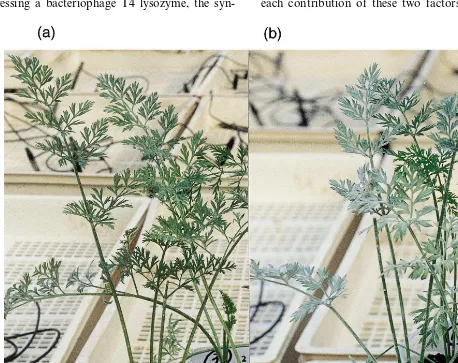
Transgenic carrots with enhanced resistance against two major
pathogens,
Erysiphe heraclei
and
Alternaria dauci
Miyuki Takaichi, Kenji Oeda *
Biotechnology Laboratory,Sumitomo Chemical Co.Ltd.,2-1,4-chome,Takatsukasa,Takarazuka,Hyogo665-8555,Japan
Received 17 June 1999; accepted 3 December 1999
Abstract
In vitro assay indicated that the human lysozyme has lytic activity against phytopathogenic fungi and bacteria. A human lysozyme gene was placed under control of the constitutive CaMV 35S promoter and the resulting expression plasmid was introduced into two cultivars (cv.) of carrot, Kurodagosun (K5) and Nantes Scarlet (NS), byAgrobacterium tumefaciens-mediated method. Seven and fourteen transgenic plants of cv. K5 and cv. NS were regenerated, respectively, and the obtained transgenic carrots of T0 generation was tested for disease resistance againstErysiphe heraclei, a pathogenic fungi causing powdery mildew. Among the tested lines, the transgenic plant No. 12-1 and 8-1 of cv. NS showed a fairly strong resistance againstE.heraclei. The strong disease resistance was also confirmed in T1 generation. Disease resistance against another pathogen of leaf blight, Alternaria dauci, were also tested using T1 transgenic lines. Significant enhanced resistance was observed in the No. 12-1 of cv. NS. Accumulation of synthesized human lysozyme protein was observed in this line, a finding consistent with observed disease resistance. © 2000 Elsevier Science Ireland Ltd. All rights reserved.
Keywords:Human lysozyme; Transgenic carrots;Agrobacterium tumefaciens; Disease resistance
www.elsevier.com/locate/plantsci
1. Introduction
Development of recombinant DNA techniques and plant genetic transformation methods facili-tate introduction of selected genes into plants to obtain transgenic ones with novel phenotypes. A variety of transgenic plants exhibiting disease re-sistance, herbicide resistance [1], delayed ripening [2], and alteration of protein composition in seeds [3] has been generated and some were tested in the field trials. Disease resistance is one of the impor-tant targets of plant breeding, because significant yield losses due to pathogenic attack limits crop productivity. Therefore, new disease control strategies to produce transgenic plants exhibiting enhanced disease resistance are now being widely
evaluated. Viral coat protein genes can be used to generate transgenic plants exhibiting virus resis-tance [4]. The cecropin gene was utilized to gener-ate disease resistant plants because cecropin contains antibacterial activity against several bac-terial pathogens [5]. Both glucanase and chitinase also exhibited antifungal activity. Transgenic plants of tobacco [5 – 7], rice [8], cucumber [9,10] expressing glucanase or chitinase genes exhibited enhanced disease resistance against fungal pathogens.
Lysozymes are basic bacteriolytic proteins widely distributed in nature. A protective and defensive role against bacterial or fungal patho-gens has been suggested for lysozymes of plant origin [11], and these lysozymes were purified from latex of papaya [12], fig [13], cultured Rubus hispiduscells [14], cultured Parthenocisis quinquifo
-liacells [15], turnip roots [16] and wheat germ [11]. Lysozymes from human [17] and hen egg [18] were also purified, and both lysozymes exhibited
bacte-Abbre6iations: CSS, chitinase signal sequence; cv., cultivar; GUS,
b-glucuronidase gene; NPT II, neomycin phosphotransferase gene. * Corresponding author. Tel.: +81-797-742121; fax: + 81-797-742133.
E-mail address:[email protected] (K. Oeda)
riolytic function. Human lysozyme cleaves the b -(1 – 4) glycosidic bond of peptidoglycan in the bacterial cell wall and of chitin in the fungal cell wall, other classes of animal lysozymes cannot hydrolyze chitin [19]. Therefore, the human lysozyme might have potential to protect plants from both bacterial and fungal diseases.
