Ultrasound Markers of Fetal Infection, Part 2
Bacterial, Parasitic, and Fungal Infections
Luiz Antonio Baila˜o, MD, PhD,* Newton G. Osborne, MD, PhD,Þ
Maria Christina S. Rizzi, MD,þ
Fernando Bonilla-Musoles, MD, PhD,§ Geraldo Duarte, MD,¶
and Teresa Cristina R. Sicchieri Baila˜o, RN||
Abstract:Up to 1% of all pregnancies have clinically overt intra-amniotic bacterial infections, and an even larger percentage of pregnant women may be affected by silent infections. Although most pregnant women with overt intra-amniotic bacterial infection have experienced prolonged rupture of membranes (PROM), sympto-matic and most silent nonviral intra-amniotic infections may occur with intact membranes.
The etiology of intra-amniotic infection after PROM is almost always polymicrobial and consists of genital tract patho-gens, such as group B streptococci, Chlamydia trachomatis, Neisseria gonorrhoeae, mycoplasmas, aerobic Gram-negative bacilli, such as the coliforms, and facultative and anaerobic endogenous organisms, such as peptococci, peptostreptococci, and Bacteroides species. These organisms gain access to the uterine cavity by the ascending route. Organisms such as Treponema pallidum, Listeria monocytogenes, Toxoplasma gondii, trypano-somes, and plasmodia are capable of gaining access to the amniotic cavity by transplacental hematogenous spread, and cause devastat-ing fetal infections.
Symptomatic intra-amniotic infection is usually a diagnosis of exclusion. Diagnostic criteria based on both clinical and laboratory findings lack sensitivity and are nonspecific. It is difficult to obtain uncontaminated intra-amniotic samples, especially when there is PROM. The problem is even greater with silent infections. In most cases, fetal infection is suspected after an unexplained and unexpected adverse outcome.
Maternal morbidity is increased with intra-amniotic infection; although maternal mortality is extremely rare in developed countries, this is not the case in societies where pregnant women have limited or no access to medical care. Although infected women who are treated early and aggressively with wide-spectrum antibiotics do well, more than 10% of these women develop bacteremia and up to half of them will require cesarean delivery because of poor uterine contractions and arrest of labor.
The overwhelming majority of term neonates exposed to intrauterine infection after PROM do well, but up to 30% of these neonates require treatment of neonatal pneumonia or bacteremia. Outcomes for preterm neonates or for neonates who experienced silent fetal infections are more severe. Morbidity and mortality rates in these cases are high, and survivors may have long-term devastating sequelae. The ability to identify ultrasound markers of fetal infection will help clinicians identify etiologic agents with greater accuracy and correlate these infections with specific antepartum and postpartum syndromes. The recognition of markers of intrauterine infection will also reduce unexpected adverse outcomes that result from undiagnosed fetal infections.
Key Words:fetal infection, placental calcifications, fetal hydrops
(Ultrasound Quarterly2006;22:137Y151)
LEARNING OBJECTIVES
After reading the article and completing the posttest, the reader should be able to
& list ultrasound markers associated with nonviral infections; & describe placental calcification patterns associated with
intrauterine infection;
& describe fetal anatomical alterations that may result from fetal bacterial, parasitic, and fungal infections;
& identify alterations in blood flow patterns of the umbilical cord and of the fetal circulation that may occur when there is fetal infection; and
& identify the sonographic features of the placenta, fetal anatomy, and fetal circulation associated with intrauterine bacterial, parasitic, and fungal infection.
M
any pregnant women are affected by overt or silent bacterial, fungal, or parasitic infections. Infections may account for as much as 20% of fetal and neonatal diseases and may, in fact, be one of the most frequent causes of perinatal mortality. Estimates of infections of embryos and fetuses and their effects on the health of the gestation are very difficult to obtain. Maternal signs and symptoms are Received for publication February 18, 2006; accepted March 29, 2006.*Professor and Co-Director, DIAGNOSIS, Ribeira˜o Preto, SP, Brazil and Department of Obstetrics and Gynecology, University of Sa˜o Paulo School of Medicine, Ribeira˜o Preto, SP, Brazil;†Professor and Chief of Gynecology, Department of Obstetrics and Gynecology, Howard University College of Medicine, Washington, DC; ‡Co-Director, DIAGNOSIS, Ribeira˜o Preto, SP, Brazil; §Professor and Chairman, Department of Obstetrics and Gynecology, University of Valencia School of Medicine, Valencia, Spain;¶Professor, Department of Obstetrics and Gynecology, University of Sa˜o Paulo School of Medicine, Ribeira˜o Preto, SP, Brazil; ||Ultrasonography Instructor, DIAGNOSIS, Ribeira˜o Preto, SP, Brazil.
The authors have disclosed that they have no interests in or significant relationships with any commercial companies pertaining to this educational activity.
