Family shuffling reaches a far greater portion of sequence space (i.e., the number of possi-ble combinations and permutations) than can be reached by single gene shuffling [24•]. Family shuffling was first used to evolve moxalactamase activity from genes encoding cephalosporinases [33••]. Moxalactamase activity in the best chimera was encoded by elements from three of four parents, and was 270-times greater than that of the most active wild-type enzyme. Shuffling of two biphenol dioxygenases produced chimeras that had substrate specificities that were different from those of both parents; some of the chimeras were active even on single aromatic hydrocarbons [37••].
Application to crop improvement
The ability to tailor proteins and enzymes has potential for broad application in crop improvement with regard to both output and input traits. For example, enzymes that control output traits, such as fatty acid or starch composition, are desirable targets for the improvement of crop storage com-pounds. Alternatively, novel enzymes and pathways could be developed to facilitate the accumulation of new compounds that represent new sources of valuable industrial feedstocks. A third application might be to create enzymes that have abnormal allosteric activation or inhibition, as has the natural mutant of ADP glucose pyrophosphorylase that is used to modify starch accumulation in potato tubers [38]. If appro-priate amino-acid substitutions to create desirable protein variants can be identified from in vitro experiments, new methodologies involving chimeric RNA/DNA oligonu-cleotides have recently been developed to perform site-specific mutations of the target gene in vivo [39•,40•]. For input traits, families of proteins, for example, Bacillus thuringiensis proteins that are involved in defense against insects, could be recombined to produce anti-insecticidal agents that have increased selective toxicity towards targeted pests. Proteins that detect and/or direct responses to chang-ing environmental conditions, such as cold [41], heat and salinity [42], could be tailored to respond to particular thresholds for improved agronomic performance.
Conclusions
Update
A major focus in directed evolution has been to mimic the pathways of natural evolution as closely as possible [24•]. This involves the use of mutagenic techniques that, for the most part, rely on point mutagenesis that results in between four and seven of the 19 possible amino-acid sub-stitutions. Miyazaki and Arnold [43•] have started to explore the inclusion of non-natural substitutions to access a greater proportion of sequence space and to enhance con-ventional directed evolution experiments. They found that non-natural substitutions allowed significant improve-ment in the thermal stability of the protease subtilisin S41. Although some amino acids are essential to protein func-tion because of the chemical properties of their side chains, others may primarily function to occupy a particu-lar space in the structure. Thus, it seems quite logical that presenting a larger variety of molecular shapes would pre-sent the best likelihood of achieving the most energetical-ly favored structure. In this context, it might be appropriate to view amino acids as ‘molecular shims’.
The past decade has seen a movement away from rational (i.e., structure-based) design and towards the new and extremely powerful techniques of directed evolution [23•,24•]. Fersht and colleagues, however, recently pub-lished a landmark paper in which these two techniques were used synergistically to engineer a new catalytic activity, phosphoribosylanthranilate isomerase, from the α/β-barrel-scaffold enzyme indole-3-glycerol-phosphate synthase [44••]. Their strategy involved first performing a 'rough cut' by rational design and comparative sequence considerations, to create an intermediate structure that the designers thought would approximate the required final design. This rough-cut was then subjected to gene shuffling and improved enzymes were selected by (par-tial or complete) complementation of tryptophan auxotrophy. The work is important because it shows that rational design and directed evolution can be used as complementary tools in protein design. Also, because the α/β scaffold is present in approximately 10% of all pro-teins, Fersht’s work elegantly demonstrates that this particularly versatile scaffold can be redesigned to have new specifications.
Acknowledgement
This work was supported by the Office of Basic Energy Sciences of the US Department of Energy.
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest
••of outstanding interest
1. Kishore GM, Shewmaker C: Biotechnology: enhancing human nutrition in developing and developed worlds.Proc Natl Acad Sci USA1999, 96:5968-5972.
