www.elsevier.com/locate/ibmb
Interaction between Manduca sexta allatotropin and Manduca sexta
allatostatin in the fall armyworm Spodoptera frugiperda
Uwe Oeh
a, Matthias W. Lorenz
a, Hubert Dyker
b, Peter Lo¨sel
b, Klaus H. Hoffmann
a,*aLehrstuhl Tiero¨kologie I, Universita¨t Bayreuth, D-95440 Bayreuth, Germany bBayer AG, Central Research, D-51368 Leverkusen, Germany
Received 31 October 1999; received in revised form 31 December 1999; accepted 25 January 2000
Abstract
A peptide that strongly stimulates juvenile hormone (JH) biosynthesis in vitro by the corpora allata (CA) was purified from methanolic brain extracts of adult Spodoptera frugiperda. Using HPLC separation followed by Edman degradation and mass spec-trometry, the peptide was identified as Manduca sexta allatotropin (Mas-AT). Treating the CA from adult S. frugiperda with synthetic Mas-AT (at 1026M) caused an up to sevenfold increase in JH biosynthesis. The stimulation of JH synthesis was dose-dependent
and reversible. Synthetic M. sexta allatostatin (Mas-AS) (1026M) did not affect the spontaneous rate of JH secretion from CA of
adult S. frugiperda, nor did any of the allatostatins of the Phe–Gly–Leu–amide peptide family tested. However, when CA had been activated by Mas-AT (1026M), addition of synthetic Mas-AS (1026M) reduced JH synthesis by about 70%. This allatostatic effect of Mas-AS on allatotropin-activated glands was also reversible. When CA were incubated in the presence of both Mas-AT (1026
M) and various concentrations of Mas-AS (from 1028to 1025M), the stimulation of JH-biosynthesis observed was inhibited in a dose-dependent manner. The experiments demonstrate a novel mechanism of allatostatin action. In S. frugiperda JH synthesis was inhibited only in those glands which had previously been activated by an allatotropin. 2000 Elsevier Science Ltd. All rights reserved.
Keywords: Noctuidae; Fall armyworm; Spodoptera frugiperda; Juvenile hormone; Corpora allata; Allatotropin; Allatostatin
1. Introduction
The pioneering investigations of Kopec (1917) were the first to infer that metamorphosis in insects is hor-monally regulated. Kopec’s experiments induced a flood of investigations on the hormonal regulation of various processes in insects. Basically, the development and metamorphosis of insects depend on precise regulation of the levels of ecdysteroids and juvenile hormones (for reviews see Nijhout, 1994; Hardie, 1995; Ga¨de et al., 1997). The juvenile hormones (JH) are unique hor-mones, sesquiterpenoids, which are important in almost every aspect of insect development and reproduction (Riddiford, 1994; Wyatt and Davey, 1996). These include embryogenesis, larval moulting, metamorphosis, vitellogenin synthesis and ovarian development,
poly-* Corresponding author. Tel.: +49-921-552650; fax: + 49-921-552784.
E-mail address: [email protected] (K.H. Hoffmann).
0965-1748/00/$ - see front matter2000 Elsevier Science Ltd. All rights reserved. PII: S 0 9 6 5 - 1 7 4 8 ( 0 0 ) 0 0 0 4 3 - 6
morphism, diapause regulation, and various aspects of metabolism associated with these functions. Juvenile hormones are synthesized and released from the corpora allata (CA), a pair of endocrine glands in the posterior region of the head, closely associated with the stomato-gastric nervous system (Tobe and Stay, 1985). Various JH homologues were found in different insect species (Schooley et al., 1984). JH III (C16-JH) is the prevalent
insect JH; the higher homologues (JH 0, I, II) are only found in Lepidoptera (Schooley et al., 1984). A new type of JH was identified in higher dipterans, the JH III bise-poxide (JHB3) (Richard et al., 1989). In many
lepidop-teran species, JH I, II, and III were found consistently in adult females, with different ones predominating in various species (Schooley et al., 1984). Moth CA seem to exhibit a biochemical sexual dimorphism in that JH acid homologues are released by CA of male moths (Bhaskaran et al., 1988; Cusson et al., 1993). Our recent studies demonstrated that CA of the fall armyworm,
Spo-doptera frugiperda, synthesize and release mainly JH III
vitro in the presence of radiolabelled methionine (Range, unpublished data).