Lippincott Continuing Medical Education Institute, Inc. has identified and resolved all faculty conflicts of interest regarding this educational activity. Reprints: Newton G. Osborne, MD, PhD, Professor and Chief of Gynecology, Department of Obstetrics and Gynecology, Howard University College of Medicine, 2041 Georgia Ave, NW, Washington, DC 20060 (e-mail: [email protected]).
frequently nonspecific. In addition, acquisition of appro-priate samples may be problematic and, at times, even if samples are obtained, accurate interpretation of the results may be difficult. To make matters more difficult, many newborn neonates with malformations or functional impair-ment are discharged without ever establishing an etiology because, in some cases, if infection occurred early in the gestation, diagnostic serological alterations may no longer be detectable by the time of birth. About 7 to 10% of newborn neonates harbor an agent of infection, many of whom have no clinical signs that would lead to a suspicion of congenital infection.
The factors that allow pathogens to invade the fetus are probably related to the severity of maternal contamination or infection, to the state of maternal cellular and humoral mechanisms of defense against infection, to the presence of subclinical co-infections, and, maybe, to certain exclusive properties of the specific pathogenic invader. It is almost universally accepted that placental villi, the amniotic membrane, and the maternal mucosal surfaces, along with their secretions, act as a protective barrier against fetal infection. In addition, there are evidences that certain microbicidal and bacteriostatic agents in the amniotic fluid inhibit the growth of pathogens that gain access to the amniotic cavity and, at times, effectively prevent fetal infection.1Y3 Unfortunately, under certain conditions, patho-gens are able to breach these barriers and initiate devastating or lethal fetal infections, which may result in overt maternal signs and symptoms or may be completely silent. Conditions that predispose to infection are more common in poor, under-nourished, or malnourished women.4Y6
A few organisms can breach the placental barrier and infect the fetus by way of the umbilical cord, regardless of the nutritional or health status of the mother. Some organisms, like Treponema pallidum, Listeria monocytogenes, and Toxoplasma gondii, to name a few, have developed effective ways of crossing the placental barriers.
Other microorganisms, such asStreptococcus agalac-tiae (group B streptococcus), unencapsulated Hemophilus influenzae,Trypanosoma cruzi, plasmodia, and a few fungi, occasionally breach the placental barrier and gain access to the fetus by the hematogenous route. Many other organisms produce a chronic placentitis, with eventual destruction of villi, which then provides the opportunity for other organisms to gain access to the fetus.7
The fetal immune system is immature. The fetal levels of immunoglobin M (IgM) produced in response to microbial invasion are relatively low; phagocytic function is not fully competent; the levels of complement are low; and T lymphocytes are still immature. These factors render the fetus vulnerable to infections acquired through the ascending or transplacental routes. Some of these fetal infections (eg, group B streptococcosis, syphilis, toxoplasmosis) may be preventable or treatable in utero. Fetal damage may be avoided in some cases if health care professionals are able to recognize incipient infection and start timely and effective antimicrobial prophylaxis or therapy.
The ability to identify ultrasound markers of infec-tion in the placenta, in the embryo, and in fetal organs
will help clinicians correlate these markers with etiologic agents of infection and with specific antepartum and postpartum syndromes (Table 1). In some cases, physicians will be able to take measures to reduce the risk of fetal and maternal adverse outcomes.8Y10 Furthermore, prenatal identification of ultrasound markers of intrauterine infec-tion is likely to reduce the problem of unexpected adverse outcomes that result from undiagnosed, silent intrauterine infection.
BACTERIAL INFECTIONS
Syphilis
Syphilis is a systemic disease caused by T. pallidum. Although the incidence rate of syphilis in pregnancy is not accurately known, it is estimated that the incidence of 15 cases per 100,000 persons in the general population probably applies also to pregnant women. There are approximately 2 cases of early congenital syphilis per hundred cases of primary or secondary syphilis in women of childbearing age. Untreated syphilis in pregnancy may result in the birth of a neonate with congenital syphilis. In fact, almost all pregnant women with untreated primary syphilis, 90% with untreated secondary syphilis, and 30% with untreated early latent syphilis will infect their fetuses.
At the onset of congenital syphilis, T. pallidum is released directly into the fetal circulation, resulting in spirochetemia with widespread dissemination of the pathogen to all fetal organs. Congenital syphilis presents as fetal hydrops, unexplained stillbirth, or neonatal hepatosplenome-galy with or without jaundice. Hemolytic anemia, pneumonia, and multiple-bone involvement, snuffles, skin lesions, and testicular masses are common. The stillborn syphilitic neonate is often macerated with a protuberant abdomen. Dermal bullous vesicular lesions contain large numbers of treponemes. Hepatomegaly with fibrosis and persistent extramedullary hematopoiesis are present.11,12 Pancreatitis may be severe.
TABLE 1. Common Ultrasound Findings Associated with Specific Fetal Infections
Infection Ultrasonography Findings
Syphilis Placentitis; IUGR; bone deformities; cardiac, retinal, intracranial, hepatic, and placental calcifications; hydrops; IUFD.
Listeriosis Hydrops, intracranial calcifications, IUFD.
Tuberculosis Hepatomegaly; echogenic bowel; IUGR; hepatic, renal, placental, and intracranial calcifications; chronic hypoxemia; IUFD; bone deformities.
Gonococcal IUGR; ophthalmic and intracranial calcifications. Fungal Placental calcifications, umbilical cord anomalies, IUFD. Toxoplasmosis Hydrocephaly; microcephaly; chronic hypoxemia;
hydrops; microophthalmia; placental, hepatic, retinal, and intracranial calcifications; IUFD.
