Cell wall-bound phenolics in cells of maize (
Zea mays
, Gramineae)
and buckwheat (
Fagopyrum tataricum
, Polygonaceae) with
different plant regeneration abilities
Vera V. Lozovaya
a,b,*, Tatyana A. Gorshkova
b, Natalja I. Rumyantseva
b,
Alexander V. Ulanov
b, Al’fya I. Valieva
b, Elena V. Yablokova
b, Chuansheng Mei
a,
Jack M. Widholm
aaDepartment of Crop Sciences,Uni6ersity of Illinois,ERML,1201W.Gregory,Urbana,IL61801,USA bInstitute of Biology of the Russian Academy of Sciences,P.O.Box30,420503Kazan,Russia
Received 4 August 1999; received in revised form 25 August 1999; accepted 26 October 1999
Abstract
Six different tissue types were used in these studies: both regenerable and nonregenerable calli of different morphotypes of buckwheat (Fagopyrum tataricum, Polygonaceae) and maize (Zea mays, Gramineae) inbred Pa91 as well as two maize inbred cultures, H99-R and H99-NR, with similar compact (embryogenic-like) structure but different regeneration abilities. The lignin levels measured by the thioglycolic acid method in the lines with different regeneration abilities 3 weeks after subculture were somewhat higher in regenerable than in nonregenerable calli. The total amount of wall bound phenolic acids in buckwheat (dicotyledonous plant) was similar to that of maize (monocotyledonous plant), but the maize cell walls contained a much higher proportion of esterified phenolic acids than the buckwheat cell walls. The buckwheat had almost equal amounts of ester and ether linked phenolic acids, while the maize cell walls had 90% or more esterified linkages. Much higher amounts of hydroxycinnamic acids are bound to the cell walls of regenerable compared with the nonregenerable buckwheat and Pa91 calli; ferulic and
p-coumaric acids in maize, and ferulic and sinapic acids in buckwheat. Room temperature alkaline treatment released almost all of the ether linked sinapic acid and about 75 – 80% of the total etherified ferulic acid from the buckwheat cell walls without breaking the ether linkages. The hydroxycinnamic acid contents were very similar in the two maize inbred H99 lines with similar morphology but different plant regeneration potential. These results indicate that wall bound phenolics, in particular ferulic acid, may be associated with a certain tissue morphological structure, that is necessary but not sufficient for plant regeneration since additional factors seem to be required for morphogenesis in vitro. © 2000 Elsevier Science Ireland Ltd. All rights reserved.
Keywords:Fagopyrum tataricum;Zea mays; Polygonaceae; Gramineae; Cell wall; Phenolics; Plant regeneration
www.elsevier.com/locate/plantsci
1. Introduction
The significance of cell wall biochemical and structural alternations in plant development has been emphasized recently in many reports [1 – 6]. The cell wall is now considered to be a vital organelle, with a composition that can change during the cell growth cycle, during growth and
development and be affected by environmental conditions and physiologically active compounds. Cell walls also contain latent regulatory molecules that can trigger and regulate developmental events. Thus the changes in cell wall metabolism needs to be investigated to understand the control of plant morphogenesis and growth.
The regulation of growth and differentiation in plants has been studied mainly in connection with alternations in cellulose [7 – 9] and wall matrix polysaccharides [3,5,10 – 16] with little research
* Corresponding author. Fax: +1-217-333-4777.
E-mail address:lozovaya@uiuc.edu (V.V. Lozovaya)
dedicated to the role of wall-bound phenolic
com-pounds in plant development. Plant cell and
tis-sue cultures provide an experimental system for the study of cell wall involvement in the communi-cation between different cells and tissues as well as in the growth and differentiation of cells and plant development. Usually studies of morphogenic pro-cesses in vitro are performed with callus tissues, where development is controlled by the culture medium as well as by the potential for regenera-tion of the callus lines. Some cultures lose their ability to regenerate plants regardless of the cul-ture medium composition usually after an ex-tended time in culture [5,17,18]. Thus regenerable and nonregenerable calli provide an appropriate experimental system to study the mechanism of morphogenesis in vitro.
