www.elsevier.com / locate / bres
Research report
Sodium valproate alters GnRH–GABA interactions during
development in seizure-prone mice
a a b a ,
*
Amanda M. Illig , Karen Melia , Peter J. Snyder , Lori L. Badura
a
Behavioral Neuroscience Division, Psychology Department, University at Buffalo, Buffalo, NY 14260, USA b
Department of Clinical Research, Pfizer Central Research, Groton, CT, USA
Accepted 29 August 2000
Abstract
During reproductive maturation, characteristic changes occur in the morphology of the gonadotropin releasing hormone (GnRH) cell population within the hypothalamus. In the early stages of development, GnRH neurons are bipolar cells; however, just before pubertal onset, the majority of these neurons transform into unipolar cells. Our laboratory has reported that valproic acid (VPA), an antiepileptic medication that has previously been shown to slow the velocity of pubertal development in both humans and seizure-prone mice, is capable of delaying the normal process of GnRH morphological differentiation. As VPA is primarily believed to act via a GABAergic mechanism, the present study investigated potential influences of VPA on GnRH–GABA interactions within the medial preoptic area (mPOA) across pubertal development (experiment 1), as well as in adult animals (experiment 2). The results from experiment 1 revealed the expected drug effects on GnRH cell morphology. For VPA animals, there was a greater percentage of bipolar neurons at every time period except for the 24-day sample. Additionally, VPA animals had greater numbers of bipolar and unipolar GnRH neurons with GABA associations across all ages. However, experiment 2 showed a lack of drug effects on GnRH–GABA interactions in adulthood. These results suggest that VPA may delay GnRH cell morphological maturation by altering the density of GABAergic inputs to GnRH neurons. These inputs may normally play a role in timing the activation of the GnRH pulse generator. However, any neuroendocrine effects of VPA in adulthood are most likely due to the actions of VPA at another level of the hypothalamic-pituitary-gonadal axis. 2000 Elsevier Science B.V. All rights reserved.
Theme: Disorders of the nervous system
Topic: Epilepsy: anti-convulsant drugs
Keywords: Medial preoptic area; Epilepsy; GnRH pulse generator; Hypothalamus; Puberty
1. Introduction years, VPA has also become increasingly popular as a
mood stabilizer in the treatment of several affective Valproic acid (VPA, 2-propylpentanoic acid) is an disorders, including major depression [21] and bipolar extremely potent and widely prescribed anticonvulsant disorder [30].
agent. Originally synthesized by Burton in 1882, VPA was Despite its widespread use, the exact mechanism of later shown to have anticonvulsant properties in 1963 [31]. action of VPA still remains relatively unclear. However, it VPA was licensed for use in the United States as an is generally agreed upon that VPA acts to potentiate anticonvulsant in 1978. Since that time, the use of VPA in GABA-mediated postsynaptic inhibition within the CNS the clinical population has increased dramatically, making via some, as of yet, unknown cellular mechanism [20]. The it one of the most frequently prescribed agents for the first major hypothesis for the cellular action of VPA was treatment of generalized seizure disorders [26]. In recent made by Godin et al. [12], who proposed that VPA produces an increase in the level of gamma-aminobutyric acid (GABA) in the CNS. Since that time, several mecha-nisms have been postulated to explain the GABA-elevating
*Corresponding author. Tel.:11-716-645-3650, ext. 220; fax:1
1-716-effects produced by VPA, including inhibition of several
645-3801.
E-mail address: [email protected] (L.L. Badura). degradative enzymes in the GABA shunt pathway; as well
as an increase in the activity of glutamic acid decarbox- inbred mice of the DBA / 2J strain [35]. This finding has ylase (GAD), the rate-limiting enzyme in the synthesis of created an animal model that has proven to be useful in GABA [10]. examining the effects of VPA on the endocrine system, and MacDonald and Bergey [28] proposed a second major in particular, attempting to determine the precise site of hypothesis for VPA’s action, suggesting that VPA may be VPA’s action on the hypothalamic-pituitary-gonadal enhancing neuronal responsiveness to GABA. These inves- (HPG) axis.
tigators demonstrated that iontophoresed VPA was capable Reproductive maturation is an extremely complex pro-of augmenting postsynaptic inhibition mediated by ion- cess that is principally regulated by both central and tophoresed GABA in cultured spinal cord neurons. Sub- peripheral mechanisms that control the development of the sequently, Kerwin et al. [22] reported the ability of VPA to HPG axis. Although the exact mechanisms that drive this potentiate GABA responses in cortical neurons in vivo. development remain to be fully elucidated, it is generally A third major hypothesis, which has received much less believed that the pulsatile release of gonadotropin releasing attention, suggests that VPA may have direct membrane- hormone (GnRH) from GnRH neurons within the hypo-stabilizing effects on neurons. However, precisely how thalamus plays a pivotal role in timing the onset of puberty VPA exerts these effects continues to be controversial. [34,38].