Levels of JH in haemolymph or tissues are affected by the rate at which the hormones are biosynthesized by the CA and the rate at which these molecules are metabolized or excreted. Although JH-degrading enzymes are known to play a role in determining physio-logical levels of JH, it is likely that the overall control of the JH level is more intimately linked to changes in the rates of its synthesis (Tobe and Stay, 1985; Weaver et al., 1998). During the last decade, interest has focused on signals or factors that regulate JH biosynthesis by the CA. Depending on the species and developmental stage, the regulatory signals may reach the glands via the hae-molymph or via nervous connections. These signals may be either stimulatory (allatotropic) or inhibitory (allatostatic) in nature (Tobe and Stay, 1985; Goodman, 1990; Stay et al., 1994). So far, a large number of neuro-peptides that are potentially inhibitory on JH production by the CA in vitro have been isolated from the brains of several insect species (moths, cockroaches, locusts, crickets, flies and bees; for reviews see Bendena et al., 1997; Ga¨de et al., 1997; Weaver et al., 1998; Hoffmann et al., 1999). These allatostatins can be divided into three groups. One peptide family is characterized by a com-mon C-terminal pentapeptide sequence Y/FXFGL/I-amide (allatostatin A family; allatostatin superfamily). In some species these peptides have no effect on the CA of the source insect, but instead exhibit myo-inhibiting properties (Duve and Thorpe, 1994). The second group consists of peptides which were isolated from Gryllus
bimaculatus and which have a common amino acid (Trp)
at positions 2 and 9 (allatostatin B family; W2W9-peptide
family) (Lorenz et al., 1995a). Manduca sexta allatosta-tin (Mas-AS) is the only known representative of the third “allatostatin family”. Mas-AS shows no sequence similarity to any other allatostatin (Kramer et al., 1991) and is the only allatostatic neuropeptide isolated from a lepidopteran species. It was first isolated from M. sexta (Kramer et al., 1991), but in Pseudaletia unipuncta a cDNA could be characterized that also encodes the 15-residue peptide (Jansons et al., 1996). Audsley et al. (1998) identified an apparently identical peptide to Mas-AS in Lacanobia oleracea. Mas-Mas-AS strongly inhibits JH biosynthesis in vitro by CA of fifth instar larvae and adult females of M. sexta. It also inhibits the CA of the moth, Heliothis virescens, but had no effect on CA of two orthopteroid species, Periplaneta americana and
Melanoplus sanguinipes, or on CA of the beetle Teneb-rio molitor (Kramer et al., 1991).
To date, only one allatotropin has been identified (Kataoka et al., 1989). This allatotropin was isolated from 10,000 heads of pharate adults of M. sexta and was called M. sexta allatotropin (Mas-AT; GFKNVEMMTARGF–NH2). Mas-AT stimulates JH
biosynthesis in vitro in CA of adults of M. sexta and H.
virescens (Kataoka et al., 1989), and also of the moth L. oleracea (Audsley et al., 1999). No CA-activating
effect by Mas-AT was found in any non-lepidopteran species (Hoffmann et al., 1999).
These results indicate an exceptional position of the lepidopteran order with respect to the regulation of CA activity. In the fall armyworm, S. frugiperda, little is known about allatoregulating peptides or the control of JH biosynthesis in general. In this paper, we report on the identification of a peptide which strongly stimulates JH biosynthesis in vitro by the CA of adult females, and we demonstrate a novel mechanism of allatoregulation, whereby JH biosynthesis is inhibited by Mas-AS only in those glands which had previously been activated by the M. sexta allatotropin.
2. Materials and methods
2.1. Insects
Larvae of S. frugiperda were reared at 27°C and ca. 70% relative humidity under a L16:D8 photoperiod and raised on an artificial diet based on bean meal. To pre-vent cannibalism, L4 larvae were maintained individu-ally in separate compartments of assortment boxes with 40 compartments (49×32×36 mm per compartment; Licefa, Bad Salzuflen, Germany). Pupae and adults were kept under the same conditions in 20×20×10 cm plastic boxes. Immediately after emergence, sexes were separ-ated and provided with water and satursepar-ated sucrose sol-ution.
2.2. Radiochemical assay for allatoregulating activity
Juvenile hormone biosynthesis was measured accord-ing to the rapid partition assay (Feyereisen and Tobe, 1981), a variant of the radiochemical assay of Tobe and Pratt (1974) and Pratt and Tobe (1974). As CA of S.
frugiperda release mainly JH III diol, and less amounts
of JH II diol (Range, unpublished data), and up to 35% of the JH III diol could remain in the aqueous, non-iso-octane phase when using the partition assay (Share and Roe, 1988), our results may not represent absolute rates of JH release by CA in vitro. The radiochemical assay was carried out as previously described (Lorenz et al., 1995a,b) with some modifications: TC 199 incubation medium with Hanks’ salts and sodium bicarbonate, with-out L-glutamine, buffered with 25 mM HEPES, sup-plemented with CaCl2to a final concentration of 10 mM
and NaCl to a final concentration of 180 mM, fortified with 1% Ficoll 400, was adjusted to pH 7.2 and sterilized by compression through a 0.2 µm filter. L-[methyl-3H]
the radiolabelled precursor was set to 200 mCi mmol21 (final L-methionine concentration ca. 0.11 mM). Ani-mals were dissected under modified cricket Ringer (Lorenz et al., 1997). CC and CA from one insect were incubated together as a complex. After a 1.5 h preincu-bation in radioactive medium, to allow equilibration of the endogenous methionine with the radiolabelled meth-ionine (Tobe and Stay, 1985), the CC–CA-complexes were incubated for 2 h in the dark with gentle shaking at 27°C. The CC–CA-complexes were then transferred to medium containing brain extracts and synthetic pep-tides, respectively, and were incubated for a second 2 h period. At the end of each incubation period, media were analysed for JH release (Feyereisen and Tobe, 1981). Rates of release were determined for the first and second incubation, respectively, and the percentage change was calculated (second incubation/first incubation21)×100.
Inhibition rates are presented as negative values, stimu-lation rates as positive values. In other experiments, three incubations (each for 2 h) were performed and either peptides were added or not, according to the details in the figure legends.