Studies of the role of cell wall pectins and proteins in intracellular attachment and morpho-genesis of embryogenic and nonembryogenic cul-tured carrot cells were carried out [5,16], but the role of wall bound phenolics was not discussed. However, Kato et al. [19] reported that feruloyl and diferuloyl esters between polysaccharides af-fect aggregation in rice suspension cultures. Some phenolic acid derivatives were suggested to be regulators of cell expansion and division since they can mimic the effects of cytokinins [20,21]. Alter-ations in phenolic acids, lignin and peroxidase activity were found to be related to the potential of sessile oak somatic embryos to convert into plants [22]. We have shown that regenerable tis-sues contain much higher levels of vanillin — presumably produced from ferulic acid by the
CuSO4– NaOH oxidation treatment of the cell
walls — than nonregenerable tissues [23]. We also have reported that the differences in cell wall phenolic compounds correlate with the develop-mental capability of callus tissues of various spe-cies [23,24]. These results indicate that the ferulic acid levels may be critical for calli regeneration ability.
Here we report that the total amounts of cell wall bound phenolic acids are similar in a grami-naceous and a polygogrami-naceous species, however, their linkages are quite different. Large differences were found in ferulic acid levels in the cell walls of buckwheat and maize regenerable and nonregener-able calli with typical morphological structure but not in maize with the same morphological structure.
2. Materials and methods
2.1. Plant material
The callus lines of maize (Zea mays L.,
Gramineae) used in this study were initiated from immature embryos resulting from self-pollination using the procedure described by Duncan et al. [25]. All cultures have been maintained by subcul-turing onto fresh D medium approximately every 3 weeks and incubated in the dark at 28°C. The age of the callus as determined by the initiation date varied from 8 – 12 months for inbred Pa91-R and Pa91-NR, 12 – 16 months for inbred H99-R, to over seven years for H99-NR.
Buckwheat (Fagopyrum tatricum, Gaertn.,
Polygonaceae, the single family comprising the order Polygonales of the subclass Caryophyllidae) calli were initiated from immature embryos and were cultivated on RX medium [26], which
con-sists of B5 mineral salts [27] and 2 mg/l thiamine –
HCl, 1 mg/l pyridoxine – HCl, 1 mg/l nicotinic
acid, 100 mg/l myo-inositol, 2 mg/l
2,4-dichlorophenoxyacetic acid, 0.5 mg/l
naphthale-neacetic acid, 0.2 mg/l kinetin, 2g/l yeast extract,
25 g/l sucrose and 8 g/l agar, pH 5.5 – 5.6.
The regenerable and nonregenerable callus was identified by morphology, color and texture as described by Duncan et al. [25] for maize and Rumyantseva et al. [26] for buckwheat. Regenera-tion was via both embryogenesis and organogene-sis. This ability was checked periodically for confirmation of the evaluation.