Using isolated frog spinal cord, Hackman et al. [13] found In rodents, the majority of GnRH cell bodies are located that low concentrations of VPA hyperpolarize dorsal roots, within the medial preoptic area (mPOA) [38]. During while higher concentrations produce depolarizations. Thus, normal sexual development, distinct morphological VPA may retard rapid neuronal firing in a manner that changes occur within the GnRH cell population prior to the mimics the actions of endogenous GABA [10]. In addition onset of puberty. It has been widely reported that early in to the GABA hypothesis, some evidence suggests that development, GnRH neurons are bipolar cells with smooth VPA may function to decrease excitatory amino acid and surfaces, however, just prior to puberty, many of these norepinephrine activity within the CNS [4,7]. neurons transform into unipolar cells with irregular spine-VPA has been shown to suppress the secretion of several like surfaces. Although the total number of GnRH cells pituitary hormones, including growth hormone (GH), does not change, the percentage of irregular, ‘spiny’ GnRH adrenocorticotrophic hormone (ACTH), follicle-stimulat- cells increases during reproductive development [34,38]. ing hormone (FSH), and luteinizing hormone (LH) It has been suggested that these morphological changes [5,18,23,27]. Additionally, reports of various symptoms associated with the pubertal development process are associated with decreased pituitary hormone release have related to an increased function of the GnRH cell popula-been described in the clinical population. In adults, VPA tion, as development of the normal diurnal pattern of LH administration has been shown to induce unresponsiveness release [37], as well as responsiveness of the hypo-to GnRH challenge, transient amenorrhea, polycystic thalamic-pituitary axis to the positive feedback [1] have ovarian syndrome, and hyperandrogenism [19,27,29]. been found to coincide with the increase in the number of
Furthermore, in a subset of the juvenile clinical popula- unipolar neurons in the preoptic region.
tion, chronic treatment with VPA has been shown to elicit a Many neurotransmitters and peptide neuromodulators retardation of pubertal development and skeletal growth have been implicated in the regulation of GnRH function. [6]. Specifically, Cook et al. [6] first described the case of In particular, several lines of evidence provide support for a 12-year-old girl who experienced an arrest of normal an inhibitory role of GABA in the modulation of the reproductive development during chronic treatment with activity of GnRH cells. Firstly, ultrastructural data indicate VPA for complex partial seizures. Shortly after this that GABAergic neurons establish symmetric (inhibitory) observation, VPA treatment was discontinued and normal synapses directly onto GnRH neurons within the mPOA pubertal development and body growth resumed 2 months [25]. Additionally, administration of GABA and GABA later. Taken together, all of this clinical evidence raises the agonists directly into the mPOA has been shown to reduce possibility that chronic VPA administration may result in GnRH gene [2], suppress the release of LH from the adverse effects on the neuroendocrine system, including anterior pituitary [24,33], and block the estrogen-induced disturbances of growth, sexual development, and fertility. LH surge that normally occurs at the time of proestrous Although a large body of literature has accumulated on [15]. More recently, infusion of GABA agonists into a the adverse effects of chronic VPA administration on the superfusion medium containing nuclei from the mPOA has neuroendocrine system, its mechanism and sites of action been found to directly decrease levels of GnRH secretion for inhibition of the reproductive axis remain to be [11].
