www.elsevier.com / locate / bres
Research report
Enhancement in activities of large conductance calcium-activated
potassium channels in CA1 pyramidal neurons of rat hippocampus
after transient forebrain ischemia
*
Liang-Wei Gong, Tian M. Gao , Xiaoming Li, Hao Huang, Zhenqing Tong
Department of Physiology, The First Military Medical University, Guangzhou 510515, PR China Accepted 29 August 2000
Abstract
It has been reported previously that the neuronal excitability persistently suppresses and the amplitude of fast afterhyperpolarization (fAHP) increases in CA1 pyramidal cells of rat hippocampus following transient forebrain ischemia. To understand the conductance mechanisms underlying these post-ischemic electrophysiological alterations, we compared differences in activities of large conductance
21
Ca -activated potassium (BKCa) channels in CA1 pyramidal cells acutely dissociated from hippocampus before and after ischemia by using inside-out configuration of patch clamp techniques. (1) The unitary conductance of BKCachannels in post-ischemic neurons (295
1
pS) was higher than that in control neurons (245 pS) in symmetrical 140 / 140 mM K in inside-out patch; (2) the membrane depolarization for an e-fold increase in open probability (P ) showed no significant differences between two groups while the membraneo
potential required to produce one-half of the maximum P was more negative after ischemia, indicating no obvious changes in channelo
21
voltage dependence; (3) the [Ca ] required to half activate BKi Cachannels was only 1mM in post-ischemic whereas 2mM in control
21
neurons, indicating an increase in [Ca ] sensitivity after ischemia; and (4) BKi Cachannels had a longer open time and a shorter closed time after ischemia without significant differences in open frequency as compared to control. The present results indicate that enhanced activity of BKCa channels in CA1 pyramidal neurons after ischemia may partially contribute to the post-ischemic decrease in neuronal excitability and increase in fAHP. 2000 Elsevier Science B.V. All rights reserved.
Theme: Disorders of the nervous system
Topic: Ischemia
1
Keywords: K channels; Ischemia; Patch clamp; Hippocampus; Rat
1. Introduction [6,42] while others showed a decreased [5,12,20] firing
rate in the CA1 region following ischemia. Recently, by Pyramidal neurons in the hippocampal CA1 region are using in vivo [14] and in vitro [40] intracellular recording particularly vulnerable to ischemic insult and display a technique, a persistent reduction in spontaneous firing rate delayed cell death fashion after transient cerebral ischemia and a progressive suppression of excitability was observed [22,35]. Neuronal hyperactivity induced by excessive in CA1 pyramidal neurons after severe forebrain ischemia accumulation of extracellular glutamate during ischemia while only a transient change in these properties was found was hypothesized to trigger the process of neuronal in CA3 neurons and dentate granule cells [13]. The degeneration [7,36]. However, the electrophysiological decrease in neuronal excitability after severe ischemia may evidence for hyperactivity in hippocampus after ischemia be due to an increase in potassium conductances. In remains controversial. Using extracellular recording tech- consistence, it was reported that the amplitude of fast niques in vivo, some investigators reported an increased afterhyperpolarization (fAHP), which is mediated by cal-cium-dependent potassium conductance, progressively in-creased in CA1 neurons following ischemia [14].
How-*Corresponding author. Tel.:186-20-8514-8216; fax: 1
86-20-8773-ever, little information is available concerning the
conduct-0321.
E-mail address: [email protected] (T.M. Gao). ance mechanisms related to the decrease in neuronal
excitability as well as the increase in fAHP in post- for 15 min. Upon release of the carotid artery clasps,
ischemic CA1 neurons. cerebral blood flow resumed immediately. Signs, such as
Single-channel studies have identified at least two types unresponsiveness, loss of righting reflex, and catatonic
21 1
of Ca -activated K channels: large conductance (BKCa) postures, were thought to be indicative of forebrain and small conductance (SKCa) channels on membranes of ischemia. Rats which showed these symptoms were al-pyramidal neurons from hippocampal CA1 region, on the lowed to survive for 2 h, and then used for later cell basis of their pharmacological and biophysical properties isolation. Rats with post-ischemic convulsions were ex-[26,37]. BKCa channel is both voltage- and calcium-depen- cluded from study.
dent. In hippocampal pyramidal neurons in vivo, BKCa
channel is thought to be activated during an action
2.2. Acute-dissociation procedures potential by membrane depolarization, together with a rise
21 1
in the intracellular Ca concentration. The resulting K
Pyramidal cells in hippocampal CA1 region were disso-current is largely responsible for action potential
repolari-ciated acutely, using procedures as described previously zation and generation of fAHP [25,41]. It was also
[2,3,15] with some modifications. In brief, rats were suggested recently that BKCa channels may play an anesthetized with chloral hydrate (i.p., 40 mg / 100 g important role in regulating neuronal excitability at the
weight) and then decapitated; brains were quickly re-resting membrane potential [21,44]. Therefore, BKCachan- moved, iced, and blocked for slicing. The blocked tissue nels are critical in setting the degree of neuronal
excitabili-was cut into 400-mm slices with a Vibroslice whilst bathed
ty, which in turn determines the rate of action potential 21
in a low Ca , HEPES-buffered salt solution containing firing and burst firing patterns.