In the present work, the gene cassette coding for human lysozyme [20] and a chitinase signal se-quence (CSS) of Azuki bean [21] was placed under a strong constitutive CaMV 35S promoter, to obtain the transgenic plants expressing the human lysozyme. The resulting construct was introduced into two different cultivars of carrot, Kurodago-sun (K5) and Nantes Scarlet (NS). More than 20 transgenic carrots were regenerated from embryo-genic calli and they were raised to mature plants. The obtained transgenic plants were tested for disease resistance against Erysiphe heracle (pow-dery mildew) and Alternaria dauci (leaf blight). Consequently, transgenic carrots of cultivar (cv.) K5 and cv. NS showed enhanced disease resistance against these pathogens. Especially, the No. 12-1 of cv. NS showed distinct disease resistance.
2. Materials and methods
2.1. In 6itro assay against phytopathogens
Fungal pathogens, Rhizoctonia and Alternaria, bacterial ones, Erwinia caroto6ora and Pseu
-domonas syringae, were used to test the effect of human lysozyme (Sigma, USA). Rhizoctonia or
Alternaria spores were inoculated on agar plates composed of PDA medium (Nissui, Japan) supple-mented with 0, 30 and 100 mg/l of human lysozyme, respectively. Each hypha length was measured after 24 h.
Bacterial cultures (50 ml) of E. caroto6ora or P.
syringae, were prepared by dilution of overnight culture, and mixed with the equal volume (50 ml) of 0, 30, 100 mg/l of human lysozyme solution, respectively. These mixtures were incubated for 1 h at room temperature, and spread on agar plates composed of PDA medium. The number of colonies was measured after 24 h.
2.2. Plant materials
Carrot (Daucus carota L.) cultivars used in this study are K5 and NS. Seeds were surface-sterilized with 70% ethanol for 30 s and disinfected with 1% chloric acid for 10 min. The seeds were rinsed 3 times in sterile distilled water and then germinated on modified MS medium (half strength Murashige and Skoog [22] salts, 2 g/l sucrose and 0.8% agar) at 25°C in the dark. Hypocotyls were dissected from 1-week-old seedlings and cut into segments of 5 – 10 mm long. The explants were used for transformation experiments of carrots.
2.3. Agrobacterium tumefaciens strains and the used plasmid
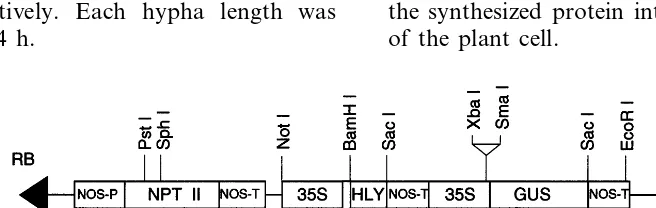
A. tumefaciens strains LBA4404 and C58C1 containing the binary plasmid pNGL2 were used to transform carrots. The plasmid pNGL2 consists of a neomycin phosphotransferase gene (NPT II), a b-glucuronidase gene (GUS) and the CaMV 35S promoter fused to a human lysozyme gene. The gene fragment coding for the CSS of Azuki bean [21] was also placed upstream of the human lysozyme gene (Fig. 1). The signal sequence is thought to be useful for efficient transportation of the synthesized protein into the intercelluar space of the plant cell.
2.4. Transformation and regeneration
Explants were inoculated in the bacterial solu-tion for 5 min, then placed on the co-cultivasolu-tion medium which consists of MS salts, 4.5 mM 2,4-dichlorophenoxyacetic acid (2,4-D), 3 g/l sucrose and 0.8 g/l agar. This medium is basal to produce embryogenic callus. Infected explants were incu-bated in the dark at 20 – 21°C for 2 – 3 days.
After co-cultivation, explants were rinsed in the liquid basal medium containing 500 mg/l cefo-taxime, and then placed on the basal medium supplemented with 300 mg/l cefotaxime for 4 weeks to prevent bacterial growth. Then, the ex-plants were transferred to the basal medium con-taining 50 mg/l kanamycin and 100 mg/l cefotaxime for 4 – 6 weeks as the first selection. The obtained embryogenic callus exhibiting kanamycin resistance was subcultured on hormone free MS medium, without kanamycin. After 2 – 4 weeks, the seedlings germinated from embryogenic calli were transferred to MS medium containg 100 mg/l kanamycin, as the second selection. After co-cultivation, the cultures were placed under con-ditions of 16 h light/8 h dark at 25°C. Transgenic seedlings were regenerated from embryogenic calli and they were examined for disease resistance.