GABA inhibition may be a key factor for the activation of Jackson laboratory (Bar Harbor, ME). Animals were group the GnRH pulse generator and, thus, the onset of puberty. housed (n54 / cage) in polypropylene cages and had access The previously discussed animal model established by to food (Agway Lab Chow 3000) and water ad libitum. All this laboratory, which mimics the clinical neuroendocrine animals were maintained from birth in a colony room abnormalities frequently associated with VPA treatment, equipped with a controlled photoperiod (12 L:12 D, with was recently utilized in a preliminary morphological study lights off at 18:00 h). All procedures were approved by the exploring the effects of VPA on the normal development of Institutional Animal Care and Use Committee at the the GnRH cell population within the mPOA [36]. Interest- University at Buffalo prior to implementation.
ingly, Snyder and Badura [36] found that chronic
adminis-tration of VPA in DBA / 2J mice delays the typical pattern 2.2. Drug administration of GnRH cell morphological changes occurring during
reproductive maturation. As the effects of VPA on GnRH Juvenile male DBA / 2J mice were weaned at 14 days of cell morphology are correlated with a slowing of gonadal age and were placed either on oral administration of VPA development, it is likely that this delayed maturation of (17 mg / kg / day) or control vehicle solution (CON). Since GnRH cells within the mPOA may lead to diminished VPA is highly water-soluble and is rapidly absorbed from anterior pituitary activity. Such a mechanism might explain the digestive tract [3], it was administered via the drinking the reproductive maturational delays that are observed in water to which 1% sucrose was added to increase some epileptic patients. palatability. This dosage of VPA was calculated to corre-Thus, if it is assumed that these morphological changes late with therapeutically relevant serum concentrations of are functional in nature, it appears that VPA may exert at VPA when used as an anticonvulsant in the clinical least some of its inhibitory effects on the HPG axis at the population [3]. Where appropriate, drug dosage was in-level of the hypothalamic GnRH cell population. Taking creased in 2-week steps in order to account for develop-into consideration both the inhibitory role of GABA as a mental differences in body weight, as well as tolerance neurochemical regulator of GnRH cells, as well as VPA’s effects. Previous work from this laboratory has revealed proposed GABAergic mechanism of action, it is possible that, under these conditions, individual mice consume 6–8 that VPA may mimic and / or elevate the activity of the ml of fluid / day and there are no differences in fluid GABAergic system to slow normal morphological matura- consumption between the VPA and CON groups [35]. tional changes occurring within the GnRH neuronal
popu-lation.
2.3. Tissue preparation The goal of the present study was to expand upon the
preliminary findings of Snyder and Badura [36] by
ex-Subsets of animals (n55–9 / age group) receiving VPA amining the putative effects of VPA on GnRH–GABA
or control solution were sacrificed via anesthetic overdose interactions within the mPOA across pubertal
develop-with sodium pentobarbital (100 mg / kg). Animals were ment, as well as in reproductively mature adult animals. As
either sacrificed at 21, 24, 28, or 32 days of age for the these mice typically become reproductively mature around
developmental portion of the study (experiment 1), or 30–45 days of age, the addition of adult animals to this
following 6 weeks of drug administration for the adult (8 study allows for an examination of any residual effects of
weeks of age) portion of the study (experiment 2). The VPA treatment on the GnRH cell population after, under
animals were then administered 0.1 ml of heparin and normal circumstances, reproductive maturity should have
perfused transcardially with 0.87% physiological saline, been reached.
followed by 4% paraformaldehyde in 0.1 M NaPO buffer4
This investigation was accomplished via a dual-labeling
(pH 7.3) using a perfusion pump. Brains were removed immunocytochemical technique for both GnRH and
and post-fixed in the same fixative overnight. Tissue was GABA. By using light level microscopy with
immuno-then cryoprotected with 4% paraformaldehyde, 20% suc-histochemical visualization techniques, this study allowed
rose (pH 7.3) for 2 days prior to frozen-sectioning (40mm) any significant gross morphological changes occurring in
through the preoptic area on a sliding microtome with a the relationship between these two cell groups as a result
freezing stage. All sections were stored in 0.1 M NaPO4
of VPA administration to be detected.
buffer (pH 7.3) until immunocytochemical staining for GnRH and GABA.