(in mM): 140 sodium isethionate, 2 KCl, 4 MgCl , 0.12
So we assumed that the post-ischemic
electrophysiologi-CaCl , 23 glucose, 15 HEPES, pH 7.4 (300–305 mOsm /2
cal changes mentioned above in CA1 pyramidal neurons
l). Slices were then incubated for 1–6 h at room tempera-may be partially due to the alterations in BKCa channel ture (20–228C) in a NaHCO -buffered saline bubbled with
3
activity. To address this question, we examined the activity
95% O / 5% CO containing (in mM): 126 NaCl, 2.5 KCl,2 2
of BKCa channels in CA1 pyramidal neurons of rat 2 CaCl , 2 MgCl , 26 NaHCO , 1.25 NaH PO , 1 pyruvic
2 2 3 2 4
hippocampus after transient forebrain ischemia using
in-acid, 0.005 glutathione, 0.1 N v-nitro-L-arginine, 1
side-out configuration of patch clamp techniques.
kynurenic acid, 10 glucose, pH 7.4 with NaOH (300–305 mOsm / l). All reagents were obtained from Sigma (St.
21
Louis, MO). Slices were then removed into the low Ca 2. Materials and methods
buffer, and CA1 region of hippocampus was dissected out under a dissecting microscope and placed into an oxy-2.1. Transient forebrain ischemia
genated chamber containing pronase (Sigma protease Type XIV, 1–1.5 mg / ml) in HEPES-buffered HBSS (Sigma) at Experimental procedures in this study were performed
338C. After 30–45 min of enzyme digestion, tissue was
within National Institutes of Health guidelines (Guide for 21
rinsed three times in the low Ca , HEPES-buffered saline the Care and Use of Laboratory Animals, NIH publication
and dissociated mechanically with a graded series of fire-93-23, revised 1985). A total of 116 male adult Wistar rats
polished Pasteur pipettes. The cell suspension was then weighing 200–250 g was used in the present study. The
plated into a 35-mm Lux petri dish mounted on the stage animals were divided into two groups with 46 rats as
of an inverted microscope containing HEPES-buffered control and the remaining 70 rats subjected to transient
HBSS saline. After allowing the cell to settle, the solution forebrain ischemia.
bathing the cells was changed to our recording solution. Transient forebrain ischemia (15 min) was induced by
the use of the four-vessel occlusion method [34] with some
modification. Briefly, on the day prior to the experiment, 2.3. Single-channel current recording rats were anesthetized with chloral hydrate (i.p., 40 mg /
100 g weight), and an occluding device (a loop of silicone Gigaseal patch recordings using the inside-out patch tubing) was placed loosely around each carotid artery to configuration as described by Hamill [16] (the feed-back allow subsequent occlusion of these vessels without inter- resistor was 50 GV) were performed on neurons with rupting carotid blood. The animals were then placed on a pyramidal shape. The pipette resistance was 8–12 MVand stereotaxic frame, and both vertebral arteries were cauter- the seal resistance was in excess of 5 GV. Single-channel ized permanently. Rats prepared in this manner showed no currents were recorded in excised inside-out patches, evidence of brain damage and were behaviorally normal. which were prepared by pulling the patch electrode away On the day of the experiment, the fully awake rats were from pyramidal cells. To remove inactivation of BKCa
of260 mV for at least 1 min. The composition of the flow unitary conductance, and sensitivity to extracellular TEA
21
solution that bathed the intracellular face of the patch and intracellular Ca . The channels recorded were
selec-1
membrane after excision was (in mM): 140 KCl, 10 NaCl; tive for K , as its channel current–voltage relations
21
10 HEPES. Free Ca concentrations of 0.01, 0.1, 0.5 and reverse at 3.1762.3 mV (n518), very close to the equilib-2mM were obtained by adding a total of 55.7, 279, 435 rium potential for potassium ion (E ), with symmetricalK
1
and 485 mM of CaCl , respectively, to a 5002 mM-EGTA K (140 / 140 mM) in both the pipette and bathing (Sigma) solution [29]. For a solution with a desired free solution. The reversal potential shifted to 262.765.9 mV
1
calcium of 5mM or higher, EGTA was omitted and CaCl2 (n517) with low concentration of 5 mM K in pipette,
1
was added as necessary. Solutions were adjusted to a final indicating a high selectivity for K . Activity of BKCa
pH of 7.40 with KOH. Pipette solution consisted of either channels recorded was affected by the concentration of
21
the bathed solution to give symmetrical charge-carrier calcium ion at the intracellular side, [Ca ] , of excisedi
1 1
distribution, or low K solution (5 mM K and 2 mM membrane patches (Fig. 2B). Whereas less than
micromo-21 1 21
Ca ) to further examine channel selectivity for K . lar concentrations of [Ca ] were sufficient to activatei
MgCl2 at 0.5 mM was routinely added to the pipette BKCa channels from the inner membrane surface, 100mM
21