Malaria Placental calcifications, hydrops, chronic hypoxemia. Trypanosomal IUGR; hydrops; chronic hypoxemia; IUFD;
placental, hepatic, and intracranial calcifications.
IUFD indicates intrauterine fetal death.
Glomerular injury caused by antigen-antibody complex deposi-tion may be present. Meningeal involvement is commonly found around the brain stem and optic chiasma. Syphilitic endocarditis may be present. Osteochondritis, periostitis, and osteomyelitis are characteristic. Severe anemia leads to fetal hydrops.
A neonate’s VDRL test titer of more than 4 times higher than the maternal level is strongly suggestive of congenital disease. The presence of fluorescent treponemal antibody IgM in the neonate’s serum confirms the diagnosis. False-positive IgM may occur by cross-reaction with other IgM antibodies. False-negative IgM may occur if maternal IgG levels are very high.
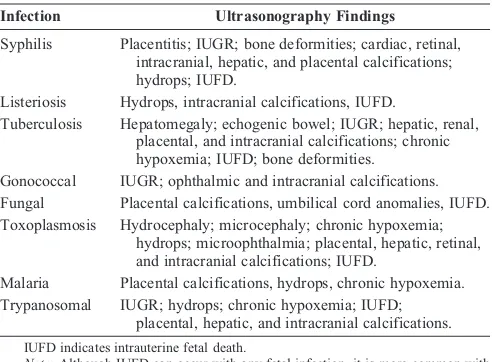
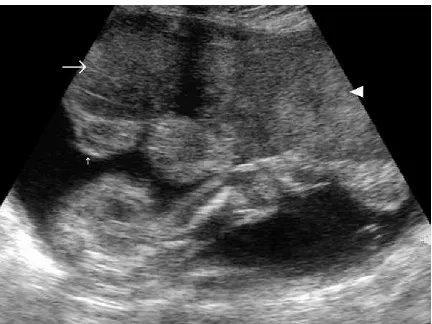
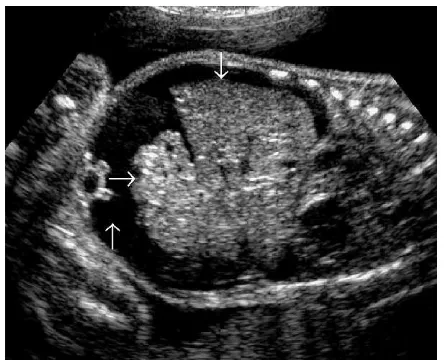
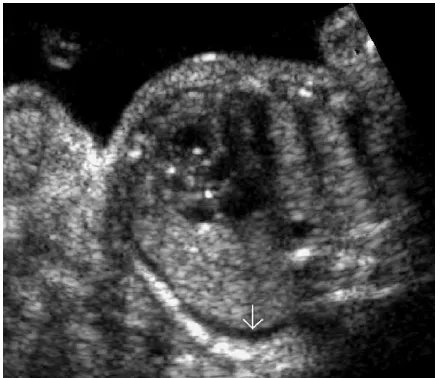
The syphilitic placenta is large, sometimes massively, but without much edema. An abnormally thick placenta is detectable with ultrasound13 (Fig. 1). The placental-fetal weight ratios in congenital syphilis are usually at least 0.5. There is a triad of changes consisting of focal villitis, vascular changes (including endothelial proliferation and perivascular inflammation), and immature villi. These changes predispose to fetal malnutrition and hypoxia (Figs. 2Y5). The decidua at the base of the placenta may have plasma cells and lymphocytes, particularly around maternal vessels. Scarring of villi occurs with chronic infections.
Unlike adult syphilis, which passes through stages, fetal or congenital syphilis has simultaneous development of lesions similar to those found at all 3 classic stages. The skin may have bullous lesions, characteristically extending to the palms and soles. Focal destruction of bony tissue between the diaphysis and epiphysis may produce characteristic bone lesions. Granulation tissue may be exuberant, producing saber shin and bossing of the forehead.BCelery stalk^lesions may occur. There may be ultrasound evidences of encephalitis, chorio-retinitis, bone deformities, hepatosplenomegaly, endocarditis, or fetal hydrops. Perivascular cuffing, deep within the tissue of the central nervous system, is a characteristic finding. Many of these changes are detectable with ultrasonography (Figs. 6Y11).
Listeria Monocytogenes
L. monocytogenesis a small, Gram-positive coccoba-cillus that is widely distributed in nature and in a variety of animal reservoirs. MostL. monocytogenesisolates that cause human disease belong to serovars 1a, 1b, and 4b.14
Human infection with L. monocytogenes has protean manifestations, of which meningitis is the most frequent form in adults. The most unique clinical form of infection with L. monocytogenes is infection of the genital tract of pregnant women with subsequent infection of the fetus or of the newborn neonate at the time of delivery. Pregnant women with genital tract infection are usually asymptomatic. Symptomatic, sys-temic maternal infection is characterized by an acute febrile illness, which may be followed by spontaneous abortion, stillborn delivery, delivery of a seriously ill premature or term neonate, or delivery of an unaffected term neonate. The case fatality rate for liveborn neonates of infected mothers is 27%.