2. Methods
2.4. Dual-labeling immunocytochemistry 2.1. Animals and housing
times for 5 min each in 0.1 M phosphate-buffered saline data obtained in experiment 1, a two-way (drug3age) with 0.2% Triton (PBS-TX, pH 7.3). Firstly, sections were analysis of variance (ANOVA) was utilized. For the adult incubated for 30 min in 0.3% H O . This was followed by2 2 data obtained in experiment 2, a one-way ANOVA for drug a 30-min blocking step in 1% normal goat serum. Next, effects was used. Significant interactions were broken sections were incubated for 24 h at 48C in rabbit anti- down using analysis of simple main effects, with Fisher’s GnRH (Incstar, LOT [ 635479, dilution51:4000), which LSD post-hoc tests where appropriate. All results were
served as the primary antibody for the first labeling considered significant if P,0.05. sequence. The following day, there was a 2-h incubation in
biotinylated goat anti-rabbit second antibody (dilution5
1:100). This was followed by a 1-h incubation in
Vectas-tain avidin-biotin complex (ABC, Vector laboratories). 3. Results
Sections were developed using 0.1% diaminobenzidine
(DAB) and 0.2% glucose-oxidase (GOD), which produced The total number of GnRH-immunoreactive neurons did a brownish-red reaction product. not differ significantly among drug or age groups in either The second labeling sequence began with a re-blocking experiment 1 or experiment 2. Stained neurons were step in 1% normal goat serum. This was followed by a divided into unipolar and bipolar groups based upon their 24-h incubation at 48C in guinea pig anti-GABA morphology. The processes of these neurons were fol-(Chemicon, LOT[ 18060181, dilution51:2000), which lowed through several planes of focus in order to ensure
served as the primary antibody for the second labeling that they extended out from the cell bodies of the neurons sequence. The following day, there was a 2-h incubation in of interest and were not part of other GnRH neurons biotinylated goat anti-guinea pig second antibody located in proximity to them.
(dilution51:100). This was followed by a 1-h incubation in Vectastain avidin-biotin complex (ABC, Vector
labora-tories). Sections were developed using 0.1% DAB and 8% 3.1. Experiment 1: effects of VPA on GnRH–GABA nickel chloride, which produced a bluish-black reaction interactions across development
product.
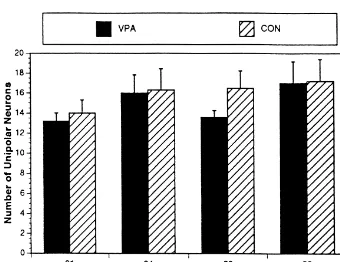
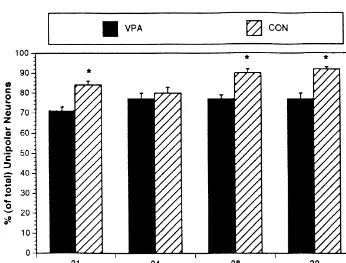
There was a significant main effect of drug (F1,375
32.972, P,0.001), but no main effect of age on the total 2.5. Histological evaluation number of bipolar neurons among groups. Follow-up analyses revealed that the VPA-treated group had more Two representative sections from each animal were bipolar neurons than the CON group at every age except subjected to histological examination with an Olympus the 24-day sample (P,0.001; Fig. 1). However, for the BH-40 microscope. The sections chosen for analysis total number of unipolar neurons, there were no significant extended in 40-mm increments beginning rostrally at the differences between groups at any time point (Fig. 2). decussation of the anterior commissure. The total number There were also no significant differences at any time of neurons immunoreactive for GnRH was counted for point for the percentage (of total) of bipolar neurons each section, and the percentages (of total) of unipolar and among groups (Fig. 3). However, for the percentage (of bipolar neurons were determined for the analyses. Addi- total) of unipolar neurons, there was a significant main tionally, the total number of unipolar and bipolar neurons effect of drug (F1,37553.958, P,0.001), but no main having GABA associations was determined. For the pur- effect of age. Follow-up comparisons revealed that the pose of this study, a GnRH-immunoreactive neuron was percentage of unipolar neurons was greater for the CON defined as having a GABA association if at least one group at all time points except for the 24-day period GABA-immunoreactive process was found to cross over (P,0.001; Fig. 4).
its cell body and / or one of its processes within the same There was a significant main effect of drug (F1,375
Fig. 1. Mean6S.E.M. number of bipolar neurons at each age group. *Significantly greater then CON group for all sampling times except 24 days,
P,0.001.
3.2. Experiment 2: effects of VPA on GnRH–GABA groups (Table 1). Likewise, there was no significant effect
interactions in adulthood of drug on the total number of unipolar neurons (Table 1). Additionally, there were no significant differences between There was no significant effect of drug on the total the two treatment groups on the mean number of bipolar or number of bipolar neurons between the two adult treatment unipolar neurons with GABA associations (Table 2).