solution purely for the convenience of easier seal forma- Ca could not activate the channels when applied only at tion in the absence of other divalent ions. All experimental the extracellular membrane surface of excised membrane solutions were made using deionized water. patches, and this was the case for all membrane potentials Sensitivity of the channel recorded to the external tested (from 250 mV to 150 mV). Current–voltage tetraethylammonium (TEA, Sigma) was examined by relations showed that the channels recorded had high using inside-out configuration when TEA was included in unitary conductance (245.44619.14 pS in symmetrical
1
the pipette as described previously [26]. 140 / 140 mM K in inside-out patch; n515). BKCa
channel in CA1 neuron was blocked by low concentration of external 0.5 mM TEA (n523) and was less sensitive to
2.4. Data collection and analysis 5 mM TEA (n521) applied to the internal side of the
patches. These points served as keys to the identification of The single-channel currents were recorded using a the channels recorded as BKCa channels.
Nihon Kohden CEZ-2300 patch clamp amplifier, with the In addition to BKCa channels, the membrane patches current filtered (23 dB, four-pole Bessel filter) at 1 kHz. typically contained other channels with smaller conduct-Data were digitized at sampling rates of 10 kHz using a ance than that of BKCa channels. These channels with TL-125 kHz interface (Scientific Solutions). The analysis smaller conductance will not be considered in this paper, routines used PCLAMP (version 5.5.1, Axon Instruments) and we have typically selected records in which they are to determine distributions for channel amplitudes, and not obviously present.
open and closed times. A 50% threshold criterion was used to determine the durations of open and closed events.
Logarithmic distributions of open and closed durations 3.1. Comparison of channel unitary conductance before were exponentially fitted with the use of the least-square and after ischemia
algorithm method. The ignored level for detecting events
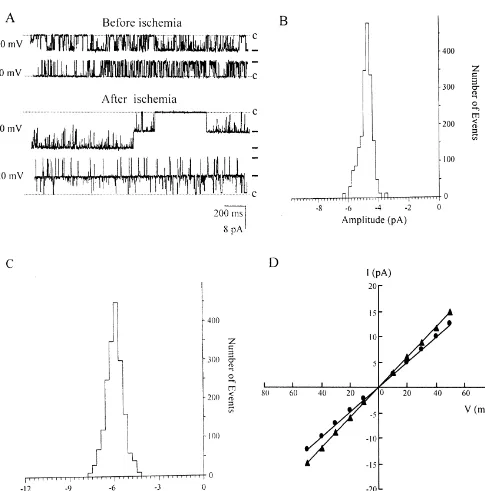
was limited to 300 ms. Channel open probability (P ) iso Fig. 1A shows original traces of single-channel current obtained by dividing NP by N [43], and NP was definedo o from CA1 neurons before and after ischemia. Amplitude as: NPo5oht112t212t31 ? ? ? 1ntnj, where N is the histograms constructed from Fig. 1A exhibited that single channel number, t , t , t are the ratios of open time to1 2 n channel current was 4.89 pA in control neuron (Fig. 1B) total time of measurement for each channel at each of the and 5.94 pA in post-ischemic neuron (Fig. 1C) at 2 mM
21
current levels [28]. Single channel data were obtained at [Ca ] with membrane potential ofi 120 mV. Unitary room temperature (20–228C). The data in text are ex- current amplitude increased across the entire membrane pressed as mean6S.D. and Student’s t-test was used for voltage range tested in both neurons from two groups. To
statistical analysis. compare unitary conductance of BKCa channels between
two groups, amplitudes of the single-channel currents were measured at a number of different membrane potentials and unitary conductance of BKCachannels was determined
3. Results by fitting a regression line through the data (Fig. 1D). The
mean slope conductance of BKCa channels in post-is-Successful recordings were made in 217 membrane chemic neurons (291.35611.48 pS, n515) was signifi-patches of CA1 pyramidal neurons obtained from 116 cantly higher than that in control neurons (245.44619.14
1
adult rats. Properties of BKCa channels in adult CA1 pS, n515) (P,0.01) in symmetrical 140 / 140 mM K , pyramidal neurons were similar to those in other prepara- and the difference in unitary conductance of BKCa
chan-1
Fig. 1. Comparisons of conductance and open probability (P ) of BKo Cachannels in CA1 neurons before and after ischemia. (A) Traces of currents recorded showing a higher amplitude in neurons after ischemia (lower) as compared to control (upper) with membrane voltage held at120 mV or220 mV and 2
21
mM [Ca ] . Outward and inward currents evoked are shown as downward and upward deflections, respectively. The dotted lines indicate the current leveli
at which all channels were closed; dashes on the sides of the records denote unitary current amplitudes. Note also that a larger open probability (P ) aftero
ischemia with two active BKCa channels in post-ischemic neuron and one in control neuron. (B and C) Amplitude histograms constructed from (A) showing that single-channel current in post-ischemic neurons (5.93 pA, C) was higher than that in control (4.96 pA, B). (D) Plots of amplitude (I ) of BKCa
channels against membrane potential (V ) in control (d) and post-ischemic (m) neurons showing a higher unitary conductance of BKCa channels after ischemia as compared with control.