Insertion of the human lysozyme gene into the plant genome was confirmed by polymerase chain reaction (PCR) analysis and the transgenic carrots were acclimatized to soil and grown at 20 – 23°C in a greenhouse. Transgenic carrots with auxetic roots were transferred to a cold room and main-tained at 5°C for at least 2 months. After this exposure to a low temperature, transgenic carrots were bolted at 20 – 23°C and self-pollinated seeds were obtained.
2.5. PCR analysis
Genomic DNA was isolated from cultured car-rot leaves, using cetyltrimethyl ammonium bro-mide [23]. PCR for the human lysozyme gene was performed with the genomic DNA of carrot plants using the synthetic oligonucleotide primers. The primers of 5% -GAACGTTGTGAATTGGCCAG-3%and 5%-GTTTTGACAGCGGTTACGCC-3%was
designed to hybridize the 5% and 3% regions of human lysozyme gene, respectively. The 42 cycle reaction, which consists of heat denaturation (95°C), annealing (55°C) and extension (72°C),
was carried out, and the reaction mixture was subjected to 0.8% agarose gel electrophoresis. Spe-cific amplification of the 350 base pairs fragment indicates that the human lysozyme gene was in-serted into the genome of the transgenic carrot plants.
2.6. Western blot analysis
Young leaves of carrots grown in a greenhouse were homogenized in phosphoric buffer (0.1 M K2HPO4, 2.5 mM EDTA, 0.1% ascorbic acid, 1%
mercaptoethanol and 0.5 mM PMSF), and the crude extract was separated by centrifugation for 10 min at 4°C (15 000×g). The supernatant (10
mg total soluble protein) was subjected to 10% SDS-polyacrylamide gel electrophoresis, and the resolved proteins were transferred onto nitrocellu-lose membranes (Schleicher Schuell, Dassel Ger-many). The human lysozyme on the membrane was detected by enzyme-linked immunostaining. The membrane was exposed to anti-human lysozyme serum from a goat (Nordic Immunology, Tilburg, The Netherlands), then to alkaline phos-phatase conjugated antibody against goat IgG from a rabbit (Vector Laboratories, CA, USA). The human lysozyme on the membrane was visu-alized by reaction with 5-bromo-4-chloro-3-indole phosphate and nitro blue tetrazolium.
2.7. Biological test for resistance against Erysiphe heraclei
Among 23 plants of transgenic carrots, 21 were transferred to the soil, and finally, 13 plants grew to the same stage of development. After 1 month of acclimatization, the obtained transgenic carrots were examined for disease resistance against E.
heraclei, the phytopathogen of powdery mildew. The diseased carrots previously infected with E.
Table 1
Disease rating on powdery mildew
Disease developmental area (%) Disease rating
0 0
0.5 0–5
5–10 1
2 10–25
25–50 4
\50 8
assay [9]. Five plants for each line were grown in vitro up to the same developmental stage, the leaves were trimmed off, and petioles were cut into 8 cm in length. Four segments were prepared from one plant and used for the biological test. Petiole fragments from transgenic and non-transgenic plants were stood upright in actively growing colony layers of the fungal pathogen on PDA medium (Nissui, Japan). The fungal pathogen, which was precultured for 7 days after inoculation, was used. The lesion length developed from the base of the petiole fragment was measured after 14 days of incubation. Lesion length on each petiole was measured.
3. Results and discussions
3.1. Lytic acti6ity of human lysozyme against
phytopathogens
Fungal pathogen, Rhizoctonia and Alternaria, and bacterial ones, E. caroto6ora and P. syringae,
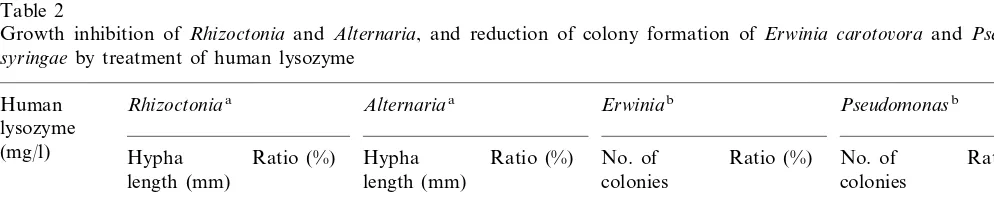
were used to test the effect of human lysozyme. Hypha length of Rhizoctonia and Alternaria were inhibited to 30 and 50% with 30 mg/l of human lysozyme, respectively (Table 2). Similarly, about 65 and 100% of bacteria, E. caroto6ora and P.
syringae, lyzed during 1 h incubation with 100 mg/l of human lysozyme, respectively (Table 2). These results indicated that the human lysozyme was effective against phytopathogenic bacteria and fungi [19].
