www.elsevier.nlrlocateraqua-online
Pilot evaluation of freeze-dried microalgae
in the mass rearing of gilthead seabream
ž
Sparus aurata larvae
/
J. Pedro Canavate
a,), C. Fernandez-Dıaz
b˜
´
´
a
CICEM El Toruno, Junta de Andalucıa, PO Box 16, E-11500 Puerto de Santa Marıa, Cadiz, Spain˜ ´ ´ ´
b ( )
Instituto de Ciencias Marinas de Andalucıa CSIC , Apartado Oficial, E-11510 Puerto Real, Cadiz, Spain´ ´
Received 14 November 1999; received in revised form 21 July 2000; accepted 2 August 2000
Abstract
Ž .
The replacement of live microalgae by freeze-dried Nannochloropsis gaditana B3 strain and
Ž . Ž .
Isochrysis galbana T-ISO strain in the mass rearing of larval seabream Sparus aurata was
studied. Total substitution of live N. gaditana by freeze-dried cells in 1 m3larval tanks produced
similar growth and survival in larvae reared from first feeding until day 43 with three different types of rotifer enrichment. Differences in growth of larvae were only due to the type of enrichment used to feed rotifers prior to their addition to larval tanks. This was regardless of the
Ž .
presence of live or freeze-dried N. gaditana in the larval tanks. Specific growth rate G of larvae
Ž .
was significantly enhanced Gs0.106"0.001; P-0.05 with a commercial enricher compared
Ž .
to N. gaditana Gs0.099"0.003 as rotifer enricher. Feeding the rotifers with freeze-dried I.
Ž .
galbana produced the same larval growth rate Gs0.109"0.002; P)0.05 as the commercial enricher, indicating the high nutritional value of these algae in a freeze-dried state. Larval survival was similar in all treatments. Water quality, in terms of dissolved oxygen and pH, was also similar
Ž .
in the different treatments. Ammonia concentration was higher P-0.05 when freeze-dried N.
gaditana was added to the larval tanks, but only during the first 15 days of culture, when no water
Ž .
exchange was used. Nitrite did not vary P)0.05 during the first 15 days, but increased more for live N. gaditana from day 16 onwards. Results of this study indicate the potential for a
)Corresponding author. Tel.:q34-956-562340; fax:q34-956-562385.
Ž .
E-mail address: [email protected] C. Fernandez-Dıaz .´ ´
0044-8486r01r$ - see front matterq2001 Elsevier Science B.V. All rights reserved. Ž .
complete replacement of live microalgae by freeze-dried microalgae throughout the whole process of mass rearing seabream larvae.q2001 Elsevier Science B.V. All rights reserved.
Keywords: Sparus aurata; Algae; Freeze-dried; Larvae; Enrichment; Rotifers; Seabream
1. Introduction
Ž .
The farming of gilthead seabream Sparus aurata is an important commercial activity in the Mediterranean area. Hatchery production of juvenile seabream has dramatically increased during the nineties, and it is a key factor in the development of this industry. Production in most hatcheries relies on feeding procedures, which are based on the use of live phytoplankton and zooplankton species. One of the features of
Ž
these feeding regimes is the use of the so-calledApseudogreen waterB Papandroulakis et
.
al., 1996 , a method characterised by a regular supply of cultured microalgae to larval tanks. The presence of microalgae in fish larval tanks has improved the growth and
Ž .
survival of the larvae e.g. Naas et al., 1992; Reitan et al., 1993, 1997 . Green water is achieved by adding small amounts of live microalgae, such as the Eustigmatophyceae,
Ž .
Nannochloropsis species Yoshimatsu et al., 1995; Hernandez-Cruz et al., 1994 . The
´
Ž .
Haptophyceae, Isochrysis galbana, is also used Howell, 1979; Reitan et al., 1993 . This latter alga is a good nutritional complement when Nannochloropsis species are added to
Ž .
the larval rearing medium Mourente et al., 1993 .
In order to reduce cost, it is desirable to replace live microalgae with long-term preserved microalgae. Obtaining long-term preserved algae that provide good culture performance may also contribute to a future diversification and a higher level of specialisation in the marine aquaculture industry. The use of preserved microalgae for
Ž .
the rearing of marine fish larvae was mentioned by Yamasaki et al. 1989 , and Sommer
Ž .
et al. 1990 reviewed the potential of processed microalgae in aquaculture. One of the initial studies using dried algae was that carried out with juvenile bivalves by Laing and
Ž .