21
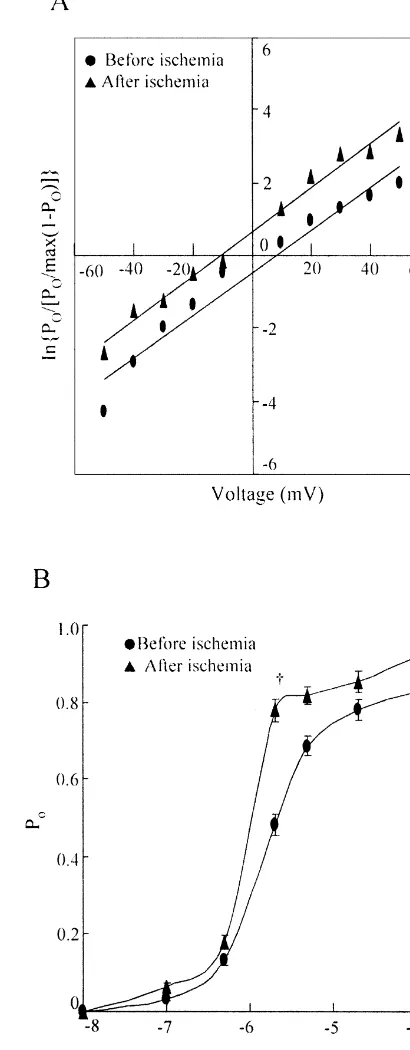
3.2. Comparison of voltage dependence and Ca (120 mV), BKCa channels in both control and
post-sensitivity of BKCa channels before and after ischemia ischemic neurons exhibited long-lasting openings while multiple openings were frequently observed in neurons Fig. 1A shows that activity of BKCa channels in post- after ischemia; at negative membrane potential (220 mV), ischemic neurons (lower trace) was greater than that in more openings were seen in patches from post-ischemic control neurons (upper trace) at fixed membrane potential than from control neurons. A larger open probability of
21
21
[Ca ] and / or a sharper voltage dependence of BKi Ca
channels in post-ischemic neurons. To analyze the differ-ences in voltage dependence of BKCa channels between two groups, individual P –V curves were fitted by theo
Boltzmann equation Po5Po,max/hPo,max1exp[(1 /
21
K )(V1 / 22V )]j with 2 mM [Ca ] . The equation wasi
transformed into the logarithmic form, V5V1 / 21K3 lnhP / [Po o,max(12P )]o j, where K is the membrane depolar-ization for an e-fold increase in P , and Vo 1 / 2 is the patch potential at which Po is one-half of the maximum Po
(Po,max). V1 / 2 and K could be obtained by plotting lnhP /o
[Po,max(12P )]o j against voltage (Fig. 2A). The values of V1 / 2and K were 2.662.1 mV and 17.060.7 mV (n510) for CA1 neurons in control and212.361.7 mV and 16.661.1 mV (n510) for CA1 neurons after ischemia, respectively. Statistical analysis showed that the K value was not significantly different (P.0.05) between two groups while the V1 / 2was more negative (P,0.01) after ischemia when compared with control.