2.2. Cell wall isolation and fractionation
Cell walls were isolated from homogenized plant material by a series of intracellular content extrac-tions with phosphate buffer (pH 6.0), ethanol, water and acetone as described [28]. A portion of
the cell wall sample was oxidized with CuSO4–
NaOH as described previously [29], while a similar sample (50 – 100 mg) was fractionated by extrac-tion with 8 ml 1 M NaOH for 24 h at room temperature. The supernatant was collected after
centrifugation at 2500×g for 10 min, transferred
to another tube and acidified with concentrated HCl to pH 1. The precipitate formed after
acidifi-cation was pelleted by centrifugation (2500×gfor
extract was acidified to pH 1 with concentrated HCl. If a precipitate formed during acidification it was treated similarly to the previous one and was
subjected to CuSO4– NaOH oxidation. The cell
wall residue after 1 M NaOH extraction was washed twice with water, dried and extracted with 4 M NaOH at 170°C for 2 h in stainless steel vessels. All acidified supernatants were extracted with diethyl ether and the organic phase was used for GC analysis. The samples were dried and
stored overnight under vacuum over CaCl2. From
30 to 100 ml of Sigma-Sil-A (Sigma, USA) were
added for 2 h and silyated derivatives were imme-diately applied to the GC (Chrom 5, Laboratorni
Pristroje, Praha) glass column (250×3 mm) filled
with 4% CE52 on Chromosorb W (100 – 120 mesh). The column temperature was programmed as follows: 145°C for 10 min, from 145 to 190°C at
2°/min, from 190 to 235°C at 5°/min and then
constant at 235°C with a detector temperature (flame-ionization) of 240°C, injector temperature
170°C and a helium gas flow of 30 ml/min.
There were two to four GC analyses done for the samples, obtained in two to three independent experiments. Lignin was assayed quantitatively by derivatization with thioglycolic acid of 10 mg of the cell wall materials as described by Bruce and West [30].
3. Results and discussion
Six different tissue types were used in these studies: both regenerable and nonregenerable calli
of different morphotypes of buckwheat (F.
tataricum) and maize (Z. mays inbred Pa91) as well as two maize cultures with similar compact (embryogenic-like) structure but different
regener-ation abilities — line H99-NR was maintained in culture since 1989 and lost plant regeneration abil-ity completely but did retain the morphology usu-ally associated with plant regeneration ability, while line H99-R initiated in 1996 was capable of forming plantlets on regeneration. The regenerable tissues of buckwheat and maize inbred line Pa91 consisted of compact embryogenic complexes sur-rounded by friable callus tissues that were nonre-generable. These tissues were separated for the analysis.
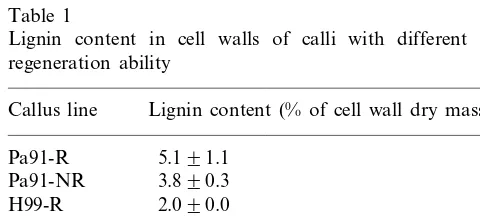
The amount of lignin was much higher in the cell walls of buckwheat cultures than in maize cultures (Table 1). There were no significant differ-ences in lignin levels measured by the thioglycolic acid method in the lines with different regenera-tion abilities 3 weeks after subculture, although regenerable calli tended to have slightly higher levels of lignin. These results are in agreement with our previous report [23] on histochemical staining
of buckwheat calli with KMnO4 (a fixative, which
primarily stains the lignin component of the cell wall according to Hepler et al. [31]). We found that the electron dense component in both types of calli was not quantitatively different but was local-ized differently on thin sections: mainly in the middle lamellae in nonregenerable callus and in the cell junctions in regenerable callus.
When wall bound hydroxycinnamic acids were extracted from callus cell walls with 1 M KOH at room temperature (esterified acids) and the residue subsequently with 4 M KOH at 170°C (etherified acids) according to Lam et al. [32] and analyzed by GC the total amount of wall bound phenolic acids in buckwheat (a dicotyledonous plant) was found to be similar to that of maize (a mono-cotyledonous plant) (Table 2). However, the maize cell walls contained a much higher proportion of esterified phenolic acids than the buckwheat cell walls. The buckwheat had almost equal amounts of ester and ether linked phenolic acids, while the maize cell walls had 90% or more esterified linkages.