Millican 1992 . Rotifer culture has also been successfully carried out using preserved
Ž . Ž
algae that was either frozen Lubzens et al., 1995 , freeze-dried Yufera and Navarro,
´
. Ž .
1995 or condensed Maruyama et al., 1997; Yoshimura et al., 1997 .
Larvae of S. aurata have been reared by the addition of frozen concentrated
Ž .
microalgae during the first 20 days of culture Papandroulakis et al., 1996 . Later,
Ž .
Navarro and Sarasquete 1998 described the laboratory growth and the histology of S.
aurata larvae during the first 15 days when freeze-dried Nannochloropsis oculata was
added to the rearing water. In the present study, the effect of using freeze-dried N.
gaditana and I. galbana on growth and survival of mass reared seabream larvae is
described. The study was performed for a longer period than Navarro and Sarasquete
Ž1998 . In addition, three different regimes of rotifer enrichment were used in the.
assessment of freeze-dried algae. The aim of both procedures was to obtain a better understanding of the effects of solely using freeze-dried microalgae. Thus, the influence
Ž
of using dry algae during the first stage of rearing seabream larvae that of rotifer
.
2. Materials and methods
2.1. Production and use of freeze-dried algae
N. gaditana was grown in 50-cm-deep, 2000-l tanks under natural radiation in a
green house. Cells were harvested by continuous centrifugation at cell densities between
6 y1 Ž y1.
50 and 70=10 cells ml average biomass from 420 to 590 mg dry weight l . I.
galbana was grown in 20-cm-diameter, 50-l acrylic tubes, placed in an algal room at
Ž y1.
208C under continuous lighting photon flux density of 150 mmol s . It was also concentrated by continuous centrifugation at an average cell density of 22=106 cells
y1 Ž y1.
ml 440 mg dry weight l . Algal pastes were frozen aty808C prior to freeze-dry-ing for 48 h. Dry algae powder was kept vacuum packaged until its use in larval rearfreeze-dry-ing. The required amount of cells was resuspended in a small volume of seawater by mixing for 1 min in a kitchen blender.
2.2. LarÕal rearing conditions
Experiments were replicated by using three batches of larvae from different spawning tanks. Eggs were obtained from captive broodstock kept under constant temperature
Ž208C and natural photoperiod. After hatching, 2-day-old larvae were stocked in 1 m. 3
round tanks to achieve an initial larval density of between 80 and 100 larvae ly1. The
tanks were supplied with seawater pumped from the San Pedro Channel, which is
Ž .
located in an estuary area connected to the Bay of Cadiz SW Spain . Water was filtered
´
through sand filters and 10 and 3mm nominal retention cartridges. There was no water exchange until day 15 of culture. Then, there was gradually increased water exchange from 20% to 80% daily up to day 45. Gentle aeration was always provided in the center of the larval tanks. Temperature and salinity in the larval tanks ranged from 198C to 218C and from 25 to 33 ppt, respectively. The light was turned off during yolk sac absorption and a permanent illumination of 1500 lux on water surface was used from initial exogenous feeding throughout the cultures. The feeding experiments lasted until larvae reached an age of 43 days, as shown in Table 1.
In each replicate, a group of three tanks received 2 g my3 of freeze-dried N.
gaditana, whereas another three tanks were supplied with an equivalent amount of live
Table 1
Feeding sequence and daily amounts of the different diets provided in tanks with seabream larvae during the mass rearing trials
Type of diet
y3 y1 y1 y3
Ž . Ž . Ž . Ž .
Days Algae g m Rotifers ind ml Artemia ind ml Dry feed g m
3–10 2 10 – –
11–15 2 15 – –
16–30 2 15 1 –
31–40 – – 3 –
Table 2
Experimental treatments followed in the evaluation of freeze-dried microalgae in the mass rearing of S. aurata larvae. F: Freeze-dried N. gaditana. I: Freeze-dried I. galbana. L: Live N. gaditana. P: Commercial enricher,
Ž .
algae in terms of dry weight. Both treatments were tested with different types of rotifer enrichment, as detailed in Table 2. When live N. gaditana was added to the larval tanks, algal cultures were previously concentrated by 500-fold in order to avoid any possible variation in water quality due to the addition of algal medium. The daily amount of
Ž . 6
algae supplied to larval tanks Table 2 provided an average cell density of 0.25=10 cells mly1. A strain of Brachionus plicatilis ranging from 120 to 230 mm in size was used. Rotifer enrichment lasted 16 h, and 200 mg ly1 of enricher was used in all cases.