21
To determine differences in sensitivity to [Ca ] ofi
BKCa channels between two groups, inside-out patch
21
recordings were obtained with different Ca concen-trations at the cytosolic surface of the membrane. In
21
constructing this plot, data with each [Ca ] were aver-i
aged from 15 different membrane patches at fixed mem-brane potential of 120 mV. A quantitative comparison of
21
sensitivity to [Ca ] of BKi Ca channels is illustrated in
21
Fig. 2B, where P is plotted as a function of [Cao ] . BKi Ca
channels in CA1 neurons showed a
concentration-depen-21
dent increase in P as [Cao ] was raised from 0.01i mM to 100 mM. P values in post-ischemic CA1 neurons wereo larger than those in control neurons, especially when the
21
[Ca ] was between 0.5 and 2.0i mM (Fig. 2B). The
21
[Ca ] required to half activate (Pi o50.5) BKCa channels was 2mM in control neurons while it was only 1mM after ischemia.
These results indicated that a larger open probability of BKCa channels from CA1 pyramidal neurons after
is-21
chemia mainly reflected more sensitivity to [Ca ] , ratheri
than voltage dependence.
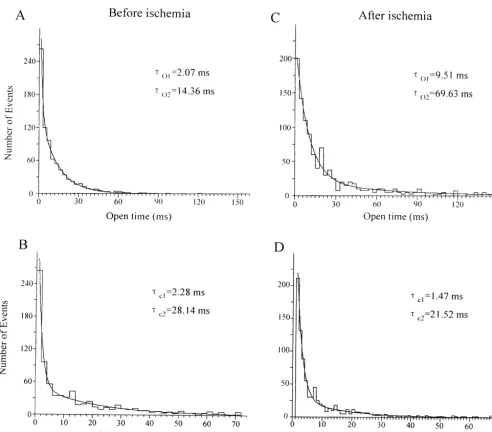
3.3. Comparison of kinetics of BKCa channels before and after ischemia
21
Kinetic analysis of BKCa channels was obtained from Fig. 2. Comparisons in voltage dependence and Ca sensitivity of BKCa
channels in control and post-ischemic CA1 neurons. (A) Logarithmic
patches in which single-channel activities were observed.
21 Boltzmann fittings of P –V curves for BKo Cachannels in control (d) and
At a given holding voltage and [Ca ] , the open prob-i post-ischemic (m) neurons with 2mM [Ca21] . After ischemia, the patch
i
ability is determined by channels’ open time and open potential required to produce one-half of the maximum open probability frequency. To understand which one is the major com- (P ) was more negative than in control but the membrane depolarizationo
ponent contributing to the differences in open probability for an e-fold increase in P showed no significant difference, indicatingo
no obvious changes in channel voltage dependence. (B) Plots of channel
between two groups, kinetics of BKCa channels were 21
open probability (P ) against log [Cao ] with membrane voltage held ati
compared between control and post-ischemic CA1
neu-120 mV in control (d) and post-ischemic (m) neurons showed that the
rons. Fig. 3 shows dwell time histograms constructed from 21
P –[Cao ] curves shifted to the left after ischemia, indicating an increasei 21
21
membrane patches held at 120 mV with 2 mM [Ca ] .i in [Ca ]i sensitivity of BKCa channels after ischemia. Values are
The distributions of open and closed times of BKCa means6S.D. The number of neurons in each group was 15. *P,0.05,
†
Fig. 3. Comparisons in kinetics of BKCachannels before and after ischemia. Distributions for open time (A and C) and closed time (B and D) of BKCa
channels in control (A and B) and post-ischemic neurons (C and D). All histograms of dwell times could be well-fitted by a two-exponential function. Time constants weret 5o1 2.07 ms andt 5o2 14.36 ms in (A),t 5c1 2.28 ms andt 5c2 28.14 ms in (B),t 5o1 9.51 ms andt 5o2 69.63 ms in (C), andt 5c1 1.47 ms
21
andt 5c2 21.52 ms in (D), respectively. Vh5 120 mV; [Ca ]i52mM for A–D. After ischemia, BKCachannels had a longer open time and a shorter closed time as compared to control.
channels from two groups could be fitted well by a two- differences were detected in open frequency (before is-exponential function (Fig. 3). It was shown clearly from chemia: 51.2621.3 events / s, after ischemia: 52.8622.3 the data summarized in Table 1 that the open time of BKCa events / s, n515, P.0.05), indicating a major contribution channels was longer with a shorter closed time after of open time to difference in open probability before and ischemia as compared with control, whereas no significant after ischemia.