2.3. Brain extracts and SEP-PAK purification
Here, 5800 brains (100 per batch) from 1- to 3-day-old adult females were dissected and stored in extraction medium (methanol/water/acetic acid, 100/10/1, v/v) at
225°C prior to purification. Each batch was
homogen-ized by sonication and centrifuged (10 min, 9000g, 2°C) three times. The supernatants were pooled and the pellets resuspended in 500 µl of extraction medium. Combined supernatants from three batches (representing 300 brains) were dried down in a vacuum concentrator to ca. 200µl, loaded onto a C18SEP-PAK cartridge (Waters)
and rinsed three times with 500µl 0.1% TFA in water. Cartridges were eluted with a stepwise gradient (4 ml each of 0.1% TFA in water, 0.1% TFA in 16% acetonitr-ile (CH3CN), 0.1% TFA in 60% CH3CN and 0.1% TFA
in 100% CH3CN). The 16–60% SEP-PAK fractions,
which contained the allatoregulating peptides (Lorenz et al., 1995b), were combined and two samples with 2800 and 3000 brain equivalents were obtained. Both samples were dried down and stored at 225°C prior to further purification by HPLC.
2.4. HPLC purification
Three reversed-phase high performance liquid chro-matography (HPLC) steps were necessary to purify the bioactive peptide. The first two HPLC runs were perfor-med on a Jasco HPLC system (Jasco Labor- und Daten-technik GmbH, Großumstadt, Germany) with the follow-ing components: two PU-980 HPLC pumps, DG-980-50 on-line degasser, 975 variable wavelength UV-detector (set to 214 nm), BFO-04 np column thermostat
Jet-stream Peltier (set to 25°C) and 7125 sample injector (Rheodyne Inc., Cotati, CA, USA). Data were processed using Borwin V 1.21 chromatographic software (JMBS Developpements, Grenoble, France) with a personal computer.
2.4.1. First HPLC run
The first purification step was performed using a ReproSil-Pur C18-AQ column, 120 A˚ , 5 µm, 250×4.6
mm, with guard column, 10×4.6 mm (same material; Maisch, Ammerbuch, Germany) and 2000 µl sample loop (Sykam GmbH, Gilching, Germany). Solvent A: 0.115% TFA in water; solvent B: 0.1% TFA in CH3CN;
gradient: 0–4 min 5% B, 4–94 min 5–50% B (linear gradient, 0.5% per min), 94–97 min 50–100% B, 97– 107 min 100% B, 107–112 min 100–5% B; flow rate: 1 ml/min. Both samples (2800 and 3000 brain equivalents) were resolved in 1 ml 5% B and separately loaded onto the HPLC column. Peaks were collected according to their UV signal. Fractions from one HPLC run (2800 brain equivalents) were tested in the radiochemical bioassay for allatoregulating activity (30 brain equiva-lents per assay). A fraction eluting between 54.5 and 55.3 min showed distinct allatotropic activity and, there-fore, was used for further purification.
2.4.2. Second HPLC run
Column: Capcell SG 120 C18, 120 A˚ , 3 µm, 150×3
mm, with guard column, 10×3 mm (same material; Grom, Herrenberg-Kayh, Germany); 500µl sample loop (Sykam GmbH, Gilching, Germany); solvent A: 0.13% HFBA in water, solvent B: 0.13% HFBA in CH3CN;
gradient: 0–45 min 10–55% B (linear gradient, 1% per min), 45–48 min 55–80% B, 48–53 min 80% B, 53–55 min 80–10% B; flow rate: 300µl/min. The sample from the first HPLC run was dried down to ca. 50µl, diluted with 10% B to a volume of 250µl and loaded onto the column. Peaks were collected and assayed for allatoreg-ulating activity (50 brain equivalents per assay). A frac-tion eluting between 53.0 and 54.8 min showed high allatotropic activity and, therefore, was chosen for the next HPLC purification step.
2.4.3. Third HPLC run
This purification step was carried out on a micro HPLC system with the following components: high pressure gradient HPLC pump Eldex Micro Pro, column thermostat Spark Mistral (set to 37°C) with built-in Rheodyne 8125 injector, 200µl sample loop and an UV-detector Spectra Flow 505 (set to 214 nm) equipped with a 35 nl ZU-flow cell (SunChrom GmbH, Friedrichsdorf, Germany). Operating conditions were as follows: col-umn ReproSil-Pur C18-AQ, 120 A˚ , 5 µm, 250×1.5 mm,
0–6 min 20% B, 6–46 min 20–36% B (linear gradient, 0.4% B per min that means 0.3% CH3CN per min), 46–
52 min 36–100% B, 52–57 min 100% B, 57–65 min 100–20% B; flow rate: 100µl/min. The sample from the second HPLC run was reduced to a volume of ca. 10
µl, filled up with 20% B to an injection volume of 100
µl and loaded onto the column. Peaks were collected and tested for allatoregulating activity (50 brain equivalent per assay). The most significant peak eluting between 26.6 and 27.4 min showed allatotropic activity and was pure enough to be analysed by mass spectrometry and Edman degradation.
2.5. Mass spectrometry and Edman degradation
For mass spectrometry and Edman degradation 1 µl of the purified sample was dried down and dissolved in 3 µl water/CH3CN (1/1, v/v). A 1 µl sample was
ana-lysed by mass spectroscopy using a MALDI-TOF instru-ment (Voyager DE-STR, PerSeptive Biosystems, Fram-ingham, MA, USA). Peptide samples were prepared using dihydroxybenzoic acid as a matrix. For external molecular weight calibration a mixture of four synthetic peptides was applied.