Artemia were used as food from day 16, and rotifer and Artemia density in the rearing
Ž .
tanks was monitored daily and adjusted to desired values Table 1 .
2.3. Analysis of growth and culture eÕaluation
Initial larval density was estimated by counting the number of larvae present in 10 different samples of 500 ml. A gentle shaking of the rearing medium with a plastic sheet allowed a uniform larval distribution to be obtained before sampling. Final density was calculated by counting fish in sub-samples after concentrating the whole population in each tank prior to being moved to larger growing tanks.
Larval dry weight was calculated by weighing a number of larvae on pre-washed and pre-weighed fiberglass filters. These filters were rinsed with distilled water and dried at 608C for 48 h. They were then weighed to the nearest 10 mg and a mean value of the individual larval dry weight was calculated. Three replicates were used at every
Ž .
sampling. Specific growth rate G was calculated from the slope of the linear regression
Ž .
of log-transformed dry weight against age. Final biomass b was calculated from dry weight and the larval density obtained at the end of the experiments. Oxygen and pH were monitored daily, and ammonia and nitrite were analyzed as described in Grasshoff
Ž .
et al. 1983 .
3. Results
3.1. Effects of algal presentation in larÕal deÕelopment
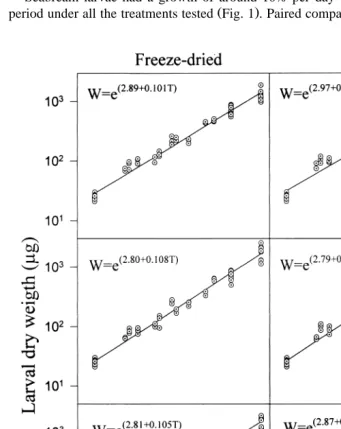
Seabream larvae had a growth of around 10% per day throughout the experimental
Ž .
period under all the treatments tested Fig. 1 . Paired comparisons between the slopes of
Ž . Ž .
Fig. 1. Growth of seabream larvae in the presence of freeze-dried left column and live right column N.
Ž gaditana supplied with rotifers previously fed N. gaditana, I. galbana and commercial enricher Protein
.
the growth equations revealed no difference when live or freeze-dried N. gaditana were used in the larval tanks. Neither was there any significant difference in growth rate
Ž
between treatments LF and FF, LI and FI, and LP and FP P)0.05; see Table 2 for
.
abbreviations . Therefore, the use of freeze-dried N. gaditana in the mass rearing of seabream larvae did not affect their growth rate with any of the three different types of rotifer enrichment used.
Slight but significant differences in growth were found between the enrichments. Larvae that received rotifers enriched with freeze-dried N. gaditana had a lower growth
Ž .
rate P-0.05 than those fed rotifers enriched with freeze-dried I. galbana or the commercial enricher. No differences in growth rate were found between the latter two
Ž .
enrichments P)0.05 .
Survival after 43 days was not affected either by the use of freeze-dried N. gaditana
Ž .
in the larval tanks or by the type of rotifer enrichment P)0.05 , achieving similar
Fig. 2. Survival and biomass obtained at the end of the experiment with seabream larvae using live and
Ž .
Ž .
values of around 15% in all instances Fig. 2 . Larval density decreased gradually, without peaks of mortality or cannibalism throughout the trials.
Ž .
The use of freeze-dried N. gaditana in larval tanks had no effect P)0.05 on final
Ž y1.
biomass mg dry weight l , as revealed by paired comparisons between treatments. On the other hand, the type of rotifer enrichment influenced the final biomass attained
ŽFig. 2 . Use of rotifers enriched with freeze-dried I. galbana or Protein Selco produced.
Ž . y1
similar biomass P)0.05 of 19"1.4 and 16.5"2.1 mg l , respectively. The biomass achieved using rotifers enriched with freeze-dried I. galbana was higher
ŽP-0.05 than that obtained with use of rotifers enriched with N. gaditana Fig. 2 .. Ž .