FIGURE 2. Spectral Doppler analysis of the umbilical arteries of the placenta shown in Figure 1 reveals an elevated RI, which indicates a risk of malnutrition and hypoxia in addition to the other risks associated with fetal infection with syphilis.
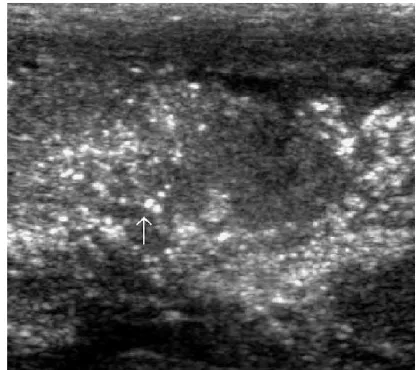
FIGURE 1. Chronic hypertrophic placentitis. Observe the marked heterogeneous echogenic thickening of the chorionic plate (small vertical arrow), along with hypoechogenic areas of the villous mass (large horizontal arrow). Maternal workup demonstrated infection withT. pallidum.
Fetal infection can occur with intact membranes.14Y17 Fetal infection may result in nonimmune hydrops and/or in fetal meningitis. Fetuses that survive an intrauterine infection withL. monocytogenesare likely to be born prematurely and to have low birth weight. Surviving fetuses usually have evidence of distress during labor and develop postnatal granulomatosis infantiseptica. When there is fetal infection, symptoms of septicemia usually develop within a few hours of birth. If evidences of infection are not obvious at birth, septicemia definitely presents within 2 days of birth in all
neonates that acquired the infection in utero, and may be associated with diarrhea, pneumonitis, seizures, and maculo-papular skin lesions of the trunk and legs. Not surprisingly, these neonates have an increased risk of neonatal mortality.
If neonatal infection results from contamination at the time of delivery, the symptoms of neonatal listeriosis appear after the fifth day of life. As is the case with survivors of intrauterine infection, infection with L. monocytogenes acquired at birth causes meningitis, widespread granuloma-tous lesions, microabscesses, and septicemia.
Chlamydia trachomatis,
Mycoplasma hominis, and
Ureaplasma urealyticum
Although C. trachomatis infection in neonates results from perinatal exposure to the mother’s infected cervix in the overwhelming majority of cases,18C. trachomatis, M. hominis, andU. urealyticummay also cause intrauterine infection.19Y26 Intrauterine infection with these pathogens may be associated with intrauterine growth restriction, preterm delivery, low birth
FIGURE 6. Chronic encephalitis with collar-of-pearl formations (arrow) along the walls of the third ventricle.
FIGURE 5. Spectral Doppler analysis of the descending aorta shows absent end-diastolic flow, indicative of visceral and placental alterations.
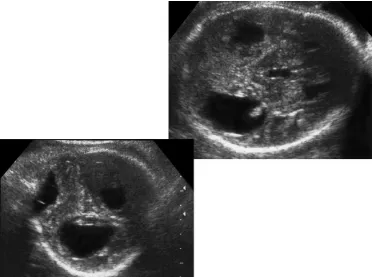
FIGURE 7. Fetal chorioretinitis. Observe the calcifications of the retina and the choroids (arrow).
weight, or neonatal death. It is likely that these pathogens are etiologic agents of these complications, particularly when the IgM antibody against them is present in fetal blood or in the blood of newborn neonates.19Y25
Neisseria gonorrhoeae
N. gonorrhoeaemay infect the cervix and contaminate the vagina of pregnant women.26The gonococcus can invade the amniotic cavity and cause fetal infection.27 More commonly, the uterus and the neonate become infected during labor and delivery. Exposed neonates who remain untreated may develop gonococcal conjunctivitis and pharyngitis.26
Intra-amniotic infection may lead to grave conse-quences. Fetal damage is directly proportional to the length
of time in the contaminated environment. There may be multiple skin abscesses, gastrointestinal abscesses, or severe genital infections. There is risk of fetal septicemia that may lead to meningoencephalitis, intrauterine growth retardation (IUGR), lesions in multiple joints, disseminated intravascular coagulopathy, and fetal death. Acute intra-amniotic infection can also lead to preterm, premature rupture of membranes (pPROM), premature labor, prematurity, and congenital conjunctivitis and ophthalmitis. In these cases, neonates may be born with pharyngeal, tracheal, stomach, or skin infections. They may also have vulvar, vaginal, urethral, or anal infection with the gonococcus.
Intrauterine infections during the first trimester may lead to septic abortion. Hematogenous fetal infection is rare, but when it occurs, there may be placentitis with eventual
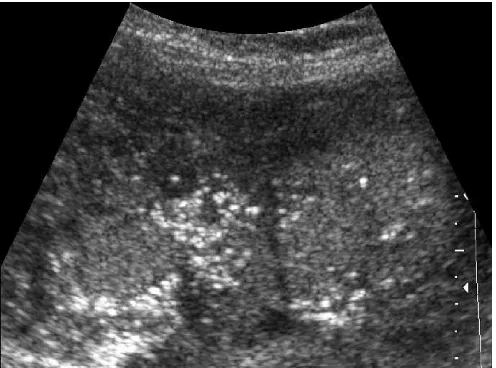
FIGURE 10. Marked fetal splenomegaly. The splenic parenchyma shows soft hypoechogenic areas that indicate edema.