Fig. 3. Mean6S.E.M. percentage (of total) of bipolar neurons at each age group.
4. Discussion ment of the GnRH neurosecretory system during the
maturational process [34,38], as well as with the mor-As expected, the total number of GnRH-immunoreactive phological study previously conducted in this laboratory neurons did not change across development. This finding is [36]. Additionally, the results obtained from this study add consistent with previously reported work on the develop- further support to the previous tenet proposed by Snyder
and Badura [35,36] that administration of VPA delays normal GnRH cell morphological differentiation. VPA-treated animals had significantly greater numbers of bipo-lar GnRH-immunoreactive neurons than CON animals at every time period except for the 24-day sampling period. Likewise, the percentage of unipolar GnRH-immuno-reactive neurons was greater for the CON group at every sampling period except for 24 days of age. If these morphological changes are, in fact, functional in preparing the GnRH pulse generator for the onset of puberty, then any alteration might explain the previously reported delays in reproductive development induced by chronic VPA administration in DBA / 2J mice [26,35].
In particular, VPA-treated animals were found to have more bipolar and unipolar GnRH-immunoreactive neurons with GABA associations when compared with
age-Fig. 5. Mean6S.E.M. number of bipolar neurons with GABA
associa-matched controls at all sampling ages utilized in the
tions. *Significantly greater than CON group at all sampling times,
present study. Thus, it appears that VPA may elicit a delay P,0.001.
in GnRH cell morphological maturation by acting on GABAergic cells that provide direct input to GnRH neurons located within the hypothalamus. However, with a lack of evidence for changes in the density of GnRH– GABA associations throughout development, it is still unclear precisely how VPA exerts these effects.
Although the sites and mechanisms of VPA’s action remain to be fully elucidated, it is generally accepted that VPA produces an increase in the level of GABA in the brain, thereby, augmenting GABA-mediated postsynaptic inhibition. Pilot work from this laboratory indicates that VPA does increase GABA release in the mPOA of male DBA / 2J mice [9], thereby, suggesting that VPA’s inhib-itory actions on GnRH cell development may be mediated by enhancing extracellular GABA concentrations within the mPOA.
Several mechanisms have been postulated to explain the
Fig. 6. Mean6S.E.M. number of unipolar neurons with GABA
associa-GABA-elevating effects produced by VPA, including
inhi-tions. *Significantly greater than CON group at all sampling times,
bition of several degradative enzymes in the GABA shunt P,0.001.
pathway, as well as an increase in the activity of GAD, the rate-limiting enzyme in the synthesis of GABA [10]. In
Table 1
particular, Harvey et al. [14] have shown that VPA is, in
Mean (6S.E.M.) total GnRH-ir cell counts across adult treatment groups
fact, capable of inhibiting GABA aminotransferase
Drug Total unipolar Total bipolar
(GABA-T) and succinic semialdehyde dehydrogenase
GnRH-ir GnRH-ir
(SSAD). These two mitochondrial enzymes are necessary
cell counts cell counts
for the intracellular degradation of GABA. In this way,
VPA 31.8960.61 4.5660.50
VPA could act to increase the amount of GABA available
CON 32.6361.07 4.8860.69
for release, which could subsequently lead to an increase in overall GABA release.
Given this evidence for at least one of VPA’s potential
Table 2
Mean (6S.E.M.) total GnRH-ir cells with GABA associations across mechanisms of action, it seems unlikely that, by simply adult treatment groups increasing GABA synthesis and release, VPA could induce
Drug Total unipolar Total bipolar such a significant increase in the overall number of
GnRH-ir GnRH-ir GABAergic inputs to GnRH neurons as was observed in cells with GABA cells with GABA this study. However, one theory can be postulated to associations associations
explain the robust nature of its effects on GnRH–GABA
VPA 6.5660.65 2.4460.60 associations throughout development. In particular, by
CON 6.8860.61 2.0060.42
maintained GABAergic contacts with GnRH neurons that the mean number of bipolar or unipolar neurons with GABA associations. These results were also expected and were already present prior to the start of drug treatment.
provided support for the final hypothesis, which stated that Although a decline in GABAergic inputs was not
any VPA-induced increase in the density of GnRH–GABA observed across developmental age, it remains possible
associations might no longer be present in reproductively that this decline could normally begin at an earlier age than
mature adult animals. Thus, it appears that VPA’s effects was examined in the present study (i.e. earlier than 21 days
on the GnRH neurosecretory system may only act to delay of age). This rapid early decline of GABAergic inputs to
reproductive development in DBA / 2J mice, but does not GnRH cells is quite possible, as most synapse elimination
stop it completely. has been shown to occur very early in postnatal life [26].