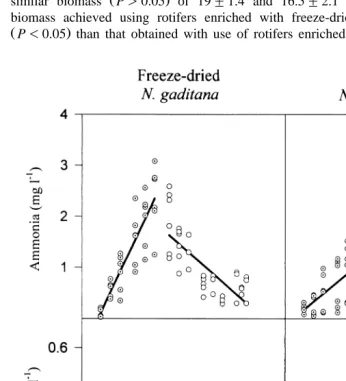
Ž .
Fig. 3. Ammonia and nitrite concentration in tanks with seabream larvae receiving freeze-dried left column
Ž .
3.2. Effects of freeze-dried algae on water quality during larÕal deÕelopment
Of the four parameters studied, ammonia was the one that varied most according to the use of freeze-dried or live N. gaditana in the larval tanks. Ammonia increased more during the period of stagnant water when freeze-dried algae was used, and was significantly higher on day 16 for freeze-dried N. gaditana compared to the use of live
Ž .
algae P-0.05, Fig. 3 . However, the increased water exchange from day 16 led to
Ž .
similar ammonia concentrations at the end of the experiment day 43 , being 0.62"0.07
y1 Ž .
and 0.53"0.08 mg l , respectively P)0.05 . The fraction of unionized ammonia
ŽNH3. was also higher for freeze-dried algae ŽP-0.05 . From day 13 to day 18 of.
Ž .
Fig. 4. pH and dissolved oxygen in tanks with seabream larvae that received freeze-dried left column and
Ž .
culture, a mean value of 0.051"0.015 mg ly1 NH3 was recorded, in comparison to 0.027"0.014 mg ly1 NH achieved for the same period when live N. gaditana was3
Ž .
present in larval tanks. The variation in pH was similar P)0.05 between treatments and decreased gradually from around 8.15 at the beginning to 7.85 at the end of the
Ž . Ž .
trials Fig. 4 . Oxygen concentration did not change P)0.05 depending on algal
Ž .
presentation and had a slow constant decrease throughout the rearing process Fig. 4 . Nitrite concentration was lower than 0.1 mg ly1 NOy and did not vary during the
2
first 15 days between tanks that received freeze-dried N. gaditana and those fed live N.
Ž .
gaditana P)0.05, Fig. 3 . From the beginning of water exchange onwards, an increase in nitrite concentration occurred when both types of algae were used. This increase was
Ž
more pronounced when live N. gaditana was added to the larval tanks P-0.05, Fig.
.
3 .
4. Discussion
Adding freeze-dried or live algae to larval tanks gave identical growth and survival rates after 43 days of culture. After this period, it is common for S. aurata fry to be weaned on to dry feed in commercial hatcheries. These results accord with those found
Ž .
for the same fish by Navarro and Sarasquete 1998 using freeze-dried N. oculata at laboratory scale during the first 15 days of larval rearing. Our study shows that there is no difference between live and freeze-dried algae on larvae beyond the age after which algae are removed. The use of freeze-dried algae is therefore suitable for use in the process of seabream fry production.
Enriching the rotifers with freeze-dried cells of I. galbana improved larval growth and led to results similar to those achieved by using a commercial enricher. The combination of both algae represents a well balanced diet in terms of essential fatty acid
Ž
requirements for marine fish larvae Watanabe et al., 1983; Mourente et al., 1993;
. Ž
Reitan et al., 1993 . Nannochloropsis species are particularly rich in EPA Ben-Amotz
.
et al., 1987; Mourente et al., 1990; Hodgson et al., 1991 and I. galbana contributes
Ž
with adequate amounts of DHA Ben-Amotz et al., 1987; Mourente et al., 1990; Sukenik
.
and Wahnon, 1991 .
Freeze-dried cells of I. galbana have been used for feeding bivalves in culture
ŽAlbentosa et al., 1997 , but there are no references to its application in the rearing of.
seabream larvae. Poor results obtained in previous studies contrast with the improvement found in this study when rotifers were enriched with freeze-dried I. galbana. Albentosa
Ž .
et al. 1997 attributed their poor results to a lower digestibility of the cell wall in freeze-dried I. galbana. An increase in cell wall thickness has been described in some
Ž .
cells after the osmotic stress produced by freezing Morris et al., 1986a . This may happen in cells having a plasmalema and a cell wall that shrink together following freezing. However, there are other types of cells undergoing plasmolysis during freezing
Ž .
where no changes occur in the cell wall due to osmotic stress Morris et al., 1986b . It should be considered that delivering freeze-dried algae to the larvae through rotifers is a procedure that may overcome possible digestibility problems due to the high digestive
Ž .
and rotifers could also explain part of the above differences for the use of freeze-dried I.