FIGURE 9. Liver of 32-week infected fetus. Observe the cluster of echogenic lesions. The absence of acoustic shadowing suggests that these echogenic foci most likely represent specular reflections from the walls of microabsceses rather than true microcalcifications. The liver is the first organ to be affected by infections that gain access by the
transplacental route.
FIGURE 11. Twenty-nine-week fetus with group B
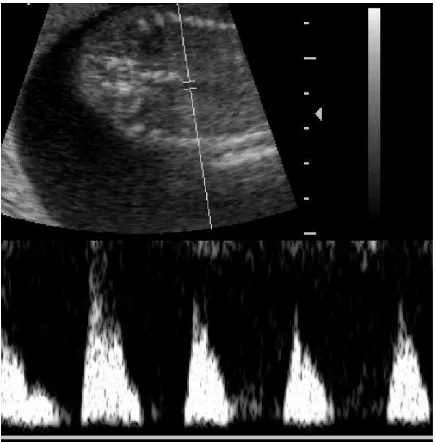
streptococcal infection. Observe the marked fetal ascites (lower vertical arrow). The bowel with marked echogenicity (middle horizontal arrow) is floating free in fluid, and the liver (upper downward arrow) is clearly visible, separated from the abdominal wall.
fetal infection via umbilical cord blood, with development of liver abscesses and septicemia.
Group B Streptococcus (Streptococcus agalactiae)
Group B streptococci are frequently cultured from the vagina and the cervical canal. Colonized pregnant women are at risk of ascending infection that may lead to pPROM and premature labor.28,29 Fetal infection can occur with intact membranes.30,31 When fetal infection occurs without pre-mature delivery, the fetus may develop skin infection. By breathing in contaminated amniotic fluid, pharyngeal and pulmonary infection may follow. The fetus may develop pleural effusions and other lesions that can be detected by ultrasonography, such as pulmonary microabscesses and areas of lung necrosis (Figs. 12, 13). On rare occasions, there may be hematogenous infections of the placenta leading to placentitis, placental infarction, and placental abscesses. Infection of the fetus by the hematogenous route is rare. When it occurs, the liver is primarily affected. Liver abscesses, septicemia, and ascites may follow, with involve-ment of multiple other organs (Fig. 9).Mycobacterium tuberculosis
Although uncommon in developed countries, congeni-tal tuberculosis is frequently underdiagnosed. Delayed diagnosis and treatment results in up to 50% mortality rate. Infected fetuses may develop hepatomegaly and splenome-galy. Neonates with congenital tuberculosis usually do not manifest signs of disease for days or weeks after delivery. The signs and symptoms of infection may be nonspecific. The neonates may develop respiratory distress, fever, poor feeding, failure to thrive, lethargy, lymphadenopathy, and abdominal distention.32 Hepatosplenomegaly may be detected on physical examination. Congenital tuberculosis has an excellent prognosis if treated at an early stage. For this
reason, early diagnosis is desirable. Mortality rate is high with untreated disease.33
The criteria for diagnosis of congenital tuberculosis34,35 are the following:
1. M. tuberculosismust be proved to be present; 2. The neonate has a primary complex in the liver; 3. The lesions are present within the first few days of life;
and
4. If the neonate has neither a proved hepatic primary complex nor lesions in the first few days of life, extrauterine infection must be excluded.
The liver is the principal site of fetal infection after the hematogenous spread of infection from the umbilical veins.
FIGURE 13. Pregnancy at 36th week of gestation with maternal history of nonspecific febrile illness. There are pulmonary hyperechogenic foci that most likely represent microabscesses and hypoechogenic areas that suggest focal parenchymal destruction. The fetus shows cardiomegaly with signs of cardiac insufficiency.
FIGURE 12. Fetus with pleural effusion. Observe the
calcifications in the heart. Cardiac calcifications are a direct sign of an inflammatory process. Pleural effusion can result from several conditions, but its association with cardiac
calcifications in this case makes pleural effusion an indirect sign of fetal infection.
Hepatic and placental granulomas can be observed with ultrasonography (Figs. 14, 15). The lungs may be infected by hematogenous spread or by inhalation of infected amniotic fluid. The central nervous system is seldom infected, but long bones, kidneys, spleen, the gastrointestinal tract, skin, and lymph nodes may be involved. Infection withM. tuberculosis causes characteristic caseating lesions. Hepatic granulo-mata are detectable with ultrasound. Severe fetal infection causes IUGR and encephalitis that may result in postnatal microcephaly.
FUNGAL INFECTIONS
Fungi may cause fetal infection by an ascending route (eg, Candida species) or by systemic spread (eg, Cocci-dioides, Cryptococcus, Blastomyces, and Sporothrix species). Systemic mycoses are extremely uncommon in immunocom-petent pregnant patients. Maternal systemic mycoses are seen in otherwise healthy patients who are first exposed in endemic areas during pregnancy or in immunosuppressed patients. Fortunately, fungi are rarely cultured in surveys of fetal or placental pathology.
Candida species
Although vaginal candidiasis is common in pregnancy, systemic maternal disease, fetal infection, and placental colonization are extremely rare.36Y38 Placental and fetal infection, when they occur, seem to occur by the ascending route. The infection is usually restricted to the placenta, umbilical cord, amniotic membrane, and fetal skin.