Table 1
a
Kinetics of BKCachannels in CA1 neurons before and after ischemia
Open time constants (ms) Closed time constants (ms)
to1 to2 tc1 tc2
Before ischemia 2.7662.11 19.20610.10 2.6961.62 26.89617.88
† † †
After ischemia 11.8066.38 51.97632.29 1.4960.90* 16.72611.12
a †
4. Discussion suggest that one more explanation for the increased
21
[Ca ]i sensitivity of BKCa channels in post-ischemic The principal finding of the present study is that the neurons may be due to changes in alternative splicing. activity of BKCa channels is enhanced in CA1 pyramidal Further experiments are needed to clarify these issues. neurons of rat hippocampus after transient forebrain is- BKCa channels have been demonstrated to contribute to chemia. This functional enhancement in BKCa channels the fAHP in hippocampal pyramidal neurons [25,41]. The may partially account for the post-ischemic changes in augmented activity of BKCa channels after ischemia found membrane properties of CA1 neurons following ischemia. in the present study may account for the result of a As shown in Fig. 1D, the conductance of BKCa channels previous report [14] showing that the amplitude of fAHP increased by about 20% after ischemia. Previous study in in CA1 neurons increased significantly from around 1 mV skeletal muscles [11] and vascular myocytes [32] showed to|5 mV following ischemia. More sensitive to
intracellu-1
that, under physiological conditions, internal Na and lar calcium, more negative V1 / 2, longer open time and
21
Mg can reduce conductance of BKCa channels by reduced closed time, and also increased unitary conduct-interacting with two independent binding sites closely ance of BKCa channels observed in post-ischemic CA1 associated with the permeation pathway of BKCa channels. neurons would all tend to make BKCa channels more
1
It might be possible that the modulation of internal Na on influential in affecting neuronal excitability. Therefore, in conductance of BKCa channels was changed after is- addition to an increase in fAHP, the enhanced activity of
chemia. BKCa channels may lead to a reduction in spontaneous
Calcium sensitivity is a critical property of BKCa firing rate and neuronal excitability observed in CA1 channels that affects their physiological function in neu- pyramidal neurons after transient forebrain ischemia
21
rons by determining the intracellular Ca level required [14,40].
for channel opening. After ischemia, the sigmoid curve In conclusion, the present results show that augmented
21
describing sensitivity to [Ca ] of BKi Ca channels as a activity of BKCa channels reflects more sensitivity to function of membrane potential shifted to the left (Fig. intracellular calcium, more negative V1 / 2, longer open time 2B), indicating an increase in the sensitivity of BKCa and reduced closed time, and also increased unitary
21
channels to [Ca ] . The negative shift of Vi 1 / 2 after conductance after transient forebrain ischemia, indicating
21
reperfusion could be accounted for by the increased Ca that the enhanced BKCa channels may contribute to the sensitivity, since we did not find a significant change in the increase in fAHP and partially account for the decrease in membrane depolarization for an e-fold increase in P . Ao neuronal excitability in CA1 pyramidal neurons after
21
change in Ca sensitivity was also observed in cultured ischemia. spinal neurons of Xenopus during development [4]. In
view of alternation in sensitivity to intracellular calcium after ischemia, we can only speculate as to the nature of
Acknowledgements this change. It seems to be possible that functional changes
in the regulatory proteins of potassium channels after
This research was supported by grants from the National ischemia, e.g. b-subunits [23], which can modulate the
Natural Science Foundation (NNSF 39970265) and Natu-calcium-sensitivity of BKCa channels when expressed with
ral Science Foundation of Guangdong Province (NSFGP the a-subunit [30], may be one candidate in interpreting
21 990395) of PR China to Tian M. Gao.
the alteration of [Ca ] sensitivity.i
Changes in phosphorylation states may be another possible mechanism whereby BKCa channel behavior can
be profoundly altered [27]. Previous studies have shown References modifications of BKCa channels by protein kinases [8–
10,31,33] and phosphatases [38,45] through phosphoryla- [1] J. Aronowski, J.C. Grotta, M.N. Waxham, Ischemia-induced
trans-2
tion and dephosphorylation. Indeed, a change in activities location of Ca / calmodulin-dependent protein kinase II: potential role in neuronal damage, J. Neurochem. 58 (1992) 1743–1752.
or translocations of protein kinases [1,19,39,46] and
phos-[2] G. Baranauskas, T. Tkatch, D.J. Surmeier, Delayed rectifier currents
phatases [17] was found in hippocampal CA1 neurons after
21 in rat globus pallidus neurons are attributable to Kv2.1 and Kv3.1 /
ischemia. So we assumed that increased [Ca ] sensitivityi 1
3.2 K currents, J. Neurosci. 19 (1999) 6394–6404.
of BKCa channels might be due to alterations in its [3] J. Bargas, A. Howe, J. Eberwine, Y. Cao, D.J. Surmeier, Cellular and
21
phosphorylation states via protein kinases or phosphatases molecular characterization of Ca currents in acutely-isolated, adult rat neostriatal neurons, J. Neurosci. 14 (1994) 6667–6686.