T1 progeny were also examined for disease resis-tance against E. heraclei. Self-pollinated seeds derived from T0 plants were sowed on soil, and the germinated seedlings were examined by PCR. Seedlings without the lysozyme gene were dis-carded. Five to ten plants for each line were grown in a greenhouse. Four weeks later, these T1 proge-nies were infected with E. heraclei, and disease rating scored as described above. A disease index (%) was also calculated by the following formula, according to the method of the Agrochemical Society of Japan.
The disease index
={%(disease rating from 0 to 8)
/(8×a number of plants)}×100
2.8. Biological test for resistance against A. dauci
T1 lines of No. 12-1 and non-transgenic plants, were tested for disease resistance against another carrot pathogen,A.dauci, using a detached petiole
Table 2
Growth inhibition of Rhizoctoniaand Alternaria, and reduction of colony formation of Erwinia caroto6ora and Pseudomonas syringaeby treatment of human lysozyme
Pseudomonasb
Erwiniab
Alternariaa
Human Rhizoctoniaa
lysozyme
(mg/l) Hypha Ratio (%) Hypha Ratio (%) No. of Ratio (%) No. of Ratio (%)
length (mm) length (mm) colonies colonies
4.5a
100 100
11.0ac
0 100 236a 100 222a
30 3.5b 32 2.3b 51 111b 47 21b 9
82b
100 3.0b 27 2.0b 44 35 0b 0
aEach spore ofRhizoctoniaandAlternariawas inoculated on agar plates composed of PDA medium supplemented with human
lysozyme. The hypha length (mm) was measured after 24 h incubation.
bEach bacterial culture ofE.caroto6oraandP.syringae, and human lysozyme solution was mixed, and incubated for 1 h. These
mixtures were spread on agar plates composed of PDA medium.
Table 3
Experimental conditions for carrot transformation
Agrobacteriumstrain
Carrot cultiver No. of explants No. of transformants Transformation efficiency (%)
86
Kurodagosun LBA4404 1 1.2
90
C58C1 6 6.7
85 10
LBA4404 11.8
Nantes Scarlet
C58C1 99 6 6.1
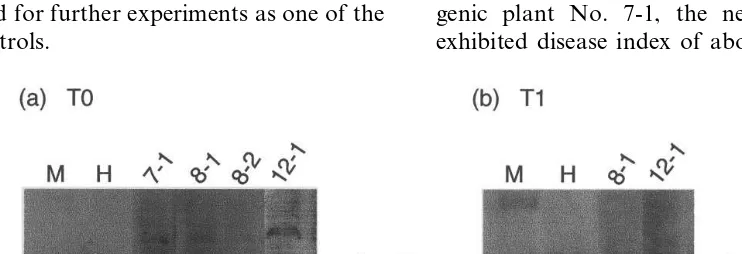
Fig. 2. Integration of the human lysozyme gene into the carrot genome. Integration of the human lysozyme gene into the carrot genome of T0 and T1 generation was confirmed by polymerase chain reaction (PCR) analysis. (a) Specific amplifications of 350 base pairs fragment of human lysozyme gene were indicated in all genomic DNA samples of T0 plants. (b) The 350 base pairs fragment was also detected in T1 plants. M was molecular marker. Plasmid pNGL2 was a positive control. All samples were cultivar (cv.) Nantes Scarlet (NS).
3.2. Regeneration and culti6ation of transgenic
carrots
The expression plasmid pNGL2 was constructed to express the human lysozyme gene in carrots (Fig. 1). The plasmid pNGL2 contains the CaMV 35S promoter and the human lysozyme gene. The obtained construct was introduced into two culti-vars of carrot, K5 and NS.
Both carrot cultivars and strains of A. tumefa
-ciens used for transformation procedure had con-siderable effects on the transformation efficiency of carrots (Table 3). When A. tumefaciens strain LBA4404 was used, only one transgenic plant was selected with cv. K5, while ten transgenic plants were obtained in the case of cv. NS. The calcu-lated transformation efficiencies were 1.2 and 11.8%, respectively. Six transgenic plants were ob-tained for both cv. K5 and cv. NS, respectively, when A. tumefaciens strain C58C1 was used. The efficiencies were estimated to be 6.7% and 6.1%, respectively. The A. tumefaciens-mediated method and electroporation [24] can be used for transfor-mation of carrots. However, the A. tumefaciens -mediated method is now widely used for carrot transformation [25 – 28], because this method is
simple and is economical of time. The efficiencies of transformation were, therefore, thought to de-pend on the combination of A. tumefaciensstrains and carrot cultivars. The other carrot cultivar ‘Nanco’ was often used as a major host cultivar because the transformation efficiency was reported to be highest among many cultivars, over 10% [25,27]. When cv. K5 and cv. NS were used in this experiment, the mean efficiency was calculated to be 6.4%, slightly lower than cv. Nanco.