2. Cahoon EB, Shanklin J, Ohlrogge JB: Expression of a coriander desaturase results in petroselinic acid production in transgenic tobacco.Proc Natl Acad Sci USA1992, 89:11184-11188.
3. Zhang C, DeLisi C: Estimating the number of protein folds.J Mol Biol1998, 284:1301-1305.
4. Brenner SE, Chothia C, Hubbard TJ: Population statistics of protein structures: lessons from structural classifications.Curr Opin
Struct Biol1997, 7:369-376.
5. Frey M, Chomet P, Glawischnig E, Stettner C, Grun S, Winklmair A, Eisenreich W, Bacher A, Meeley RB, Briggs SP et al.: Analysis of a chemical plant defense mechanism in grasses.Science1997,
277:696-699.
6. Glawischnig E, Grun S, Frey M, Gierl A: Cytochrome P450 monooxygenases of DIBOA biosynthesis: specificity and conservation among grasses.Phytochemistry1999, 50:925-930.
7. van de Loo FJ, Broun P, Turner S, Somerville C: An oleate 12-hydroxylase from Ricinus communisL. is a fatty acyl
desaturase homolog.Proc Natl Acad Sci USA1995, 92:6743-6747.
8. Lee M, Lenman M, Banas A, Bafor M, Singh S, Schweizer M, • Nilsson R, Liljenberg C, Dahlqvist A, Gummeson PO et al.:
Identification of non-heme diiron proteins that catalyze triple bond and epoxy group formation.Science1998, 280:915-918. The authors use their knowledge of reaction chemistry and predicted simi-larities with desaturase enzymes to identify the genes that encode two desaturase-related enzymes. These enzymes introduce triple bonds and epoxy groups into fatty acids.
9. Cahoon EB, Carlson TJ, Ripp KG, Schweiger BJ, Cook GA, Hall SE, • Kinney AJ: Biosynthetic origin of conjugated double bonds:
production of fatty acid components of high-value drying oils in transgenic soybean embryos.Proc Natl Acad Sci USA1999,
96:12935-12940.
The authors successfully use a genomics approach to solve the longstand-ing biochemical problem of how conjugated double bonds are introduced into fatty acids.
10. Gerlt JA, Babbitt PC: Mechanistically diverse enzyme superfamilies: the importance of chemistry in the evolution of catalysis.Curr Opin Chem Biol1998, 2:607-612.
11. Yuan L, Voelker TA, Hawkins DJ: Modification of the substrate specificity of an acyl–acyl carrier protein thioesterase by protein engineering.Proc Natl Acad Sci USA1995, 92:10639-10643.
12. Facciotti MT, Bertain PB, Yuan L: Improved stearate phenotype in
•• transgenic canola expressing a modified acyl–acyl carrier protein thioesterase.Nat Biotechnol1999, 17:593-597.
Key amino-acid residues were manipulated to improve the substrate speci-ficity of mutant enzyme with respect to 18:0. This is a pioneering example of tailored enzymes being introduced into crop plants to improve their agronomic properties.
13. Cahoon EB, Lindqvist Y, Schneider G, Shanklin J: Redesign of soluble fatty acid desaturases from plants for altered substrate specificity and double bond position.Proc Natl Acad Sci USA
1997, 94:4872-4877.
14. Lindqvist Y, Huang W, Schneider G, Shanklin J: Crystal structure of delta9 stearoyl–acyl carrier protein desaturase from castor seed and its relationship to other di-iron proteins.EMBO J1996,
15:4081-4092.
15. Black ME, Newcomb TG, Wilson HM, Loeb LA: Creation of drug-specific herpes simplex virus type 1 thymidine kinase mutants for gene therapy.Proc Natl Acad Sci USA1996, 93:3525-3529.
16. Broun P, Shanklin J, Whittle E, Somerville C: Catalytic plasticity of
•• fatty acid modification enzymes underlying chemical diversity of plant lipids.Science1998, 282:1315-1317.