Large differences in the levels of wall bound phenolics exists between regenerable and nonre-generable calli of buckwheat and maize Pa91. Much higher amounts of hydroxycinnamic acids are bound to the cell walls in regenerable calli of these lines (Tables 2 – 4); specifically ferulic and
p-coumaric acids in maize, and ferulic and sinapic
acids in buckwheat. Three-fold higher ferulic acid
Table 1
Lignin content in cell walls of calli with different plant regeneration ability
Table 2
Total phenolic acids bound to the cell wall via ester and ether linkages in maize and buckwheat calli
Ester (mg/100 g of cell wall Ether (mg/100 g of cell wall Regenerability
Plant type Total (mg/100 g of cell wall
dry mass) dry mass)
dry mass)
208 237
R 445
Buckwheat
34 113 147
NR
1695 64
R 1709
Maize Pa91
294
NR 5 299
574 50
R 624
Maize H-99
588 42
NR 630
Table 3
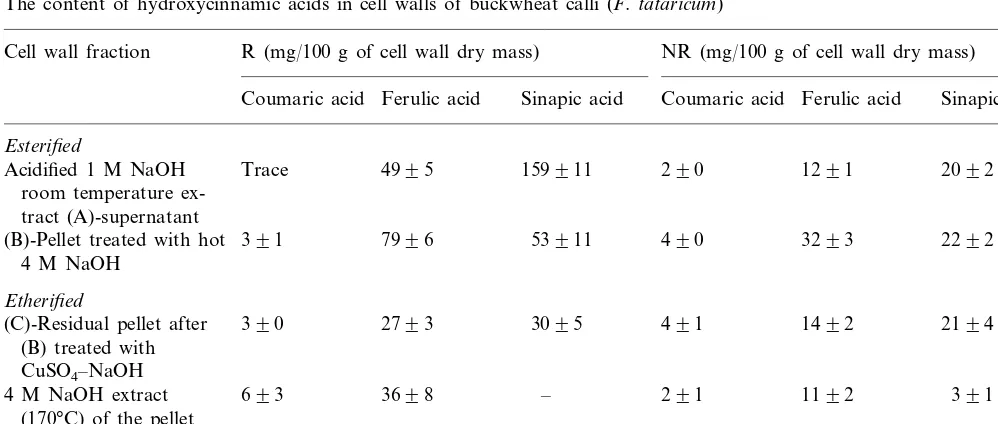
The content of hydroxycinnamic acids in cell walls of buckwheat calli (F.tataricum)
Cell wall fraction R (mg/100 g of cell wall dry mass) NR (mg/100 g of cell wall dry mass)
Ferulic acid Sinapic acid Coumaric acid
Coumaric acid Ferulic acid Sinapic acid
Esterified
2092 Acidified 1 M NaOH Trace 4995 159911 290 1291
room temperature ex-tract (A)-supernatant
391 7996 53911 490
(B)-Pellet treated with hot 3293 2292
4 M NaOH
Etherified
2194
390 2793 3095 491
(C)-Residual pellet after 1492
(B) treated with CuSO4–NaOH
391
693 3698 – 291
4 M NaOH extract 1192
(170°C) of the pellet after 1 M NaOH treatment
contents were reported recently for cell walls of sessile oak somatic embryos capable of converting into plantlets while nonconverting embryos had higher levels of cell wall bound sinapic acid [22]. Based on existing data with maize and buckwheat (presented here), oak [22] and strawberry tissue cultures [23], we can assume that ferulic acid may play a specific role in modification of cell wall structure and composition during development in vitro, since the ferulic acid level was higher in tissue cultures capable of regeneration in all the species studied. Ferulic acid may be involved in cross-linking the primary wall during differentia-tion through ester- and ether-bridges thus restrict-ing cell expansion.