after ischemia. Heterologous expression study has shown
21
[4] L.A.C. Blair, V.E. Dionne, Developmental acquisition of Ca
-that alternative splicing of a common RNA precursor 1
sensitivity by K channels in spinal neurons, Nature 315 (1985)
produces functional diversity of the expressed Slowpoke
329–331.
channel, a Drosophila calcium-activated potassium chan- [5] G. Buzsaki, T.F. Freund, F. Bayardo, P. Somogyi, Ischemia-induced´
21
nel, properties in terms of unit conductance, Ca sen- changes in the electrical activity of the hippocampus, Exp. Brain
[6] H.S. Chang, T. Sasaki, N.F. Kassel, Hippocampal unit activity after of potassium channels in rat hippocampal neurons in culture, J. transient ischemia in rats, Stroke 20 (1989) 1051–1058. Neurosci. 11 (1991) 23–30.
[7] D.W. Choi, S.M. Rothman, The role of glutamate neurotoxicity in [27] I.B. Levitan, Modulation of ion channels by protein phosphorylation hypoxic-ischemic neuronal death, Annu. Rev. Neurosci. 13 (1990) and dephosphorylation, Annu. Rev. Physiol. 56 (1994) 193–212. 171–182. [28] H. Liu, E. Moczydlowski, G.G. Haddad, O2 deprivation inhibits
21 1
[8] T.M. Egan, D. Dagan, I.B. Levitan, Properties and modulation of Ca -activated K channels via cytosolic factors in mice neocortical calcium-activated potassium channels in rat olfactory bulb neurons, neurons, J. Clin. Invest. 104 (1999) 577–588.
J. Neurophysiol. 69 (1993) 1433–1442. [29] K.L. Magleby, B.S. Pallotta, Calcium dependence of open and shut [9] M. Esguerra, J. Wang, C.D. Foster, J.P. Adelman, R.A. North, I.B. interval distributions from calcium-activated potassium channels in
21 1
Levitan, Cloned Ca -dependent K channels modulated by a cultured rat muscle, J. Physiol. 344 (1983) 585–604.
functionally associated protein kinase, Nature 369 (1994) 563–565. [30] O.B. McManus, L.M.H. Helms, L. Pallanck, B. Ganetzky, R.
21
[10] D.A. Ewald, A. Williams, I.B. Levitan, Modulation of single Ca - Swanson, R.J. Leonard, Functional role of the beta subunit of high
1
dependent K -channels activity by protein phosphorylation, Nature conductance calcium-activated potassium channels, Neuron 14
315 (1985) 503–506. (1995) 645–650.
21
[11] W.B. Ferguson, Competitive Mg block of a large-conductance, [31] P. Meera, K. Anwer, R. Monga, C. Oberti, E. Stefani, L. Toro, B.M.
21 1 21 21
Ca -activated K channels in rat skeletal muscle. Ca , Sr , and Sanboru, Relaxin stimulates myometrical calcium-activated
potas-21
Ni also block, J. Gen. Physiol. 98 (1991) 163–181. sium channels activity via protein kinase A, Am. J. Physiol. 269 [12] K. Furukawa, K. Yamana, K. Kogure, Postischemic alterations of (1995) C312–C317.
spontaneous activities in rat hippocampal CA1 neurons, Brain Res. [32] E. Morales, W.C. Cole, C.V. Remillard, N. Leblanc, Block of large
21 1
530 (1990) 257–260. conductance Ca -activated K channels in rabbit vascular
21 1
[13] T.M. Gao, E.M. Howard, Z.C. Xu, Transient neurophysiological myocytes by internal Mg and Na , J. Physiol. 495 (1996) 701– changes in CA3 neurons and dentate granule cells after severe 716.
forebrain ischemia in vivo, J. Neurophysiol. 80 (1998) 2860–2869. [33] N.B. Prevarskaya, R.N. Skryma, P. Vacher, J. Djiane, B. Dufy, Role [14] T.M. Gao, W.A. Pulsinelli, Z.C. Xu, Changes in membrane prop- of tyrosine phosphorylation in potassium channels activation. Func-erties of CA1 pyramidal neurons after transient forebrain ischemia tional association with prolaction receptor and JAK2 tyrosine in vivo, Neuroscience 90 (1999) 771–780. kinase, J. Biol. Chem. 270 (1995) 24292–24299.