Microscopic examination of the umbilical cord reveals multiple yellow circular plates spanning the length of the umbilical cord and, occasionally, appears histologically as superficial microabscesses comprised of neutrophil polymor-phonuclear cells, lymphocytes, and large mononuclear cells. The lesions penetrate Wharton jelly but do not invade deeper vascular structures. Neither the chorionic villi nor the
decidual plate are involved. Chorioamnionitis is commonly seen. Fetal infection has been associated with premature delivery and fetal death. There may be nonspecific ultrasound abnormalities of the placenta and umbilical cord.39,40
Coccidioides immitis
C. immitis, the etiologic agent of coccidioidomycosis, is a dimorphic fungus that lives in the soil of highly restricted geographic areas. The organism is restricted to the south-western United States and contiguous areas of northern Mexico. This fungus can also be found in a few areas of Central and South America that have semiarid climate, alkaline soil, and characteristic plants and rodents of this type of semidesert environment. The risk ofC. immitis dissemina-tion is higher in the third trimester of pregnancy, probably because of an intrinsic susceptibility of the mother and the fetus when levels of estradiol are higher.41,42
Placental infection withC. immitisleads to microscopic foci of necrosis. The necrotic foci contain dead villi, fibrin deposits, and granulomatous reactions. Fetal demise may accompany severe maternal disease and placental damage. The placental barrier seems to be efficient in preventing fetal disease. The necrotic villi may lead to infarction of placental cotyledons. These changes are detectable with ultrasound and by the absence of flow when observed with color Doppler echocardiography. The infection-induced, chronic, atrophic placentitis provokes IUGR and chronic hypoxemia, and may cause perinatal death.
Cryptococcus, Blastomyces, and
Sporothrix species
Cryptococcus neoformansis one of the few organisms of the genus Cryptococcus that is pathogenic to humans.C. neoformansis an encapsulated yeast that is normally found in soil and avian feces. Cryptococcosis, the disease that results from infection with C. neoformans after airborne exposure and inhalation of the yeast, initially affects the lungs. The organism may metastasize from the lungs to virtually any organ in the body. This organism is ubiquitous in nature, yet the incidence rate of cryptococcosis is low.
Blastomyces dermatitidis, a thermally dimorphic fun-gus, is the causative agent of blastomycosis, a chronic lung infection characterized by granulomatous and suppurative lesions that occurs after inhalation of the organism. Most infections are subclinical and resolve spontaneously. Calci-fication is uncommon; therefore, there is little radiological or histopathologic evidence of residual blastomycotic lesions. Although there is preferential spread from the lungs to the skin and bones in subclinical infections, dissemination may occur to any organ with severe infections.
Sporothrix schenckiiis a thermally dimorphic fungus that causes sporotrichosis, a chronic infection of the lymphatics and cutaneous and subcutaneous tissues. The infection follows traumatic implantation of the organism into the skin. Secondary spread may involve lymph nodes, underlying muscle, bone, and, occasionally, lungs, central nervous system, and the genitourinary tract. Sporotrichosis is considered an occupational disease of forest rangers, horticulturists, and other agricultural workers.
C. neoformans, B. dermatitidis, and S. schenckii are known to be able to cause systemic maternal infection. However, placental involvement and fetal infection have not been well documented with these organisms. It is likely that the scant documentation of the effects of fetal infection with these organisms is due to a low incidence rate of maternal infection, along with a low index of suspicion when there is unexplained IUGR, fetal flow centralization, fetal organ calcification, or abnormal placental calcification.
PARASITIC INFECTIONS
Toxoplasma gondii
T. gondii is an obligate intracellular parasite of, primarily, domestic cats and other felines. However, the organism is known for its lack of host specificity. It may be found in several primates, carnivores, ungulates, birds, and rodents. The organism causes toxoplasmosis in humans. Acquisition ofT. gondiifollows the consumption of infected undercooked meat or exposure to oocysts in cat excreta or infected soil. Most maternal infections are asymptomatic or result in a mild influenzalike illness.43Y46
Acute infection during pregnancy, accompanied by parasitemia, can lead to placental infection. Transmission to the fetus depends on gestational age. In early pregnancy, the transmission rate is only about 10%; however, severe lesions can affect 90% of infected fetuses. In the last trimester of pregnancy, the rate of vertical transmission rises to about 45%; however, the rate of severe fetal lesions drops to less
than 5%. Fetal infection may result in encephalomyelitis, central nervous system necrosis and calcification, and ophthalmic calcifications (Figs. 16Y18).