Although VPA is capable of increasing the number of In other words, CON animals may have had higher
GnRH–GABA associations during development via the numbers of GnRH–GABA associations at an earlier age
mechanism proposed above, it may be possible that this group than was assessed in the present study, at which time
effect diminishes over time. The VPA-induced increase in they would have been no different from VPA-treated
GABAergic activity might decline in response to an animals on this variable. But sometime between 14 (the
increase in stimulatory inputs (including NE and gluta-initiation of drug treatment) and 21 days of age, the
mate) to the GnRH cell group. In particular, it has density of these GABAergic inputs in the CON group may
previously been mentioned that NE decreases mPOA have begun to decline rapidly, reaching somewhat of a
GABA release both in vitro and in vivo [16,17]. Thus, stabilization around 21 days of age.
given enough time, these stimulatory inputs might override It is generally believed that mPOA GABA levels are
the increased GABAergic inhibition on the GnRH cell higher prior to the onset of puberty in order to maintain
group induced by VPA, thereby, resulting in normal GnRH tonic inhibition on the GnRH cell group [8,32]. If this is
cell differentiation, activation of the GnRH pulse genera-presumed to be true, then a greater number of GnRH–
tor, and the onset of reproductive maturity. This would GABA interactions early in postnatal life could result from
explain the fact that, by 8 weeks of age, VPA-treated higher levels of activity in the GABAergic cell population.
animals no longer differed from CON animals on either This activity increase could elevate extracellular GABA
endocrine or gonadal measures. levels, thereby, augmenting the degree of inhibition on
However, in the clinical population, various neuroen-GnRH neurons. Likewise, a rapid decline in GABAergic
docrine abnormalities are still reported in adults inputs with increasing developmental age might represent a [17,27,29]. Although these side effects are somewhat less normal form of ‘plasticity’ that exists within the hypo- severe than those observed in juvenile patients, they are thalamic-pituitary axis. In this manner, the HPG axis could still common. Based on the findings from this study, it prepare for the eventual activation of the GnRH pulse appears that the side effects observed in adult epileptic generator, and the subsequent onset of puberty. patients might be due to the actions of VPA at yet another VPA may be capable of interfering with this ‘plasticity’ level of the HPG axis, possibly at the level of the anterior by maintaining high levels of activity in the GABAergic pituitary itself.
cell group. In accordance with the activity-dependent In conclusion, this series of experiments not only phenomenon of synapse elimination, these high activity provides further knowledge regarding VPA’s actions at the levels could strengthen the connections between GABAer- level of GnRH neurons within the mPOA during develop-gic and GnRH neurons. This would then result in the ment, but it also suggests that VPA has yet another stabilization and maintenance of a large number of GnRH– potential mechanism of action on the HPG axis, by which GABA associations throughout reproductive development, it exerts its commonly reported neuroendocrine side effects thereby, explaining the results obtained from experiment 1 in adulthood. Future investigations should focus on at-of the present study. By maintaining an increased number tempting to elucidate this, as of yet, unknown mechanism of GnRH–GABA interactions within the mPOA during of action.
development, VPA could enhance GABA-mediated inhibi-tion on GnRH neurons, thus, delaying their differentiainhibi-tion,
the activation of the GnRH pulse generator, and the Acknowledgements eventual onset of puberty.
Finally, the data obtained from experiment 2 of this This work was supported by NSF grant IBN9603780 study indicate that, by 8 weeks of age (following 6 weeks and institutional funds from the Dean of Arts and Sciences of drug treatment), there was no longer any effect of VPA at the University at Buffalo to Lori L. Badura, Ph.D. on either the number of bipolar or unipolar GnRH neurons
within the mPOA. These results were as expected based on
the preliminary study using this animal model, in which References VPA-treated animals no longer differed from their control
counterparts on measures of endocrine or gonadal status at [1] W.W. Andrews, G.J. Mizejewski, S.R. Ojeda, Development of
female rat: a quantitative study, Endocrinology 109 (1981) 1404– [21] L.I. Kemp, Sodium valproate as an antidepressant, Br. J. Psychiatry
1413. 160 (1992) 121–123.