Edman sequencing was performed using the auto-mated protein sequencer 494 cLC from ABI-Perkin-Ellmer (Perkin ABI-Perkin-Ellmer Biosystems, Warrington, UK). The Fast A software program was used to search for sequence similarities in SwissProt and PIR protein data-bases.
2.6. Coelution of synthetic and native peptides
Synthetic Mas-AT was purchased from Bachem (Bubendorf, Switzerland) and coeluted with native pep-tide using the micro HPLC system as in the third HPLC purification step, with the following modifications and chromatographic conditions: 10µl sample loop; column: YMC-Pack ODS-AQ, 120 A˚ , 5 µm, 150×0.5 mm; sol-vent A: 0.113% TFA in 5% CH3CN, solvent B: 0.1%
TFA in 80% CH3CN; gradient: 0–6 min 20% B, 6–46
min 20–36% B (linear gradient, 0.4% B per min that means 0.3% CH3CN per min), 46–52 min 36–100% B,
52–57 min 100% B, 57–65 min 100–20% B; flow rate: 10µl/min. After initial runs with synthetic Mas-AT and native peptide separately, both peptides were coinjected in equal amounts of about 20 pmol.
3. Results
3.1. Purification and identification of the allatotropic peptide
In the first HPLC step bioactive material eluted between 54.5 and 55.3 min (Fig. 1). This fraction caused
an eightfold increase in JH biosynthesis (30 brain equiv-alents per assay) and, therefore, was selected for further purification. Allatotropic material from the second HPLC run (53.0–54.8 min, not shown) eluted from the third HPLC-system as a single peak at around 26.6 min (Fig. 2) and was the only fraction to induce a distinct allatotropic response (50 brain equivalents per assay). The total yield of allatotropic material was estimated at about 70 pmol, which corresponded to an amount of ca. 16 fmol/brain. The sample was subjected to sequence analysis and mass spectrometry. Edman sequencing indi-cated an amino acid sequence GFK(N/D)VEMMxARGF which is similar to Mas-AT. MALDI-TOF analysis showed a mass of 1486.11 Da (M+H+) which corre-sponded to the theoretical value of 1486.79 Da (M+H+) for Mas-AT. Thus, the uncertain amino acids on pos-itions 4 and 9 could be designated as N and T, respect-ively, and the peptide was tentatively identified as
Mas-AT with an amino acid sequence
GFKNVEMMTARGF–NH2. For additional
confir-mation of the primary structure, synthetic Mas-AT and native peptide were coinjected onto a YMC-Pack HPLC column in equal amounts of ca. 20 pmol. A single peak eluted at 28.05 min (not shown), which had almost a double peak area (ca. 200,000 units) compared to the single peak areas from previous runs of both peptides alone (each ca. 110,000 units).
3.2. Effects of synthetic Mas-AT and Mas-AS on JH release in vitro
with-Fig. 1. Chromatographic steps of S. frugiperda allatotropin isolation from 5800 brains of adult females. (A) Chromatogram of the first HPLC step. (B) Chromatogram of the third (last) HPLC step. For details see Section 2. Peaks were collected from the HPLC runs and their allatoregulating activity measured with the rapid partition assay with CC–CA-complexes from 2-day-old females at a concentration of 30 brain equivalents per assay (A, lower graph) and 50 brain equivalents per assay (B, lower graph), respectively. Mean values of five determinations. Mas-AT: peak identified as M. sexta allatotropin.
Fig. 2. Dose response for stimulation of CC–CA-complexes from 2-day-old females of S. frugiperda by Mas-AT. Mean values±SE of 10 determinations. Rate of JH release by untreated glands was ca. 3 pmol h21animal21.
out Mas-AT. The basal rate was ca. 3 pmol h21animal21 and the stimulated rate was about 21 pmol h21animal21. Addition of Mas-AS at concentrations of 1025and 1026 M significantly reduced the rates of JH release. This sug-gests that Mas-AS strongly suppressed the allatotropic effect of Mas-AT. This inhibiting effect was reduced at 1027 M Mas-AS concentration, and disappeared altog-ether at 1028 M concentration. In another experiment,
three successive 2 h incubations were carried out and either a single peptide or two peptides were added to the medium, according to the figure legend (Fig. 5). Basal rates of JH release were relatively constant during three consecutive 2 h incubation periods with ca. 4 pmol h21 animal21 (Fig. 5, first group). The second group con-firmed earlier results that Mas-AS does not affect JH release of CA from adult females of S. frugiperda. Mas-AT acted as a stimulant, and the glands returned within 2 h to unstimulated values in medium without Mas-AT (Fig. 5, third group). When Mas-AS was added together with Mas-AT, JH release rates were less than half of the hormone production rates obtained with Mas-AT alone (Fig. 5, last three groups). The inhibiting effect of Mas-AS on allatotropin activated glands was reversible (Fig. 5, sixth group).