Severe neonatal manifestations are low birth weight, jaundice, hepatomegaly, thrombocytopenia, meningoence-phalitis, and hydrocephalus. Hydrocephalus, intracranial calcification, and bilateral retinochoroiditis are the classical
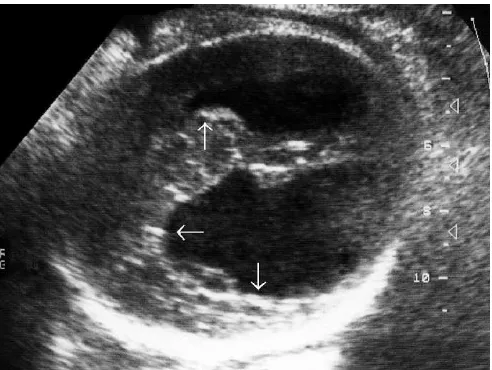
FIGURE 16. Fetus at 36th week of gestation with chronic encephalitis. Observe the asymmetric dilatation of the lateral ventricles and enlargement of the cisterna magna. There is also vascular calcification (arrow on vasculith) next to the fissure of Sylvius. This fetus also had splenomegaly.
triad of congenital toxoplasmosis. These common fetal and neonatal lesions are detectable with ultrasound. The accurate diagnosis of disease in pregnancy is desirable because antenatal therapy seems to reduce the severity of postnatal manifestations.45,46
Malaria (Plasmodium falciparum)
Congenital malaria has been documented with all major species of plasmodia. Most diseases related to malaria in pregnancy are secondary to placental involvement and its subsequent effects upon the fetus. Pregnancy seems to be associated with increased maternal parasitemia. Prenatally acquired malaria is found primarily in neonates of non-immune women who contract malaria during pregnancy. It is rare among the neonates of indigenous women of malarial areas, despite heavy infestation of the placenta. The incidence rate of abortion is inversely proportional to the degree of maternal immunity.47Y49
Placental infection is often associated with severe maternal anemia, preterm labor, fetal growth restriction, and low birth weight. Fever, anemia, and splenomegaly occur in neonates with congenital infection. Reticulocytosis, jaundice, and hyperbilirubinemia are frequently seen. Hepatomegaly is less frequently seen.
Schistosomiasis
Schistosoma haematobiumis likely to migrate preferen-tially to the pelvic veins after penetration, in cercarial form, through the skin. Excretion of eggs in the uterus and placenta can occur both withS. haematobium and with Schistosoma mansoni.50,51
Eggs are found in the intervillous spaces, with or without an accompanying inflammatory response in the form
of a chronic granulomatous lesion. Fetal disease results from placental compromise secondary to massive egg deposition and the associated inflammatory reaction. Direct fetal infection has not been documented. The observable ultra-sound changes occur in the placenta.
Trypanosomiasis
T. cruzi, the agent of Chagas disease, is the most important cause of trypanosomal congenital infection.52 Infected mothers are commonly asymptomatic during pregnancy. The incidence rate of congenital disease is higher if the mother is symptomatic, presumably because of greater parasitemia. Recurrent congenital disease is possible.
The placenta becomes infected after maternal para-sitemia. Amastigotes are subsequently found in the chorionic villi and plate. Less frequently, they are found in the membranes. Villous edema and granulomatous reaction may follow placental infection. Fetal infection occurs predominantly through the umbilical cord but may also occur from contaminated amniotic fluid. Fetal infection involves the heart, skeletal muscle, brain, skin, the gastro-intestinal tract, and the reticuloendothelial system. Infection leads to severe anemia, fetal edema, hepatosplenomegaly, jaundice, and encephalitis. Extramedullary hematopoiesis is increased. The disease may resemble erythroblastosis fetalis. Infection may lead to abortion, stillbirth, prematurity, low birth weight, or neonatal death.
DISCUSSION
Although the advances in knowledge and the develop-ment of newer laboratory methods, such as the polymerase chain reaction and nucleic acid hybridization technique, have improved our ability to detect maternal infections, many (if not most) infections that can cause serious fetal damage or death remain undetected. Placental atrophy, amniotic volume alterations, fetal growth restriction, depletion or failure of development of fetal fat stores, chronic hypoxemia, mal-formations, and perinatal death are nonspecific events that can result from intrauterine infection. Intrauterine infection may affect the brain, eyes, heart, lungs, liver, spleen, kidneys, or bones of the fetus in ways that may not be clinically obvious until weeks, months, or years after birth.53
Most of the anatomical malformations, placental structural alterations, and umbilical cord changes that occur with fetal infection are detectable with good-quality ultra-sound machines. Our experience suggests that appropriate screening ultrasonography schedules can be used to identify certain markers of fetal infection. We are proposing a minimum of 4 ultrasound examinations (at the 13th, 22nd, 30th, and 36th gestational weeks) for the following reasons:
1. Prenatal and postnatal clinical detection of congenital silent infections is low.
2. Many of the sequelae of silent chronic encephalitis become evident during the school years. At times, the sequelae of silent fetal infection are erroneously attrib-uted to events that occur during labor and delivery. 3. Many conditions that occur later in the life of affected
individuals are late manifestations of undiagnosed
FIGURE 18. Pregnancy at 34th week of gestation with fetal toxoplasmosis. The fetus has severe, chronic encephalitis. Observe the asymmetric cerebral atrophy with calcifications of the ventricular walls (horizontal arrow) and the cerebral parenchyma (vertical upward arrow). There is great destruction of the parenchyma to the extent that the ventricle
congenital infection categorized as idiopathic or attrib-uted to postnatal events. Late manifestations of chronic inflammation in organs such as the heart, lungs, liver, kidneys, and other organs may, in fact, be sequelae of undetected congenital infection that could have been detected prenatally by identification of ultrasound markers and confirmation with appropriate prenatal laboratory tests.