Petiole, cotyledon, hypocotyl, root of carrot seedling and even callus could be used for trans-formation experiments, but the transtrans-formation efficiency was also greatly affected by used ex-plants and the developmental stage [25 – 28]. In this experiment, hypocotyls of 1-week-old seedlings were used as explants because the highest regeneration efficiency was obtained.
Table 4
The number of regenerated, acclimatized and self-pollinated transgenic carrots
No. of transformants Carrot
cultiver
Regeneration Acclimatiza- Self-pollination tion
Kurodagosun 7 7 5
16 14 10
Nantes Scarlet
Self-pollinated seeds were obtained from trans-genic carrots of cv. K5 and cv. NS, respectively. These seeds were germinated and five to ten seedlings for each line were grown in a green-house. T1 transgenic plants were also subjected to Western blot analysis to determine expression level of the human lysozyme. Especially, expression level was high and stable in samples of lines No. 8-1 and 12-1 of cv. NS. (Fig. 3b).
3.4. Resistance against pathogens
3.4.1. E. heraclei
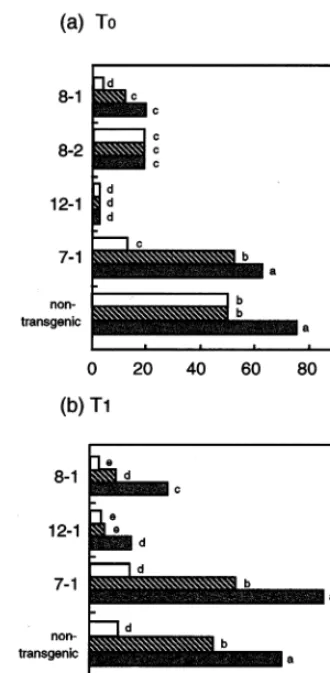
3.4.1.1. T0 generation. To observe disease resis-tance of the obtained transgenic carrots of cv. NS, the resistance against E. heraclei was measured. The disease rating was first measured 1 week after inoculation, to observe early symptom of diseased plants. The disease rating was successively mea-sured twice every week, and the development of powdery mildew was investigated. Symptoms in all transgenic plants were moderate (disease index lower than 20%) compared to that of non-trans-genic plants (disease index of 50%), when disease rating was scored 1 week after inoculation. How-ever, symptoms of powdery mildew developed on the upper leaves at a later stage, and the degree of symptoms varied among tested plants. When dis-ease rating was scored 3 weeks later after inocula-tion, transgenic carrots No. 8-1, 8-2 and 12-1 showed a disease index of 30%, while other trans-genic plants showed 50 – 80% of the disease index. At that time, non-transgenic plants and the trans-genic plant No. 7-1, the negative control line, exhibited disease index of about 80% (Fig. 4a). seeds were produced. Similarly, 14 transformants
of cv. NS were acclimatized to obtain mature plants. Finally, 10 independent transgenic plants of cv. NS self-pollinated and T1 seeds were pro-duced (Table 4). The amplified DNA bands were also observed in these T1 plants (Fig. 2b).
3.3. Western blot analysis
Mature plants of each line were subjected to Western blot analysis to evaluate accumulation of the synthesized human lysozyme.
A specific signal of the 14 kDa protein band was detected in almost all transgenic plants. The signal for the human lysozyme was detected in almost all samples from the T0 carrots of cv. K5 and NS. Expression levels of the human lysozyme were relatively high in samples of No. 8-1, 8-2 and 12-1 of cv. NS (Fig. 3a). The transgenic plant No. 7-1, which contained the human lysozyme gene, did not produce human lysozyme. This might be caused by a strong co-suppression [29]. This line was also used for further experiments as one of the negative controls.