[15] L.W. Gong, T.M. Gao, H. Huang, Z. Tong, Redox modulation of [34] W.A. Pulsinelli, J.B. Brierley, A new model of bilateral hemispheric large conductance calcium-activated potassium channels in CA1 ischemia in the unanesthetized rat, Stroke 10 (1979) 267–272. pyramidal neurons from adult rat hippocampus, Neurosci. Lett. 286
[35] W.A. Pulsinelli, J.B. Brierley, F. Plum, Temporal profile of neuronal (2000) 191–194.
damage in a model of transient forebrain ischemia, Ann. Neurol. 11 [16] O.P. Hamill, A. Marty, E. Neher, B. Sakmann, F.J. Sigworth,
(1982) 491–498. Improved patch clamp techniques for high-resolution current
record-[36] S.M. Rothman, J.W. Olney, Glutamate and the pathophysiology of ing from cells and cell-free membrane patches, Pflugers Arch. 391
hypoxic-ischemic brain damage, Ann. Neurol. 19 (1986) 105–111.
(1981) 85–100. 21 1
[37] P. Sah, Ca -activated K currents in neurons: types, physiological [17] H. Hara, H. Onodera, H. Kata, T. Araki, K. Kogure,
Autoradiog-roles and modulation, Trends Neurosci. 19 (1996) 150–154. raphic analysis of second messenger and neurotransmitter system
[38] S.C. Sansom, J.D. Stockand, D. Hall, B. Williams, Regulation of receptors in the gerbil hippocampus following transient forebrain
large calcium-activated potassium channels by protein phosphatase ischemia, Brain Res. 545 (1991) 87–96.
21 2A, J. Biol. Chem. 272 (1997) 9902–9906.
[18] G.A. Hicks, N.V. Marrion, Ca -dependent inactivation of large
21 1 [39] D.A. Shackelford, R.Y. Yeh, M. Hsu, G. Buzsaki, J.A. Zivin, Effect
conductance Ca -activated K (BK) channels in rat hippocampal
of cerebral ischemia on calcium / calmodulin-dependent protein neurons produced by pore block from an associated particle, J.
kinase II activity and phosphorylation, J. Cereb. Blood Flow Metab. Physiol. 508 (1998) 721–734.
15 (1995) 450–461. [19] B.R. Hu, T. Wieloch, Tyrosine phosphorylation and activation of
[40] K. Shinno, L. Zhang, J.H. Eubanks, P.L. Carlen, M.C. Wallace, mitogen-activated protein kinase in the rat brain following transient
Transient ischemia induces an early decrease of synaptic transmis-cerebral ischemia, J. Neurochem. 62 (1994) 1357–1367.
sion in CA1 neurons of rat hippocampus: electrophysiological study [20] H. Iomn, A. Mitani, Y. Andou, T. Arai, K. Kataoka, Delayed
in brain slices, J. Cereb. Blood Flow Metab. 17 (1997) 955–966. neuronal death is induced without postischemic hyperexcitability:
[41] J.F. Storm, Action potential repolarization and a fast after-hyperpo-continuous multiple-unit recording from ischemic CA1 neurons, J.
larization in rat hippocampal pyramidal cells, J. Physiol. 385 (1987) Cereb. Blood Flow Metab. 11 (1991) 819–823.
733–759. [21] J. Kang, J.R. Huguenard, D.A. Prince, Development of BK channels
[42] R. Suzuki, T. Yamaguchi, C.L. Li, I. Klatzo, The effects of 5-minute in neocortical pyramidal neurons, J. Neurophysiol. 76 (1996) 188–
ischemia in mongolian gerbils. II. Changes of spontaneous neuronal 198.
activity in cerebral cortex and CA1 sector of hippocampus, Acta [22] T. Kirino, Delayed neuronal death in gerbil hippocampus following
Neuropathol. (Berl.) 60 (1983) 217–222. ischemia, Brain Res. 237 (1982) 59–69.
[43] D. Thuringer, I. Findlay, Contrasting effects of intracellular redox [23] H.-G. Knaus, K. Folander, M. Garcia-Calvo, M.-L. Garcia, G.J.
couples on the regulation of maxi-K channels in isolated myocytes Kaczorowski, M. Smith, R. Swanson, Primary sequence and
immu-from rabbit pulmonary artery, J. Physiol. 500 (1997) 583–592. nological characterization of beta-subunit of high-conductance
21 1 [44] K.T. Wann, C.D. Richards, Properties of single calcium-activated
Ca -activated K channels from smooth muscle, J. Biol. Chem.
potassium channels of large conductance in rat hippocampal neurons 269 (1994) 17274–17278.
in culture, Eur. J. Neurosci. 6 (1994) 607–617. [24] A. Lagrutta, K.Z. Shen, R.A. North, J.P. Adelman, Functional
[45] R.E. White, A. Schonbrunn, D.L. Armstrong, Somatostatin stimu-differences among alternatively spliced variants of slowpoke, a
21 1
lates Ca -activated K channels through protein
dephosphoryla-Drosophila calcium-activated potassium channel, J. Biol. Chem. 269
tion, Nature 351 (1991) 570–573. (1994) 20347–20351.