Ž
galbana. Conditions during rotifer enrichment particularly density of feed and feeder,
.
water movement and tank design are more favourable for a rapid ingestion of particulate feeds in suspension. This is of special importance for freeze-dried I. galbana, a type of particle with a lower capacity to remain in suspension in comparison to the live state of this flagellate.
One of the main factors for the successful use of N. gaditana in the larval tanks could be associated with the similar buoyancy of live or freeze-dried cells of these coccoid microalgae. The good suspension performance of N. gaditana is probably a
Ž .
consequence of its small size Lubian and Yufera, 1989 . This alga has a very thick cell
´
Ž .
wall Lubian, 1981 that does not seem to negatively affect its digestibility for fish larvae when it is delivered through rotifers.
The conservation of cell integrity after resuspension of freeze-dried I. galbana is an important factor when this alga is used in rearing systems, such as those for bivalves, where these inert particles are suspended in water for a longer time before they are ingested. This is due to the fact that a prolonged exposure to water of inert cells with a disrupted protoplasm may lead to excessive leaching of essential nutrients. From cryopreservation research, it is known that I. galbana is very sensitive to freezing, in
Ž .
comparison to other marine microalgae Canavate and Lubian, 1995 . It is obvious that
˜
freeze-drying produced cells with a much-altered protoplasm caused by membrane disruption. These cells, encasing a disrupted cytoplasmic material, were found to keep integrity for at least 4 h after resuspension, time enough for rotifers to fill their guts. The stability after longer periods in water is not known, particularly those aspects concerning leaching rates of essential nutrients out of the freeze-dried cells. This is a factor that might noticeably affect the nutritional quality of freeze-dried I. galbana when used in rearing systems in which ingestion by feeders does not occur as rapidly as for rotifers. Different beneficial effects of green water have been reported in the rearing of marine fish larvae. Growth and survival are generally improved when unicellular green algae
Ž
are present in larval tanks Naas et al., 1992; Reitan et al., 1993; Navarro and
.
Sarasquete, 1998 . It has been suggested that algae, by releasing attractant compounds,
Ž .
may stimulate the appetite of larvae Stottrup et al., 1995 . Algae will also tend to change light conditions in the tanks and improve the feeding behaviour of the larvae
ŽNaas et al., 1992 . All these positive aspects of using algae in marine fish larviculture.
do not seem to be noticeably affected by whether N. gaditana is live or freeze-dried.
Ž .
Skjermo and Vadstein 1993 indicated that algae might influence bacterial populations in the rearing water and contribute in establishing an early gut microbial flora in the larvae. Possible changes in bacterial populations due to the use of inert microalgae are not yet known. In another study, the presence of algae in the rearing tanks improved
Ž
growth of Dicentrarchus labrax when a compound diet was fed to larval stages Cahu
.
et al., 1998 . These authors demonstrated that algae acted by triggering digestive enzyme production. It would be highly interesting to know whether such capacity would occur when algae are freeze-dried.
Some differences occurred in water quality as a consequence of using freeze-dried N.
gaditana. The most important one was the higher increase of ammonia during the initial
the stagnant water period is just below the levels, producing a significant mortality in S.
Ž .
aurata larvae, according to Parra 1998 . The unionized ammonia peaks for both inert
and live N. gaditana were within a range that was found to depress growth in Pagrus
Ž .
major larvae Guillen et al., 1993 . In the case that a similar effect should be detected in S. aurata larvae, an earlier use of water exchange would easily overcome this constraint.
Nitrite increased at similar rates throughout the cultures. Nevertheless, the increase of this compound could not be suspected of causing negative effects since concentrations
Ž .
were always far below toxic levels found for S. aurata larvae by Parra 1998 . The almost parallel development in pH and oxygen concentrations under the presence of live and inert N. gaditana suggests that this alga was not playing an important role in contributing to an improved water quality in larval tanks.