Fig. 4. Disease index against powdery mildew of transgenic lines of cultivar (cv.) Nantes Scarlet (NS). After 1 month of acclimatization, the obtained transgenic carrots were exam-ined for disease resistance against powdery mildew. The dis-eased carrots previously infected withErysiphe heraclei were randomly placed among the tested transgenic and non-trans-genic plants, and co-cultivated in a greenhouse at 21 – 23°C for 1 week. Disease rating was classified into six ranks based on the average area of disease in plants, as defined in Table 1. The disease rating was first measured 1 week after inocula-tion, to observe early symptoms of diseased plants. The disease ratings were successively measured twice every week, and the development of powdery mildew was investigated. (a) T0 generation, investigated on 1 week (), 2 weeks () and 3 weeks (b) after the pathogen inoculation. (b) T1 genera-tion, investigated on 1 week (), 2 weeks () and 3 weeks (b). The disease index test of T1 generation of transgenic line No. 8-2 was not performed, because T0 generation of this line did not set seeds. Disease index (%)={(Disease rating from 0 to 8)/(8×number of plants)}×100. Mean values obtained for five to ten plants per line. Within each generation and line, numbers followed by the same letter are not significantly (0.05%) different according to LSD test.
8-1 showed about 40% of the disease index, while the No. 12-1 exhibited a very low disease index (15%) (Figs. 4b and 5). A difference of disease development was observed at early stages of pathogen inoculation as found in two tests of non-transgenic plants (Fig. 4a,b). This fluctuation at early stages might be caused by different timing of pathogen infections. However, disease develop-ment at later stages was basically the same. Inter-estingly, the disease index of powdery mildew differed among the carrot cultivars. In cv. K5 development of powdery mildew was slightly de-layed compared to findings in cv. NS (data not shown). It was also reported that the process of disease development varied in the carrot cultivars, when transgenic carrots expressing chitinase gene were tested [9].
3.4.2. A. dauci
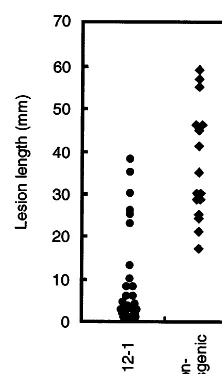
The detached petiole assay was done to evaluate disease resistance of T1 generation of the line No. 12-1 and non-transgenic plants against A. dauci. Five plants for each line were grown in vitro up to the same developmental stage, the leaves were trimmed off, and petioles were cut into 8 cm in length. Four segments were prepared from one plant and used for the biological test. The No. 12-1 was selected based on data concerning the disease index against powdery mildew. This line exhibited very high disease resistance against pow-dery mildew, and also showed high level and stable accumulation of the human lysozyme (Figs. 3 and 4).
The lesion lengths of non-transgenic plants were dispersed between 10 and 60 mm at 2 weeks after inoculation. On the contrary, the transgenic line No. 12-1 was mostly collected below 25 mm of lesion development (Fig. 6). The detached petiol assay was repeated twice, and the obtained results were basically the same between two tests (data not shown). Thus, development of pathogen was considered to be inhibited in the No. 12-1 in comparison with the non-transgenic plants. Thus, the No. 12-1 exhibited the disease resistance against pathogens, both E. heraclei and A. dauci. In this resistant line, the production level of the human lysozyme was high and stable compared with those of other lines, which is consistent with the acquired resistance against these pathogens. Taken together, the human lysozyme gene will prove useful for production of transgenic crops 3.4.1.2. T1 progeny. The disease indexes of all T1
and vegetables to enhance disease resistance against both bacterial and fungal pathogens.
A human lysozyme has strong bacteriolytic ac-tivity against bacteria and fungi [30]. In a previous work, T0 generations of transgenic tobacco plants expressing the human lysozyme showed enhanced disease resistance against Erysiphe cichoracearum
and P. syringae [20]. In the present work, the
stable production of the human lysozyme was further confirmed in T1 as well as T0 generations of the transgenic carrots and also usefulness of the human lysozyme gene to confer the enhanced dis-ease resistance.
Leaf extracts of transgenic tobacco plants ex-pressing a hen egg white lysozyme (HEWL) con-tained only about 30 ng of HEWL per mg of leaf tissue (0.003%) [31]. In transgenic potato plants expressing a bacteriophage T4 lysozyme, the
syn-thesized lysozyme level was estimated to be about 0.001% of total soluble proteins [32]. On the con-trary, the production level of the human lysozyme in the transgenic line No. 12-1 was calculated to be about 0.5% per total soluble protein. Thus, the expression level of human lysozyme was very high compared with those of the above cases. The gene fragment coding for the CSS of Azuki bean [21] was placed upstream of the human lysozyme gene. The CSS might cause highly efficient transporta-tion of the synthesized protein to extracellular spaces.