[2] H.T. Bergen, J.F. Hejtmancik, D.W. Pfaff, Effects of gamma-amino- [22] R.W. Kerwin, H.R. Olpe, M. Schmutz, The effect of sodium-n-butyric acid receptor agonists and antagonist on LHRH-synthesizing dipropyl acetate on GABA-dependent inhibition in the rat cortex and neurons as detected by immunocytochemistry and in situ hybridiza- substantia nigra in relation to its anticonvulsant activity, Br. J. tion, Exp. Brain Res. 87 (1991) 46–56. Pharmacol. 71 (1980) 545–551.
[3] J. Bruni, B.J. Wilder, Valproic acid: review of a new antiepileptic [23] R.K. Kritzler, E.P. Vining, L.P. Plotnick, Sodium valproate and drug, Arch. Neurol. 36 (1979) 393–398. corticotropin suppression in the child treated for seizures, J. Pediatr. [4] A.G. Chapman, B.S. Meldrum, E. Mendes, Acute anticonvulsant 102 (1983) 142–143.
activity of structural analogues of valproic acid and changes in brain [24] R. Lamberts, E. Vijayan, M. Graf, T. Mansky, W. Wuttke, In-GABA and aspartate levels, Life Sci. 32 (1983) 2023–2031. volvement of preoptic-anterior hypothalamic GABA neurons in the [5] M.J. Conran, P.J. Kearney, M.N. Callaghan, D. Murphy, T. Goggin, regulation of pituitary LH and prolactin release, Exp. Brain Res. 52
Hypothalamic pituitary function testing on children receiving car- (1983) 356–362.
bamazepine or sodium valproate, Epilepsia 26 (1985) 585–588. [25] C. Leranth, N.J. MacLusky, H. Sakamoto, M. Shanabrough, F. [6] J.S. Cook, J.F. Bale, R.P. Hoffman, Pubertal arrest associated with Naftolin, Glutamic acid decarboxylase-containing axons synapse on valproic acid therapy, Pediatr. Neurol. 8 (1992) 229–231. LHRH neurons in the rat medial preoptic area, Neuroendocrinology [7] J.M. Crowder, H.F. Bradford, Common anticonvulsants inhibit 40 (1985) 536–539.
11
Ca uptake and amino acid transmitter release in vitro, Epilepsia [26] J.W. Lichtman, S.J. Burden, S.M. Culican, R.O.L. Wong, Synapse 28 (1987) 378–382. formation and elimination, in: M.J. Zigmond, F.E. Bloom, S.C. [8] J. Demling, E. Fuchs, M. Baumert, W. Wuttke, Preoptic catechol- Landis, J.L. Roberts, L.R. Squire (Eds.), Fundamental Neuroscience,
amine, GABA, and glutamate release in ovarianectomized estrogen Academic Press, San Diego, CA, 1999, pp. 547–580.
primed rats utilizing push-pull cannula technique, Neuroendocrinol- [27] B. Lundberg, A. Nergardh, E.M. Ritzen, K. Samuelsson, Influence ogy 41 (1985) 212–221. of valproic acid on the gonadotrophin-releasing hormone test in [9] J.C. Dodge, A.M. Illig, P.J. Snyder, L.L. Badura, GABA levels puberty, Acta Pediatr. Scand. 75 (1986) 787–792.
within the medial preoptic area: effects of chronic administration of [28] R.L. MacDonald, G.K. Bergey, Valproic acid augments GABA-sodium valproic acid, Psychoneuroendocrinology (2000), In press. mediated postsynaptic inhibition in cultured mammalian neurons, [10] C.L. Faingold, R.A. Browning, Mechanisms of anticonvulsant drug Brain Res. 170 (1979) 558–562.
action II. Drugs primarily used for absence epilepsy, Eur. J. Pediatr. [29] J.W. Margraf, F.E. Dreifuss, Amenorrhea following initiation of 146 (1987) 8–14. therapy with valproic acid, Neurology 31 (1981) 159.