3.3. Effects of synthetic peptides of the FGL-amide peptide family on JH synthesis
In a first experiment, members of the FGL-amide alla-tostatin family which had been isolated from
Helico-verpa armigera (Duve et al., 1997), and an allatostatin
of the W2W9-peptide family isolated from the cricket G. bimaculatus (Lorenz et al., 1995a,b), were tested for
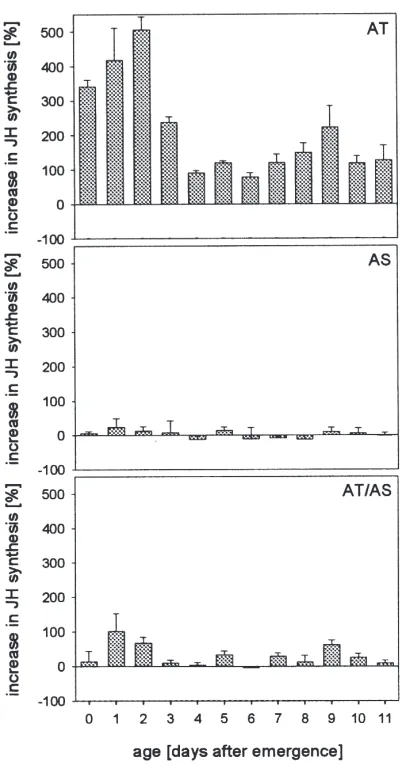
Fig. 3. Effects of Mas-AT and Mas-AS on JH release in CC–CA-complexes from females of S. frugiperda at the first 11 days after emergence. Mean values of five determinations. Peptides were added during a second 2 h incubation at a concentration of 1026M. Mas-AT
(AT), Mas-AS (AS), Mas-AT plus Mas-AS (AT/AS).
Fig. 4. Dose response for the inhibition of the allatotropic effect of Mas-AT by Mas-AS in CC–CA-complexes from 2-day-old females of
S. frugiperda. Mean values±SE of five determinations. Rate of JH release by untreated glands was ca. 3 pmol h21 animal21. Peptides were added during the second 2 h incubation. Mas-AT (AT), Mas-AT plus Mas-AS (AT/AS). Negative log peptide concentrations [M] are given in parentheses.
peptides significantly inhibited JH release in vitro (not shown). In a second experiment, the same peptides were used for measuring a possible allatostatic effect on CC– CA-complexes which had been activated by Mas-AT (Table 1). Again, however, none of these peptides sig-nificantly reduced JH release during the second 2 h incu-bation period. Of all known allatostatins, only Mas-AS is able to inhibit JH release in allatotropin activated CC– CA-complexes of S. frugiperda.
4. Discussion
Severance of nervous connections from the brain to the CA demonstrated the role of neurosecretory sub-stances with respect to their regulatory impact on JH biosynthesis in the CA. When axons from the brain to the CA were cut in the cockroach, Leucophaea maderae, neurosecretory material accumulated proximal to the cut, and an increased CA bioactivity was observed (Scharrer, 1952). Brain implantation and removal experiments con-firmed the hypothesis that neurosecretory material syn-thesized in the brain causes a down-regulation of the CA activity leading to a decreasing rate of JH biosynthesis (Tobe and Stay, 1985). Rankin et al. (1986) provided a good indication of the peptidergic nature of the inhibi-tory material. Soon thereafter, efforts were undertaken to identify and characterize the amino acid sequence of these peptides. To date, more than 60 potentially allato-inhibiting factors have been found in a variety of insect species (for reviews see Bendena et al., 1997; Ga¨de et al., 1997; Weaver et al., 1998; Hoffmann et al., 1999). They were termed “allatostatins” because of their attri-bute of inhibiting JH biosynthesis in the CA (Woodhead et al., 1989).
Additional findings indicated the existence of neuro-secretory factors which are able to stimulate CA bioac-tivity, the so-called “allatotropins”. Implantation of a fourth-instar larval brain of Galleria mellonella into a fifth-instar larva led to an additional moult in intact lar-vae, but not in allatectomized larvae (Granger and Sehnal, 1974). This effect was abolished when certain cell groups of the brain (medial neurosecretory cells, M-NSC) had been removed before transplantation, indicat-ing that allatotropic factors are synthesized in the M-NSC, and that they act, at least in part, humorally. Fac-tors with allatotropic activity are known from various insect species. Brain extracts of Locusta migratoria (Gadot and Applebaum, 1985) and extracts from the suboesophageal ganglia of G. bimaculatus and Acheta
domesticus (Lorenz and Hoffmann, 1995) increase JH
Fig. 5. Three successive incubations of CC–CA-complexes from 2-day-old females of S. frugiperda. Mean values±SE of five determinations. No addition to medium (0), addition of Mas-AS (AS), addition of Mas-AT (AT), addition of Mas-AT plus Mas-AS (AT/AS). Peptide concentrations: 1026M. First incubation (white columns), second incubation (grey columms), third incubation (dark grey columns).
Table 1
Inhibition of JH release in CC–CA-complexes from 2-day-old females of S. frugiperda by various peptides in a medium containing 1026M AT. During the first 2 h incubation the medium contained Mas-AT, during the second 2 h incubation Mas-AT plus the indicated pep-tide or only Mas-AT (control). Peppep-tide concentrations: 1026M.