Differences of the expression level were ob-served between the obtained transgenic carrot lines, which is mainly caused by two factors, the positional effects of the inserted gene and the copy number. It is very difficult to discriminate between each contribution of these two factors to the
Fig. 6. Biological test for disease resistance againstAlternaria dauci. T1 lines of No. 12-1 ( ) and non-transgenic plants lines (wild type control,"), were tested forA.dauci, using a detached petiole assay. Five plants for each line were grown in vitro up to the same developmental stage, the leaves were trimmed off, and petioles were cut into 8 cm in length. Four segments were prepared from one plant and used for this test. Petiole fragments from transgenic and non-transgenic plants were stood upright in actively growing colony layers of the fungal pathogen on PDA medium. The fungal pathogen, which was pre-cultured for 7 days after inoculation, was used. The lesion length on each petiole developed from the base of the petiole fragment was measured after 14 days of incuba-tion. Each dot in the figure represented the obtained individ-ual data.
Acknowledgements
We thank T. Chikahisa and S. Sakamoto for technical assistance and M. Ohara for critical comments on the manuscript.
References
[1] M. Mannerlof, S. Tuvesson, P. Steen, P. Tenning, Trans-genic sugar beet tolerant to glyphosate, Euphytica 94 (1997) 83 – 91.
[2] A.J. Reed, K.M. Magin, J.S. Anderson, M.W. Naylor, K.A. Kretzmer, H.J. Klee, An assessment of fruit riping traits and food safety of tomato plants expressing ACC deaminase protein, Plant Physiol. 105 (Suppl. 1) (1994) 116.
[3] J. Kohno-Murase, M. Murase, H. Ichikawa, J. Imamura, Improvement in the quality of seed storage protein by transformation ofBrassica napuswith an antisense gene for cruciferin, Theor. Appl. Genet. 91 (1995) 627 – 631. [4] R. Di, V. Purcell, G.B. Collins, S.A. Ghabrial,
Produc-tion of transgenic soybean lines expressing the bean pod mottle virus coat protein precursor gene, Plant Cell Rep. 15 (1996) 746 – 750.
[5] Y. Huang, R.O. Nordeen, M. Di, L.D. Owens, J.H. McBeath, Expression of an engineered cecropin gene cassette in transgenic tobacco plants confers disease resis-tance to Pseudomonas syringae pv.tabaci, Phytopathol-ogy 87 (5) (1997) 494 – 499.
[6] T. Terakawa, N. Takaya, H. Horiushi, M. Koike, A fungal chitinase gene fromRhizopus oligosporus confers antifungal activity to transgenic tobacco, Plant Cell Rep. 16 (1997) 439 – 443.
[7] G. Jach, B. Gornhardt, J. Mundy, J. Longemann, E. Pinsdorf, R. Leah, J. Schell, C. Maas, Enhanced quanti-tative resistance against fungal disease by combinatorial expression of different barley antifungal proteins in transgenic tobacco, Plant J. 8 (1) (1995) 97 – 109. [8] W. Lin, C.S. Anuratha, K. Datta, I. Potrykus, S.
Muthukrishnan, S.K. Datta, Genetic engineering of rice for resistance to sheath blight, Bio/Technology 13 (1995) 686 – 689.
[9] Z.K. Punja, S.H. Raharjo, Response of transgenic cu-cumber and carrot plants expressing different chitinase enzymes to inoculation with fungal pathogens, Plant Dis. 80 (9) (1996) 999 – 1005.
[10] Y. Tabei, S. Kitade, Y. Nishizawa, N. Kikuchi, T. Kayano, T. Hibi, K. Akutsu, Transgenic cucumber plants harboring a rice chitinase gene exhibit enhanced resistance to gray mold (Botrytis cinerea), Plant Cell Rep. 17 (1998) 159 – 164.
[11] P. Audy, N. Benhamou, J. Trudel, A. Asselin, Immuno-cytochemical localization of a wheat germ lysozyme in wheat embryo and coleoptile cells and cytochemical study of its interaction with the cell wall, Plant Physiol. 88 (1988) 1317 – 1322.
[12] J.B. Howard, A.N. Glazer, Papaya lysozyme. Terminal sequences and enzymatic properties, J. Biol. Chem. 244 (1969) 1399 – 1409.
[13] A.N. Glazer, A.O. Barel, J.B. Howard, D.M. Brown, Isolation and characterization of fig lysozyme, J. Biol. Chem. 14 (13) (1969) 3583 – 3589.