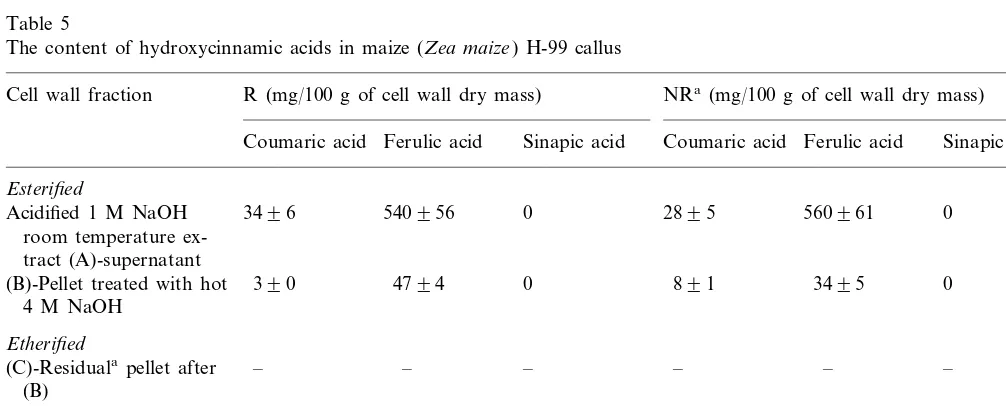
When wall phenolics were compared between two maize inbred H99 lines with similar morphol-ogy but different plant regeneration potential, very little difference was found both in lignin or
hot concentrated alkali treatment which breaks the ether linkages. In the present study we also found that a pellet was formed with the regenerable and nonregenerable buckwheat and H99 maize calli but not in the case of Pa91 (Tables 3 – 5). The amounts of ether-linked phenolic acids released by such treatment with buckwheat and H99 were higher than those found in the 4 M hot alkali extract. Room temperature alkaline treatment extracted
almost all of the ether linked sinapic acid and about 75 – 80% of total etherified ferulic acid found in the cell wall. The amount of both esterified and etherified wall-bound phenolic acids was much higher in regenerable than in nonregenerable buck-wheat callus. In maize calli the amounts of ether-bound phenolic acids (mainly, ferulic acid) found in the room temperature alkaline extract were less than one-tenth that of the ester-bound acids (Table 4).
Table 4
The content of hydroxycinnamic acids in cell walls of maize (Zea mays) Pa91 calli
Cell wall fraction R (mg/100 g of cell wall dry mass) NR (mg/100 g of cell wall dry mass)
Ferulic acid
Coumaric acid Ferulic acid Sinapic acid Coumaric acid Sinapic acid
Esterified
14329418
213934 0 1593 279925 0
Acidified 1 M NaOH room temperature ex-tract (A)-supernatant
– –
– –
– –
(B)-Pelletatreated with hot 4 M NaOH
Etherified
–
(C)-Residual pelleta after – – – – –
(B) treated with CuSO4–NaOH
6491
Trace Trace 591 0
4 M NaOH extract 0
(170°C) of the pellet af-ter 1 M NaOH treat-ment
aFraction was not formed.
Table 5
The content of hydroxycinnamic acids in maize (Zea maize) H-99 callus
R (mg/100 g of cell wall dry mass) NRa(mg/100 g of cell wall dry mass) Cell wall fraction
Ferulic acid Sinapic acid Coumaric acid Ferulic acid Sinapic acid Coumaric acid
Esterified
0 0
540956
3496 2895 560961
Acidified 1 M NaOH room temperature ex-tract (A)-supernatant
0 (B)-Pellet treated with hot 390 4794 0 891 3495
4 M NaOH
Etherified
–
(C)-Residualapellet after – – – – –
(B)
4 M NaOH extract Trace Trace 0 Trace Trace 0
(170°C) of the pellet af-ter 1 M NaOH treat-ment
Even though cell wall bound ferulic acid is considered to be a unique feature of grasses and some Caryophyllales family dicots [35,36], our re-sults show that it can also be a significant cell wall constituent of tissue cultures of buckwheat, a plant that belongs to the Polygonaceae dicot family. We also found ferulic acid in the cell walls of dicot plants including soybean leaves and cultured cells and flax stem tissues and buckwheat callus [29]. It should be noted that Bate-Smith [37] reported that the presence of significant amounts of sinapic acid in cell walls was confined to a few plant families. Transgenic plants and mutants that have altered phenolic metabolism have shown that there is significant plasticity in the wall phenolic biosyn-thesis in plants [38,39]. The possibility of
incorpo-ration of numerous phenolic precursors (in
addition to the three main monolignols) into the lignin molecule has been demonstrated [40 – 44]. Interestingly, the levels of wall bound phenolic acids can be regulated by gene manipulation. Thus cinnamoyl-CoA oxidoreductase depressed trans-genic tobacco lines had approximately 10-fold higher levels of ferulic acid in the cell walls along with dramatic decreases in lignin [45].