Per-centage inhibition was calculated as (12second incubation/first incubation)×100. Basal rate of JH release (first incubation) was about 18 pmol h21animal21. Mean values of five determinations±SE
Peptide Inhibition of JH release [%]
Control 4.1±6.3
pEVRFRQCYFNPISCF–OH2 63.6±4.0
(Mas-AS)
AQHQYSFGL–NH2(Grb-AS 8.2±3.9
A1)a
GWQDLNGGW–NH2(Grb-AS 9.7±5.7
B1)b
LPVYNFGL–NH2c 28.6±9.6 SPHYDFGL–NH2c 12.6±7.0
SRPYSFGL–NH2c 212.9±13.5
aLorenz et al. (1995b). b Lorenz et al. (1995a). cDuve et al. (1997).
research work was focused on the characterization of the allatotropic substances, the amino acid sequence of only one allatotropin, the Mas-AT, has so far been identified (Kataoka et al., 1989). Mas-AT was isolated from the heads of pharate adults of M. sexta and it stimulates CA activity in adult animals but not in larvae or pupae. In addition, Mas-AT stimulates JH release from CA of adult female H. virescens (Kataoka et al., 1989) and adult male, female and also larval L. oleracea (Audsley et al., 1999).
In this work we demonstrate the existence of Mas-AT in the brain of another lepidopteran insect, the fall armyworm S. frugiperda. The native as well as the syn-thetic peptide strongly stimulated CA activity in a hom-ologous bioassay and caused an up to sevenfold increase in JH release. The effect was dose-dependent, saturable and reversible. The rates of increase in JH release are similar to those found in M. sexta (Kataoka et al., 1989). The estimated amount of 16 fmol Mas-AT per brain seems to be relatively low compared to results from immunoassay studies for this neuropeptide in other insect species. In P. americana 1.9 pmol allatotropin-immunoreactive peptide were found per brain (Veenstra and Hagedorn, 1993). Our estimation does not include the loss of bioactive material during the purification steps.
The detection of functional Mas-AT in S. frugiperda supports the hypothesis that the allatotropic function of this peptide is restricted to Lepidoptera, although pep-tides with Mas-AT-like immunoreactivity were also found in non-lepidopteran species (L. migratoria: Pae-men et al., 1991; P. americana: Veenstra and Hage-dorn, 1993).
modulation) of the CA and not to a dynamic one (short-term modulation) as obtained with Mas-AT in Lepidop-tera (for details see Unnithan et al., 1998).
Using our in vitro assay no allatostatic activity could be detected in S. frugiperda brain extracts. However, this does not mean that allatostatins do not have a role in regulation of CA activity in this species. The results from our in vitro incubations of CC–CA-complexes with synthetic Mas-AS suggest that this neuropeptide does not affect the basal levels of JH biosynthesis in S.
frugip-erda at any adult age (Fig. 3). This is in contrast to the
results of Jansons et al. (1996), in which CA of P.
unipuncta showed significant changes in responsiveness
to Mas-AS with age.
However, when CC–CA-complexes of S. frugiperda were assayed in a medium containing AS and Mas-AT, the allatostatin showed significant potency to inhibit or even abolish the allatotropic action of Mas-AT (Fig. 3). This allatostatic effect of Mas-AS on allatotropin-activated glands is dose-dependent (Fig. 4) and revers-ible (Fig. 5).
Thus, a novel mechanism in the regulation of CA activity by humoral brain factors has to be discussed. To date, allatostatins and allatotropins were considered to act directly and separately on the target gland, inhibiting or activating, respectively. Allatostatins are thought to inhibit JH synthesis by binding to putative allatostatin receptor proteins (Cusson et al., 1991) in the CA, which causes a cascade of specific signal transduction (Stay et al., 1994). Although little is known about the exact mechanisms, the role for cyclic nucleotides (cAMP, cGMP) and Ca2+as second messengers has been
investi-gated (Aucoin et al., 1987; Allen et al., 1992a,b; Cusson et al., 1992; Rachinsky et al., 1994; Rachinsky and Tobe, 1996). For Mas-AT, similar modes of action involving a receptor second messenger system have been suggested (Unni et al., 1991).
Our results indicate that Mas-AS may control the CA of S. frugiperda indirectly. While the allatostatin alone has no direct effect on the basal activity of the CA, it inhibits allatotropin activated glands or, in other words, it decreases the allatotropic effect of Mas-AT. This sug-gests that high rates of JH synthesis maintained by Mas-AT are down-regulated by an interaction with Mas-AS. Whether Mas-AS acts as an antagonist of a putative alla-totropin receptor or whether other modes of action are involved in the regulation of JH synthesis remains to be investigated. The allatostatic action on allatotropin-activated CA seems to be restricted to Mas-AS, since none of the other tested allatostatins or allatostatin-like peptides showed this effect (Table 1). Further studies will focus on the isolation of the Mas-AS from the brain of S. frugiperda.
Acknowledgements
This work was supported by the Bayer AG, Central Research, Leverkusen, and by an award from American Cyanamid Corporation. We thank Dr Horst Ahorn, Wien, for carrying out the mass spectrometry and Edman degradation. We are grateful to Professor Dr Joseph Woodring (Baton Rouge) for critical reading of the manuscript.
References
Allen, C.U., Herman, B., Granger, N.A., 1992a. Fura-2 measurement of cytosolic free Ca2+concentration in corpus allatum cells of larval Manduca sexta. J. Exp. Biol. 166, 253–266.
Allen, C.U., Janzen, U.P., Prestwich, G.D., Granger, N.A., 1992b. Manipulation of intracellular calcium affects in vitro juvenile hor-mone synthesis by larval corpora allata of Manduca sexta. Molec. Cell. Endocrinol. 84, 227–241.