[11] C. Feleder, H. Jarry, S. Leonhardt, W. Wuttke, J.A. Moguilevsky, [30] S.L. McElroy, P.E. Keck, H.G. Pope, J.I. Hudson, Valproate in the The GABAergic control of gonadotropin-releasing hormone secre- treatment of bipolar disorder: literature review and clinical guide-tion in male rats during sexual maturaguide-tion involves effects on lines, J. Clin. Psychopharmacol. 12 (1992) S42–S52.
hypothalamic excitatory and inhibitory amino acid systems, Neuro- [31] H. Meunier, G. Carraz, Y. Meunier, P. Eymard, M. Aimard, endocrinology 64 (1996) 305–312. Proprietes pharmacodynamique de l’acide n-di-propylacetique, [12] Y. Godin, L. Heiner, J. Mark, P. Mandel, Effects of di-n-pro- Therapie 18 (1963) 435–438.
pylacetate, an anticonvulsant compound, on GABA metabolism, J. [32] D. Mitsushima, D.L. Hei, E. Terasawa,g-aminobutyric acid is an Neurochem. 16 (1969) 869–873. inhibitory neurotransmitter restricting the release of luteinizing [13] J.C. Hackman, V. Grayson, R.A. Davidoff, The presynaptic effects of hormone-releasing hormone before the onset of puberty, Proc. Natl.
valproic acid in the isolated frog spinal cord, Brain Res. 220 (1981) Acad. Sci. 91 (1994) 395–399.
269–285. [33] C.J. Scott, I.J. Clarke, Inhibition of luteinizing hormone secretion in [14] P.K. Harvey, H.F. Bradford, A.N. Davison, The inhibitory effect of ovariectomized ewes during the breeding season byg-aminobutyric sodium-n-dipropylacetate on the degradative enzymes of the GABA acid (GABA) is mediated by GABA-A receptors, but not GABA-B shunt, FEBS Lett. 52 (1975) 251–254. receptors, Endocrinology 132 (1993) 1789–1796.
[15] A.E. Herbison, R.G. Dyer, Effect on luteinizing hormone secretion [34] A.J. Silverman, I. Livne, J. Witkin, The gonadotropin-releasing of GABA receptor modulation in the medial preoptic area at the hormone (GnRH) neuronal systems; immunocytochemistry and in time of proestrous luteinizing hormone surge, Neuroendocrinology situ hybridization, in: E. Knobil, J.D. Neill (Eds.), 2nd Edition, The 53 (1991) 317–320. Physiology of Reproduction, Raven Press, New York, 1994, pp. [16] A.E. Herbison, R.P. Heavens, R.G. Dyer, Oestrogen and norad- 1683–1709.
renalin modulate endogenous GABA release from slices of the rat [35] P.J. Snyder, L.L. Badura, Chronic administration of sodium valproic medial preoptic area, Brain Res. 486 (1989) 195–200. acid slows pubertal maturation in inbred DBA / 2J mice: skeletal, [17] A.E. Herbison, R.P. Heavens, R.G. Dyer, Oestrogen modulation of histological, and endocrinological evidence, Epilepsy Res. 20 (1995)
excitatory A1 noradrenergic input to rat medial preoptic gamma 203–311.
aminobutyric acid neurones demonstrated by microdialysis, Neuro- [36] P.J. Snyder, L.L. Badura, A putative mechanism underlying slowed endocrinology 52 (1990) 161–168. pubertal maturation following administration of sodium valproic [18] C. Invitti, L. Danesi, A. Dubini, F. Cavagnini, Neuroendocrine acid, Neurology 50 (1998) 922–925.
effects of chronic administration of sodium valproate in epileptic [37] H.F. Urbanski, S.R. Ojeda, The juvenile-peripubertal transition patients, Acta Endocrinol. 118 (1988) 381–388. period in the female rat: establishment of a diurnal pattern of [19] J.I.T. Isojarvi, T.J. Laatikainen, A.J. Pakarinen, K.T.S. Juntunen, V.V. pulsatile luteinizing hormone secretion, Endocrinology 17 (1985)
Myllyla, Polycystic ovaries and hyperandrogenism in women taking 644–649.
valproate for epilepsy, N. Engl. J. Med. 329 (1993) 1383–1388. [38] S. Wray, G. Hoffman, Postnatal morphological changes in rat LHRH [20] D. Johnston, Valproic acid: update on its mechanisms of action, neurons correlated with sexual maturation, Neuroendocrinology 43