Based on our data we can conclude that the development of plant tissue in vitro is associated with the modification of cell wall phenolics. The common feature of such alterations is enhanced amounts of wall bound ferulic acid in regenerable tissues found in different plant materials (maize, buckwheat and strawberry (present study) and [23] and oak [22]). An increased level of ferulic acid in regenerable tissues is likely needed for the forma-tion of the compact tissue morphotype of calli capable of regeneration. However, other factors are also needed to allow plant development in vitro as seen with the two H99 maize callus lines of similar morphotype but different regenerability where the phenolic composition is not different. The nature of the linkages between ferulic acid and other cell wall constituents was different in the plant materials analyzed: in maize most of the phenolic acids were esterified to cell wall compo-nents, while in buckwheat both ester- and ether-wall bound phenolic acids were present. Further studies could elucidate if the same difference in the bonding of ferulic acids to the cell wall exists in
other monocotyledonous and dicotyledonous
plants and if esterified and etherified phenolic acids perform different functions in plants. The
possible biological role of cell wall bound phenolic acids in plant development processes remains to be elucidated, but the use of tissue cultures, trans-genic plants and mutants is providing material useful for initial basic studies.
Acknowledgements
This work was supported in part by funds from The Illinois Agricultural Experiment Station, US National Academy of Sciences Program and the Russian Foundation of Basic Research (grant 98-04-50020).
References
[1] F. Berger, A. Taylor, C. Brownlee, Cell fate determina-tion by the cell wall in early Fucus development, Science 263 (1994) 1421 – 1423.
[2] F. Liners, T. Gaspar, V. Cutsem, Acetyl- and methyl-es-terification of pectins of friable and compact sugar-beet calli: consequences for intercellular adhesion, Planta 192 (1994) 545 – 556.
[3] S.C. Fry, Polysaccharide-modifying enzymes in the plant cell wall, Ann. Rev. Plant Physiol. Plant Mol. Biol. 46 (1995) 497 – 520.
[4] A.J. Fleming, S. McQueen-Mason, T. Mandel, P. Kulle-meier, Induction of leaf primordia by the cell wall protein expansion, Science 276 (1997) 1415 – 1418. [5] S. Satoh, Functions of the cell wall in the interactions of
plant cells: analysis using carrot cultured cells, Plant Cell Physiol. 39 (1998) 361 – 368.
[6] U.-D. Yeo, M. Kohmura, N. Nakagawa, N. Sakurai, Quantitative and qualitative changes of cell wall polysac-charides during somatic embryogenesis and plantlet de-velopment of asparagus (Asparagus officinalis L.), Plant Cell Physiol. 39 (6) (1998) 607 – 614.
[7] D.J. Cosgrove, How do plant walls extend?, Plant Phys-iol. 102 (1993) 1 – 6.
[8] D.P. Delmer, Y. Amor, Cellulose biosynthesis, Plant Cell 7 (1995) 987 – 1000.
[9] A.E. Vasil’ev, Comparison of structure and functions of cytoskeleton between animals and higher plants, Russ. J. Gen. Biol. 57 (1996) 293 – 325.
[10] P. Albersheim, A.G. Darvill, Oligosaccharins, Sci. Am. 253 (1985) 44 – 50.