This paper describes the first example of work in which an enzyme was delib-erately altered so as to change the function of the enzymatic reaction from double-bond insertion to hydroxylation. This work provides a key step for-ward in generating enzymes that will put a desired functional group at a par-ticular location in a fatty acid.
17. Van den Burg B, Vriend G, Veltman OR, Venema G, Eijsink VG: • Engineering an enzyme to resist boiling.Proc Natl Acad Sci USA
1998, 95:2056-2060.
Comparative genetic and theoretical approaches are used to engineer a protease that is capable of functioning at 100°C in the presence of denaturing agents.
18. Arnold FH: When blind is better: protein design by evolution.Nat
Biotechnol1998, 16:617-618.
20. Street AG, Mayo SL: Computational protein design.Struct Fold Des1999, 7:105-109.
21. Gordon DB, Marshall SA, Mayo SL: Energy functions for protein design.Curr Opin Struct Biol1999, 9:509-513.
22. Chen K, Arnold FH: Tuning the activity of an enzyme for unusual environments: sequential random mutagenesis of subtilisin E for catalysis in dimethylformamide.Proc Natl Acad Sci USA1993,
90:5618-5622.
23. Arnold FH: Design by directed evolution.Acc Chem Res1998, • 31:125-131.
A persuasive argument for the use of directed evolution instead of rational design for changing the properties of enzymes in desired directions. This paper is especially helpful in identifying both the potential and the limitations of various techniques. This is a well-written personal account by a leader in the field of directed evolution.
24. Minshull J, Stemmer WP: Protein evolution by molecular breeding.
• Curr Opin Chem Biol1999, 3:284-290.
A review of protein evolution by molecular breeding by authors from the lead-ing company that develops and uses gene shuffllead-ing. A review of the meth-ods used to generate protein variants and the screening strategies used to identify variants of interest.
25. Stemmer WP: Rapid evolution of a protein in vitroby DNA shuffling.Nature1994, 370:389-391.
26. Fromant M, Blanquet S, Plateau P: Direct random mutagenesis of gene-sized DNA fragments using polymerase chain reaction.Anal
Biochem1995, 224:347-353.
27. You L, Arnold FH: Directed evolution of subtilisin E in Bacillus
subtilisto enhance total activity in aqueous dimethylformamide.
Protein Eng1996, 9:77-83.
28. Zhao H, Arnold FH: Directed evolution converts subtilisin E into a functional equivalent of thermitase.Protein Eng1999, 12:47-53.
29. Zhang JH, Dawes G, Stemmer WP: Directed evolution of a fucosidase from a galactosidase by DNA shuffling and screening.
Proc Natl Acad Sci USA1997, 94:4504-4509.
30. Reetz MT, Zonta A, Schimossek K, Liebeton K, Jaeger KE: Creation of enantioselective biocatalysts for organic chemistry by in vitro
evolution.Angew Chem Int Ed Engl1997, 36:2830-2833.
31. Joo H, Arisawa A, Lin Z, Arnold FH: A high-throughput digital imaging screen for the discovery and directed evolution of oxygenases.Chem Biol1999, 6:699-706.
32. Joo H, Lin Z, Arnold FH: Laboratory evolution of peroxide-mediated
•• cytochrome P450 hydroxylation.Nature1999, 399:670-673. Cytochrome P450s are one of the most versatile group of enzymes that mediate oxygen chemistry with respect to a variety of substrates. This paper describes a way to access the wealth of possible applications of this chemistry using direct-ed evolution by the development of a clever screen to identify divergent activities.
33. Crameri A, Raillard SA, Bermudez E, Stemmer WP: DNA shuffling of
•• a family of genes from diverse species accelerates directed evolution.Nature1998, 391:288-291.
The work described in this paper clearly demonstrates the power of family shuf-fling in the evolution of novel proteins that have vastly improved kinetic proper-ties. Family shuffling combines the natural evolution of successful solutions to a catalytic problem with access to a higher proportion of potentially successful recombinant events (i.e., improved coverage of relevant sequence space).