The potential for using freeze-dried algae indicates that this type of feed may promote easier management in fish larvae production. Obtaining a freeze-dried product that ensures similar properties to live algae and that can be transported and stored for long periods offer great benefits to the fish culturist.
Acknowledgements
This study was supported by the Regional Council for Fisheries and Agriculture
ŽJunta de Andalucia . The manuscript was revised by Matthew Harffy of English.
Language Consultants.
References
Albentosa, M., Perez-Camacho, A., Labarta, U., Fernandez-Reiriz, M.J., 1997. Evaluation of freeze-dried´
microalgal diets for the seed culture of Ruditapes decussatus using physiological and biochemical parameters. Aquaculture 154, 305–321.
Ben-Amotz, A., Fishler, R., Schneller, A., 1987. Chemical composition of dietary species of marine unicellular algae and rotifers with emphasis on fatty acids. Mar. Biol. 95, 31–36.
Cahu, C.L., Zambonino Infante, J.L., Peres, A., Quazuguel, P., Le Gall, M.M., 1998. Algal addition in sea´
Ž .
bass Dicentrarchus labrax larvae rearing: effect on digestive enzymes. Aquaculture 161, 479–489. Canavate, J.P., Lubian, L.M., 1995. Relationship between cooling rates, cryoprotectant concentrations and˜
salinities in the cryopreservation of marine microalgae. Mar. Biol. 124, 325–334.
Grasshoff, K., Ehrhardt, M., Kremling, K., 1983. Methods of Seawater Analysis. Verlag Chemie, Weinheim, 419 pp.
Guillen, J.L., Endo, M., Turnbull, J.F., Kawatsu, H., Richards, R.H., Aoki, T., 1993. Depressed growth rate and damage to the cartilage of read sea bream larvae associated with exposure to ammonia. Nippon Suisan Gakkaishi 59, 1231–1234.
Hernandez-Cruz, C.M., Salhi, M., Fernandez-Palacios, H., Izquierdo, M.S., 1994. Improvements in the culture´ ´
of Sparus aurata L. larvae in relation to the use of antibiotics, phytoplankton and rearing system. Aquaculture 124, 269–274.
Hirayama, K., Takagi, K., Kimura, H., 1979. Nutritional effect of eight species of marine phytoplankton on population growth of the rotifer Brachionus plicatilis. Bull. Jpn. Soc. Sci. Fish. 45, 11–16.
Hodgson, P.A., Henderson, R.J., Sargent, J.R., Leftley, J.W., 1991. Patterns of variation in the lipid class and
Ž .
Howell, B.R., 1979. Experiments on the rearing of larval turbot Scophtamus maximus L. Aquaculture 18, 215–225.
Laing, I., Millican, P.F., 1992. Indoor nursery cultivation of juvenile bivalve molluscs using diets of dried algae. Aquaculture 102, 231–243.
Lubian, L.M., 1981. Crecimiento en cultivo de cuatro cepas de Nannochloris. Estudio de su citologia y composicion de pigmentos como base para el establecimiento de su situacion taxonomica. PhD thesis,´ ´
University of Seville, Seville, Spain, 186 pp.
Lubian, L.M., Yufera, M., 1989. Coleccion de cepas de microalgas marinas del Instituto de Ciencias Marinas´
Ž . Ž .
de Andalucia CSIC . In: Yufera, M. Ed. , Acuicultura Intermareal. Consejo Superior de Investigaciones Cientificas, Cadiz, Spain, pp. 66–78.
Ž
Lubzens, E., Gibson, O., Zmora, O., Sukenik, A., 1995. Potential advantages of frozen algae
Nannochlorop-. Ž .
sis sp. for rotifer Brachionus plicatilis culture. Aquaculture 133, 295–309.
Maruyama, I., Nakao, I., Shigueno, Y., Ando, Y., Hirayama, K., 1997. Application of unicellular algae
ChlorellaÕulgaris for the mass culture of marine rotifer Brachionus. Hydrobiologia 358, 133–138. Morris, G.J., Winters, L., Coulson, G.E., Clarke, K.J., 1986a. The effect of osmotic stress on the ultrastructure
and viability of the yeast Saccharomyces cereÕisiae. J. Gen. Microbiol. 129, 2023–2034.
Morris, G.J., Coulson, G.E., Engels, M., 1986b. A cryomicroscopic study of Cylindrocystis brebissonii De
Ž .