[11] K. Tran Than Van, S. Mutaftschiev, Signals influencing cell elongation, cell enlargement, cell division and mor-phogenesis, in: H.J.J. Nikamp, L.H.W. Van der Plas, J. Aarriyk (Eds.), Progress in Plant Cellular and Molecular Biology, Kluwer, Dordrecht, 1990, pp. 504 – 513. [12] C. Aldington, S.C. Fry, Oligosaccharins, in: Advances in
[13] G.P. Bolwell, Dynamic aspects of the plant extracellular matrix, Int. Rev. Cytol. 146 (1993) 261 – 324.
[14] V.V. Lozovaya, O.A. Zabotina, N.I. Rumyantseva, R.G. Malihov, M.V. Zihareva, Stimulation of root develop-ment on buckwheat thin cell-layer explants by pectic fragments from pea stem cell walls, Plant Cell Rep. 12 (1993) 530 – 533.
[15] A. Kikuchi, S. Satoh, N. Nakamura, T. Fujii, Differ-ences in pectic polysaccharides between embryogenic and non-embryogenic calli, Plant Cell Rep. 14 (1995) 279 – 284.
[16] R. Pennell, Cell walls: structures and signals, Curr. Opin. Plant Biol. 1 (1998) 504 – 510.
[17] F.C. Steward, M.O. Mapes, K. Mears, Growth and organized development of cultured cells. II Organization in cultures grown from freely suspended cells, Am. J. Bot. 45 (1958) 705 – 708.
[18] S. Satoh, M. Kamada, M. Harada, T. Fujii, Auxin-con-trolled glycoprotein release into the medium of embryo-genic carrot cells, Plant Physiol. 81 (1986) 931 – 933. [19] Y. Kato, H. Yamanouchi, K. Hinata, C. Ohsumi, T.
Hayashi, Involvement of phenolic esters in cell aggrega-tion suspension cultured rice cells, Plant Physiol. 104 (1994) 147-152.
[20] A.N. Binns, R.H. Chen, H.N. Wood, D.H. Lynn, Cell division promoting activity of naturally occurring dehy-drodiconiferyl glucosides: do cell wall components con-trol cell division?, Proc. Natl. Acad. Sci. USA 84 (1987) 980 – 984.
[21] D.G. Lynn, R.H. Chen, K.S. Manning, H.N. Wood, The structural characterization of endogenous factors from
Vinca roseacrown gall tumors that promote cell division of tobacco cells, Proc. Natl. Acad. Sci. USA 89 (1987) 615 – 619.
[22] M. Cvikrova´, J. Mala´, J. Eder, M. Hrubcova´, M. Vagner, Abscisic acid, polyamines and phenolic acids in sessile oak somatic embryos in relation to their conver-sion potential, Plant Phys. Biochem. 36 (3) (1998) 247 – 255.
[23] V. Lozovaya, T. Gorshkova, E. Yablokova, et al., Callus cell wall phenolics and plant regeneration ability, J. Plant Physiol. 148 (1996) 711 – 717.
[24] D.R. Duncan, J.M. Widholm, Differential response to potassium permanganate of regenerable and of non-re-generable tissue cell walls from maize callus cultures, Plant Sci. 61 (1989) 291 – 301.
[25] D.R. Duncan, M.E. Williams, B.E. Zehr, J.M. Widholm, The production of callus capable of plant regeneration from immature embryos of numerous Zea mays geno-types, Planta 165 (1985) 322 – 332.
[26] N.I. Rumyantseva, N.V. Sergeeva, L.E. Khakimova, V.V. Salnikov, E.A. Gumerova, V.V. Lozovaya, Organo-genesis and somatic embryoOrgano-genesis in tissue culture of two buckwheat species, Russ. Plant Phys. 36 (1989) 187 – 194.
[27] O.L. Gamborg, R.A. Miller, K. Ojima, Nutrient require-ments of suspension cultures of soybean root cells, Exp. Cell Res. 50 (1968) 151 – 158.