34. Shao Z, Zhao H, Giver L, Arnold FH: Random-priming in vitro
recombination: an effective tool for directed evolution.Nucleic
Acids Res1998, 26:681-683.
35. Zhao H, Giver L, Shao Z, Affholter JA, Arnold FH: Molecular evolution by staggered extension process (StEP) in vitro
recombination.Nat Biotechnol1998, 16:258-261.
36. Kikuchi M, Ohnishi K, Harayama S: Novel family shuffling methods for the in vitroevolution of enzymes.Gene1999, 236:159-167.
37. Kumamaru T, Suenaga H, Mitsuoka M, Watanabe T, Furukawa K: •• Enhanced degradation of polychlorinated biphenyls by directed
evolution of biphenyl dioxygenase.Nat Biotechnol1998,
16:663-666.
The authors demonstrate the applicability of gene shuffling to multi-subunit enzymes and to bioremediation. They report the discovery of some unexpected and useful substrate specificities with respect to polychlorinat-ed biphenyls (which are particularly resistant to catalytic degradation).
38. Stark DM, Timmerman KP, Barry GF, Preiss J, Kishore GM:
Regulation of the amount of starch in plant tissues by ADP glucose pyrophosphorylase.Science1992, 258:287-292.
39. Beetham PR, Kipp PB, Sawycky XL, Arntzen CJ, May GD: A tool for
• functional plant genomics: chimeric RNA/DNA oligonucleotides cause in vivogene-specific mutations.Proc Natl Acad Sci USA
1999, 96:8774-8778.
The authors report how in vivomutagenesis in dicotyledonous plants pro-vides both an exciting new opportunity to manipulate existing genes in their natural context and an interesting technology for crop design.
40. Zhu T, Peterson DJ, Tagliani L, St. Clair G, Baszczynski CL, Bowen B: • Targeted manipulation of maize genes in vivousing chimeric
RNA/DNA oligonucleotides.Proc Natl Acad Sci USA1999,
96:8768-8773.
The authors show that in vivomutagenesis [39•] also works in
monocotyle-donous plants.
41. Jaglo-Ottosen KR, Gilmour SJ, Zarka DG, Schabenberger O, Thomashow MF: ArabidopsisCBF1 overexpression induces COR genes and enhances freezing tolerance.Science1998,
280:104-106.
42. Lee JH, Van Montagu M, Verbruggen N: A highly conserved kinase is an essential component for stress tolerance in yeast and plant cells.Proc Natl Acad Sci USA1999, 96:5873-5877.
43. Miyazaki K, Arnold FH: Exploring nonnatural evolutionary pathways
• by saturation mutagenesis: rapid improvement of protein function. J Mol Evol 1999, 49:716-720.
The authors demonstrate that non-natural amino-acid substitutions can sig-nificantly improve enzyme activity.
44. Altamirano MM, Blackburn JM, Aguayo C, Fersht AR: Directed
•• evolution of new catalytic activity using the alpha/beta-barrel scaffold. Nature 2000, 403:617-622.
This paper describes a tour de force of enzyme redesign that clearly demon-strates the power of combining the best of rational design with directed evo-lution. The authors also demonstrate that the TIM-barrel fold can be redesigned to perform quite different chemistries.
45. Naki D, Paech C, Granshaw G, Schellenberger V: Selection of a subtillisin-hyperproducing Bacillus in a highly structured environment. Appl Microbiol Biotechnol 1998, 49:290-294.
46. Gao C, Lin CH, Lo CHL, Mao S, Wirsching P, Lerner RA, Janda KD:
Making chemistry selectable by linking it to infectivity. Proc Natl
Acad Sci USA 1997, 94:11777-11782.
47. Moore JC, Arnold FH: Directed evolution of a para-nitrobenzyl esterase for aqueous-organic solvents. Nat Biotechnol 1996,