Bary and two spacies of Micrasterias Ralfs Conjugatophyceae, Chlorophyta during freezing and
Ž .
thawing. J. Exp. Bot. 37 179 , 842–856.
Mourente, G., Lubian, L.M., Odriozola, J.M., 1990. Total fatty acid composition as a taxonomic index of some marine microalgae used as food in marine aquaculture. Hydrobiologia 203, 147–154.
Mourente, G., Rodriguez, A., Tocher, D.R., Sargent, J.R., 1993. Effects of dietary docosahexaenoic acid
ŽDHA; 22:6 ny3 on lipid and fatty acid compositions and growth in gilthead seabream Sparus aurata L.. Ž .
larvae during first feeding. Aquaculture 112, 79–98.
Ž .
Naas, K.E., Naess, T., Harboe, T., 1992. Enhanced first feeding of halibut larvae Hippoglossus hippoglossus in green water. Aquaculture 105, 143–156.
Navarro, N., Sarasquete, M.C., 1998. Use of freeze-dried microalgae for rearing gilthead seabream, Sparus
aurata, larvae: Part I. Growth, histology and water quality. Aquaculture 167, 179–193.
Papandroulakis, N., Kentouri, M., Stefanakis, S., Tredidi, M., Divanach, P., 1996. Comparative value of live
Ž . Ž .
and frozen marine Chlorella for seabream Sparus aurata larviculture with the pseudo green water technique. Proceeding of the Conference of IIR Commission C2. Refrigeration and Aquaculture, Burdeaux, France, pp. 73–81.
Ž
Parra, M.G., 1998. Fisiologia y balance energetico durante el desarrollo larvario de peces marinos Solea
.
senegalensis Kaup y Sparus aurata Linneo en cultivo. PhD thesis, University of Cadiz, Cadiz, Spain, 175
pp.
Reitan, K.I., Rainuzzo, J.R., Oie, G., Olsen, Y., 1993. Nutritional effects of algal addition in first feeding of
Ž .
turbot Scophthalmus maximus L. larvae. Aquaculture 118, 257–275.
Reitan, K.I., Rainuzzo, J.R., Oie, G., Olsen, Y., 1997. A review of the nutritional effects of algae in marine fish larvae. Aquaculture 155, 207–221.
Skjermo, J., Vadstein, O., 1993. The effect of microalgae on skin and gut bacterial flora of halibut larvae. In:
Ž .
Reinertsen, H., Dahle, L.A., Jorgensen, L., Tvinnereim, K. Eds. , Proceeding from International Confer-ence of Fish Farming Technology, Trondheim, Norway, August 1993, pp. 61–67.
Sommer, T.R., Potts, W.T., Morrissy, N.M., 1990. Recent progress in the use of processed microalgae in aquaculture. Hydrobiologia 204r205, 435–443.
Stottrup, J.G., Gravningen, K., Norsker, N.H., 1995. The role of different algae in the growth and survival of
Ž .
turbot larvae Scophthalmus maximus L. in intensive rearing system. ICES Mar. Sci. Symp. 201, 173–186.
Sukenik, A., Wahnon, R., 1991. Biochemical quality of marine unicellular algae with special emphasis on lipid composition: Part I. Isochrysis galdana. Aquaculture 97, 61–72.
Watanabe, T., Kitajima, C., Fujita, S., 1983. Nutritional values of live organisms used in Japan for mass propagation of fish: a review. Aquaculture 34, 115–143.
Ž
Yamasaki, S., Tanabe, K., Hirata, H., 1989. Efficiency of chilled and frozen Nannochloropsis sp. marine
.
Chlorella for culture of rotifer. Mem. Fac. Fish., Kagoshima Univ. 38, 77–82.
requirement of larvae redlip mullet for essential fatty acids, and the supplemental effect of
Nannochlorop-Ž .
sis to the rearing water. Nippon Suisan Gakkaishi 61 6 , 912–918.
Yoshimura, K., Usuki, K., Yoshimatsu, T., Kitajima, Ch., Hagiwara, A., 1997. Recent development of a high density mass culture system for the rotifer Brachionus rotundiformis Tschugunoff. Hydrobiologia 358, 139–144.
Yufera, M., Navarro, N., 1995. Population growth dynamic of the rotifer Brachionus plicatilis cultured in´