Aucoin, R.R., Rankin, S.M., Stay, B., Tobe, S.S., 1987. Calcium and cyclic AMP involvement in the regulation of juvenile hormone biosynthesis in Diploptera punctata. Insect Biochem. 17, 965–969. Audsley, N., Weaver, R.J., Edwards, J.P., 1998. Enzyme linked immu-nosorbent assay for Manduca sexta allatostatin (Mas-AS), isolation and measurement of Mas-AS immunoreactive peptide in Lacanobia
oleracea. Insect Biochem. Molec. Biol. 28, 775–784.
Audsley, N., Weaver, R.J., Edwards, J.P., 1999. Juvenile hormone syn-thesis by corpora allata of tomato moth, Lacanobia oleracea (Lepidoptera: Noctuidea) and the effects on allatostatin and allato-tropin in vitro. In: Abstracts of the 7th International Congress of Juvenile Hormones 1999, Jerusalem.
Bendena, W.G., Garside, C.S., Yu, C.G., Tobe, S.S., 1997. Allatostat-ins: diversity in structure and function of an insect neuropeptide family. Ann. New York Acad. Sci. 814, 53–66.
Bhaskaran, G., Sparagana, S.P., Dahm, K.H., Barrera, P., Peck, K., 1988. Sexual dimorphism in juvenile hormone synthesis by corpora allata and in juvenile hormone acid methyltransferase activity in corpora allata and accessory sex gland of male Lepidoptera. Int. J. Invertebr. Reprod. Develop. 13, 87–100.
Cusson, M., Prestwich, G.B., Stay, B., Tobe, S.S., 1991. Photoaffinity labeling of allatostatin receptor proteins in the corpora allata of the cockroach, Diploptera punctat. Biochem. Biophys. Res. Commun. 181, 736–742.
Cusson, M., Yagi, K.J., Tobe, S.S., McNeil, J.N., 1993. Identification of release products of corpora allata of male and female armyworm moths Pseudaletia unipuncta. J. Insect Physiol. 39, 775–783. Cusson, M., Yagi, K.J., Xue-Chen, G., Tobe, S.S., 1992. Assessment
of the role of cyclic nucleotides in allatostatin-induced inhibition of juvenile hormone biosynthesis in Diploptera punctata. Molec. Cell. Endocrinol. 89, 121–125.
Duve, H., Johnsen, A.H., Maestro, J.-L., Scott, A.G., Winstanley, D., Davey, M., East, P.D., Thorpe, A., 1997. Lepidopteran peptides of the allatostatin superfamily. Peptides 18, 1301–1309.
Duve, H., Thorpe, A., 1994. Distribution and functional significance of Leu-callatostatins in the blowfly Calliphora vomitoria. Cell Tissue Res. 276, 367–379.
Feyereisen, R., Tobe, S.S., 1981. A rapid partition assay for routine analysis of juvenile hormone release by insect corpora allata. Anal. Biochem. 111, 372–375.
Ga¨de, G., Hoffman, K.H., Spring, J., 1997. Hormonal regulation in insects: facts, gaps and future directions. Physiol. Rev. 77, 963– 1032.
corpora allata by extracted locust brain allatotropic factor. Arch. Insect Biochem. Physiol. 2, 117–129.
Goodman, W.G., 1990. Biosynthesis, titer regulation and transport of juvenile hormones. In: Gupta, A.P. (Ed.), Morphogenetic Hor-mones of Arthropods. Discoveries, Synthesis, Metabolism, Evol-ution, Modes of Action and Techniques, Vol. 1. Rutgers University Press, New Brunswick, NJ, pp. 83–124.
Granger, N.A., Sehnal, F., 1974. Regulation of larval corpora allata in
Galleria mellonella. Nature 251, 415–417.
Hardie, J., 1995. Hormones and reproduction. In: Leather, S.R., Har-die, J. (Eds.), Insect Reproduction. CRC Press, Boca Raton, FL, pp. 95–108.
Hoffmann, K.H., Meyering-Vos, M., Lorenz, M.W., 1999. Allatostat-ins and allatotropAllatostat-ins: is the regulation of corpora allata activity their primary function? Eur. J. Entomol. 96, 256–266.
Jansons, I., Cusson, M., McNeil, J.N., Tobe, S.S., Bendena, W.G., 1996. Molecular characterization of a cDNA from Pseudaletia
unipuncta encoding the Manduca sexta allatostatin peptide
(Mas-AST). Insect Biochem. Molec. Biol. 26, 767–773.
Kataoka, H., Toschi, A., Li, J.P., Carney, L., Schooley, D.A., Kramer, S.J., 1989. Identification of an allatotropin from adult Manduca
sexta. Science 243, 1481–1483.
Kopec, S., 1917. Experiments on metamorphosis of insects. Bull. Int. Acad. Cracov. B, 57–60.
Kramer, S.J., Toschi, A., Miller, C.A., Kataoka, H., Quistad, G.B., Li, J.P., Carney, L., Schooley, D.A., 1991. Identification of an allatos-tatin from the tobacco hornworm Manduca sexta. Proc. Nat. Acad. Sci. USA 88, 9458–9462.
Lorenz, M.W., Hoffmann, K.H., 1995. Allatotropic activity in the suboesophageal ganglia of crickets, Gryllus bimaculatus and
Ach-eta domesticus (Ensifera, Gryllidae). J. Insect Physiol. 41, 191–196.