[14] P. Bernasconi, J. Jolles, P.E. Pilet, Increase of lysozyme and chitinase inRubus callicaused by infection and some polymers, Plant Sci. 44 (1986) 79 – 83.
[15] P. Bernasconi, R. Locher, P.E. Pilet, J. Jolles, P. Jolles, Purification and N-terminal amino-acid sequence of a basic lysozyme from Parthenocissus quinquifoliacultured in vitro, Biomed. Biophys. Acta 915 (1987) 254 – 260. [16] I. Bernier, E. Leemputten, M. Horisberger, D.A. Bush,
P. Jolles, The turnip lysozyme, FEBS Lett. 14 (1971) 100 – 104.
[17] M. Muraki, Y. Jigami, H. Tanaka, N. Harada, N. Kishimoto, H. Agui, S. Ogino, S. Nakasato, Expression of synthetic human lysozyme gene in Escherichia coli, Agric. Biol. Chem. 50 (1986) 713 – 723.
[18] H.R. Ibrahim, H. Hatta, M. Fujiki, M. Kim, T. Ya-mamoto, Enhanced antimicrobial action of lysozyme against gram-negative and gram-positive bacteria due to modification with perilladehyde, J. Agric. Food Chem. 42 (1994) 1813 – 1817.
[19] P. Jolles, J. Jolles, What’s new in lysozyme research?, Mol. Cell Bioche. 63 (1984) 165 – 189.
[20] H. Nakajima, T. Muranaka, F. Ishige, K. Akutsu, K. Oeda, Fungal and bacterial disease resistance in trans-genic plants expressing human lysozyme, Plant Cell Rep. 16 (1997) 674 – 679.
[21] F. Ishige, H. Mori, K. Yamazaki, H. Imazeki, Cloning of a complementary DNA that encodes an acidic chiti-nase which is induced by ethylene and expression of the corresponding gene, Plant Cell Physiol. 34 (1) (1993) 103 – 111.
[22] T. Murashige, F. Skoog, A revised medium for rapid growth and bioassay with tobacco tissue cultures, Phys-iol. Plant 15 (1962) 69 – 76.
[23] S.O. Roger, A.J. Bendich, Extraction of DNA from milligrams of fresh, herbarium and mummified plant tissue, Plant Mol. Biol. 5 (1985) 69 – 76.
[24] W.H.R. Langridge, B.J. Li, A.A. Szalay, Electric field mediated stable transformation of carrot protoplasts with naked DNA, Plant Cell Rep. 4 (1985) 355 – 359. [25] M.O. Gilbert, Y.Y. Zhang, Z.K. Punja, Introduction and
expression of chitinase encoding genes in carrots follow-ing Agrobacterium-mediated transformation, In Vitro Cell 32 (1996) 171 – 178.
[26] E. Wurtele, K. Bulka, A simple, efficient method for the Agrobacterium-mediated transformation of carrot callus cells, Plant Sci. 61 (1989) 253 – 262.
[27] N. Pawlicki, S. Rajbir, B. Sangwan, S. Sangwan-Norreel, Factors influencing theAgrobacterium tumefaciens -medi-ated transformation of carrot (Daucus carota L.), Plant Cell Tissue Organ Culture 31 (1992) 129 – 139.
[28] J.C. Thomas, M.J. Guiltinan, S. Bustos, T. Thomas, C. Nessler, Carrot (Daucus carota) hypocotyl transforma-tion usingAgrobacterium tumefaciens, Plant Cell Rep. 8 (1989) 354 – 357.
[29] J.J. English, M. Mueller, D.C. Baulcombe, Suppression of virus accumulation in transgenic plants exhibiting silencing of nuclear genes, Plant Cell 8 (1996) 179 – 188. [30] Y. Matsuki, H. Gomi, S. Nabeshima, Jpn. Patent
2-27988 (1990).
[31] J. Trudel, C. Potvin, A. Asselin, Expression of active hen egg white lysozyme in transgenic tobacco, Plant Sci. 87 (1992) 55 – 67.
[32] K. During, P. Porsch, M. Fladung, H. Lorz, Transgenic potato plants resistant to the phytopathogenic bacterium Erwinia caroto6ora, Plant J. 3 (4) (1993) 587 – 589. [33] F. Ishige, M. Takaichi, R. Foster, Nam-Hai Chua, K.
Oeda, A G-box motif (GCCACGTGCC) tetramer con-fers high-level constitutive expression in dicot and mono-cot plants, Plant J. 18 (4) (1999) 443 – 448.