[28] K.W. Talmadge, K. Keegstra, W.D. Bauer, P. Alber-sheim, The structure of the plant cell walls, 1. The
macromolecular components of the pectic polysaccha-rides, Plant Physiol. 51 (1973) 158 – 173.
[29] V.V. Lozovaya, T.A. Gorshkova, E.V. Yablokova, et al., Cold alkali can extract phenolic acids that are ether linked to cell wall components in dicotyledonous plants (buckwheat, soybean and flax), Phytochemistry 50 (1999) 395 – 400.
[30] R.J. Bruce, C.A. West, Elicitation of lignin biosynthesis and isoperoxidase activity by pectic fragments in suspen-sion cultures of castor bean, Plant Physiol. 91 (1989) 889 – 897.
[31] P.K. Hepler, D.E. Fosket, E.H. Newcomb, Lignification during secondary wall formation in Coleus: an electron microscopic study, Am. J. Bot. 57 (1970) 85 – 96. [32] T.B.T. Lam, K. Iiyama, B.A. Stone, Distribution of free
and combined phenolic acids in wheat internodes, Phyto-chemistry 29 (1990) 429 – 433.
[33] M.M. Yeoman, E. Forche, Cell proliferation and growth in callus cultures, Int. Rev. Cytol. Suppl. 11A (1980) 1 – 24.
[34] M.W. Bayliss, Chromosomal variation in plant tissues in culture, Int. Rev. of Cytol. Suppl. 11A (1980) 113 – 143. [35] P.J. Harris, M.R. Kelderman, M.F. Kendon, R.J. McKenzie, Monosaccharide compositions of unlignified cell walls of monocotyledons in relation to the occur-rence of wall-bound ferulic acid, Biochem. Syst. Ecol. 25 (1997) 167 – 179.
[36] R.J. Henry, P.J. Harris, Molecular distinction between monocotyledons and dicotyledons: more than a simple dichotomy, Plant Mol. Biol. Rep. 15 (1997) 216 – 218. [37] E.C. Bate-Smith, The phenolic constituents of plants and
their taxonomic significance, J. Linn. Soc. (Bot.) 58 (1962) 95 – 173.
[38] N.G. Lewis, A 20th century roller coaster ride: a short account on lignification, Curr. Opin. Plant Biol. 2 (1999) 153 – 162.
[39] R.R. Sederoff, J.J. MacKay, J. Ralph, R.D. Hatfield, Unexpected variation in lignin, Curr. Opin. Plant Biol. 2 (1999) 145 – 152.
[40] A.M. Boudet, C. Lapierre, J. Grima-Pettenati, Tansley Review No. 80, Biochemistry and molecular biology of lignification, New Phytol. 129 (1995) 203 – 236.
[41] A.M. Boudet, D.P. Goffner, J. Grima-Pettenati, Lignins and lignification: recent biochemical and biotechnologi-cal developments, C.R. Acad. Sci. Paris Life Sci. 319 (1996) 317 – 331.
[42] M.M. Campbell, R.R. Sederoff, Variation in lignin con-tent and composition, Plant Physiol. 110 (1996) 3 – 13. [43] R.A. Dixon, C.J. Lamb, S. Masoud, V.J.H. Sewalt, N.L.
Paiva, Metabolic engineering: prospects for crop im-provement through the genetic manipulation of phenyl-propanoid biosynthesis and defense responses — a review, Gene 179 (1996) 61 – 71.
[44] M. Baucher, B. Monties, M. Van Montagu, W. Boerjan, Biosynthesis and genetic engineering of lignin, Crit. Rev. Plant Sci. 17 (1998) 125 – 197.
[45] J. Piquemal, C. Lapierre, K. Myton, et al., Down-regula-tion of cinnamoyl-CoA reductase induces significant changes in lignin profiles in transgenic tobacco plants, Plant J. 13 (1998) 71 – 83.