Lorenz, M.W., Kellner, R., Hoffmann, K.H., 1995a. A family of neuro-peptides that inhibit juvenile hormone biosynthesis in the cricket,
Gryllus bimaculatus. J. Biol. Chem. 270, 21103–21108.
Lorenz, M.W., Kellner, R., Hoffmann, K.H., 1995b. Identification of two allatostatins from the cricket, Gryllus bimaculatus de Geer (Ensifera, Gryllidae): additional members of a family of neuropep-tides inhibiting juvenile hormone biosynthesis. Regul. Pept. 57, 227–236.
Lorenz, J.I., Lorenz, M.W., Hoffmann, K.H., 1997. Factors regulating juvenile hormone synthesis in Gryllus bimaculatus (Ensifera: Gryllidae). Eur. J. Entomol. 94, 369–379.
Nijhout, H.F., 1994. Insect Hormones. Princeton University Press, Princeton, NJ.
Paemen, L., Tips, A., Schoofs, L., Proost, P., VanDamme, J., DeLoof, A., 1991. Lom–AG-myotropin: a novel myotropic peptide from the male accessory glands of Locusta migratoria. Peptides 12, 7–10. Pratt, G.E., Tobe, S.S., 1974. Juvenile hormones radiobiosynthesized
by corpora allata of female locusts in vitro. Life Sci. 14, 575–586. Rachinsky, A., Tobe, S.S., 1996. Role of second messenger in the regulation of juvenile hormone production in insects, with parti-cular emphasis on calcium and phosphoinositide signaling. Arch. Insect Biochem. Physiol. 33, 259–281.
Rachinsky, A., Zhang, J., Tobe, S.S., 1994. Signal transduction in the inhibition of juvenile hormone biosynthesis by allatostatins: roles
of diacylglycerol and calcium. Molec. Cell. Endocrinol. 105, 89– 96.
Rankin, S.M., Stay, B., Aucoin, R.R., Tobe, S.S., 1986. In vitro inhi-bition of juvenile hormone synthesis by corpora allata of the viviparous cockroach Diploptera punctata. J. Insect Physiol. 32, 151–156.
Richard, D.S., Applebaum, S.W., Sliter, T.J., Bader, F.C., Schooley, D.A., Reuter, C.C., Henrich, V.C., Gilbert, L.I., 1989. Juvenile hor-mone bisepoxide biosynthesis in vitro by the ring gland of
Droso-phila melanogaster: a putative juvenile hormone in the higher
Dip-tera. Proc. Natl. Acad. Sci. USA 86, 1421–1425.
Riddiford, L.M., 1994. Cellular and molecular actions of juvenile hor-mone I. General considerations and premetamorphic actions. Adv. Insect Physiol. 24, 213–274.
Scharrer, B., 1952. Neurosecretion. XI. The effects of nerve section on the intercerebralis–cardiacum-allatum system of the insect
Leuc-ophaea maderae. Biol. Bull. Woods Hole 102, 261–272.
Schooley, D.A., Baker, F.C., Tsai, L.W., Miller, C.A., Jamieson, G.C., 1984. Juvenile hormones 0, I and II exist only in Lepidoptera. In: Hoffmann, J., Porchet, M. (Eds.) Biosynthesis, Metabolism and Mode of Action of Invertebrate Hormones. Springer-Verlag, Berlin, pp. 373–383.
Share, M.R., Roe, R.M., 1988. A partition assay for the simultaneous determination of insect juvenile hormone esterase and epoxide hydrolase activity. Anal. Biochem. 169, 81–88.
Stay, B., Tobe, S.S., Bendena, W.G., 1994. Allatostatins: identification, primary structures, functions and distribution. Adv. Insect Physiol. 25, 267–337.
Tobe, S.S., Pratt, G.E., 1974. The influence of substrate concentrations on the rate of insect juvenile hormone biosynthesis by corpora allata of the desert locust in vitro. Biochem. J. 144, 107–113. Tobe, S.S., Stay, B., 1985. Structure and regulation of the corpus
alla-tum. Adv. Insect Physiol. 18, 305–432.
Unni, B.G., Bhaskaran, G., Dahm, K.H., Hayes, T.K., 1991. Stimu-lation of juvenile hormone biosynthesis by analogues of a Manduca
sexta allatotropin: in vitro studies. Arch. Insect Biochem. Physiol.
17, 129–142.
Unnithan, G.C., Sutherland, T.D., Cromey, D.W., Feyereisen, R., 1998. A factor causing stable stimulation of juvenile hormone synthesis by Diploptera punctata corpora allata in vitro. J. Insect Physiol. 44, 1027–1037.
Veenstra, J., Hagedorn, H., 1993. Sensitive enzyme immunoassay for
Manduca allatotropin and the existence of an
allatotropin-immuno-reactive peptide in Periplaneta americana. Arch. Insect Biochem. Physiol. 23, 99–109.
Weaver, R.J., Edwards, J.P., Bendena, W.G., Tobe, S.S., 1998. Struc-tures, functions and occurrence of insect allatostatic peptides. In: Coast, G.M., Webster, S.G. (Eds.), Recent Advances in Arthropod Endocrinology. Cambridge University Press, Cambridge, pp. 3–32. Woodhead, A.P., Stay, B., Seidel, S.L., Khan, M.A., Tobe, S.S., 1989. Primary structure of four allatostatins: neuropeptide inhibitors of juvenile hormone synthesis. Proc. Natl. Acad. Sci. USA 86, 5997–6001.