4. At the 13th week of gestation, we can identify abnormal nuchal translucency, abnormal ductus venosus flow, and several fetal anomalies. Besides being a marker for certain chromosomal anomalies and recognized syn-dromes, nuchal translucency and abnormal ductus venousus flow are also markers of fetal infection during the first trimester and, especially, of infections that compromise fetal cardiovascular function.
5. Ultrasound evaluation during the 22nd, 30th, and 36th gestational week will reveal signs of placentitis, altera-tions of amniotic fluid volume, fetal dysmorphology, hydrops, IUGR, and chronic hypoxemia.
Signs of chronic hypoxemia can be detected by performance of a simplified fetal biophysical profile (respira-tory movements, body movements, and cardiac reactivity), along with Doppler study of cerebral arteries. Doppler studies are also useful for the evaluation of cardiac, cerebral, renal, and placental functions, and for evaluation of functional alterations caused by inflammatory processes. These studies also help to evaluate calcifications, granulomas, and disrup-tions in the various tissues that are strong markers of infec-tion. In many of the ultrasound images, hyperechogenic foci identified as Bcalcifications^have no acoustic shadowing as would be expected with truly calcified lesions (Figs. 9, 13, 15). Without pathological correlation, it is impossible to state unequivocally that these images are definite examples of microcalcification. These echogenic lesions most likely rep-resent specular reflections from the walls of microabscesses that may eventually evolve into truly calcified lesions.
Infections that reach the fetus by the placental route are characteristically associated with inflammation of the placental villi. There may be focal infiltration of villi with lymphocytes and histiocytes. This inflammatory reaction eventually leads to necrosis, granulation tissue formation, fibroblastic prolifera-tion, and fibrosis. Vasculitis of villus stem arteries may cause thrombosis and atrophy of villi. Villitis caused by subclinical infection can lead to progressive placental atrophy and abnormal calcification. These placental changes lead to progressive restriction of fetomaternal exchange, to fetal growth restriction, and to the most severe forms of chronic fetal hypoxia. The diagnosis of villositary atrophy can be made with Doppler scanning by detection of an increase in the resistive index (RI) of the umbilical arteries. Doppler scanning of fetal cerebral arteries, aorta, renal vessels, and venous system can also be used to evaluate the necessary vascular adjustments of the fetus to compensate for chronic hypoxemia. Fetal hydrops and pathological calcification are 2 common sequelae of intrauterine infection. A variety of structural abnormalities, which primarily interfere with the fetoplacental circulation, usually obstructing venous return
from the placenta, are causally related to hydrops. Obstruc-tion to venous return from the placenta leads to increased venous pressure, which, in turn, leads to placental edema, impaired gas exchange, fetal hypoxia, fetal capillary damage, loss of plasma proteins, and, eventually, to fetal hydrops.
The so-calledTORCHinfections (toxoplasmosis,other infections, rubella, cytomegalovirus infection, and herpes simplex), syphilis, coxsackie virus infection, Parvovirus infection, malaria, and infection from organisms that cause fetal myocarditis can initiate the changes that ultimately result in fetal hydrops. In cases of fetal hydrops (Fig. 11), the placenta is often extremely thick and may weigh more than 1 kg (Fig. 1). In addition, there may be marked edema of the umbilical cord.
Placental edema suggests that the underlying cause induced severe fetal anemia and/or obstruction to venous return from the placenta. The 3 most important failures of fetal homeostasis, which are associated with hydrops, are (1) severe, chronic anemia, (2) hypoproteinemia, and (3) fetal cardiac failure. Complications arise as fluid balance deterio-rates. Eventually, all 3 mechanisms may contribute to the worsening of the hydropic state.
Placental edema interferes with maternal-fetal trans-port.54Y56This event contributes to the worsening of the fetal condition by contributing to a decrease in fetal plasma protein because of poor protein transport, and to hypoxic damage to fetal capillaries caused by less efficient oxygenation, which, in turn, leads to the loss of even more plasma proteins into the interstitial space.
Although placental alterations may give a clue to the etiology of fetal disorders, lesions such as villitis, atrophy, and thickening are generally nonspecific. Frequently, pla-cental lesions are markers of problems that may range from malformations and infection to serious metabolic disorders.
Hydrops is one of the most common nonspecific disorders related to fetal abnormalities and acquired disease. Besides certain viral infections, noninfectious fetal condi-tions, and a few maternal disorders,57syphilis, leptospirosis, S. agalactiae(group B streptococcus) septicemia, toxoplas-mosis, and Chagas disease can start the chain reaction that results in hydrops fetalis.
Silent congenital infections can also cause nonspecific changes in the amniotic fluid. Volume may be reduced because of placental atrophy, chronic vascular lesions, a reduction in fetal pulmonary or renal perfusion or function, an alteration in fetal metabolism, or a reduction in fetal-maternal exchange. Infections are prominent among the many etiologies for polyhydramnios. Infection causes disturbances in the control of fetal blood levels of protein, interferes with placental venous return, and induces cardiovascular and hematological alterations. These disturbances interfere with the normal control of amniotic fluid volume. When there is intra-amniotic microbial invasion, the amniotic fluid density may increase because of the proliferation of microorganisms and because of the accumulation of debris that originates from meconium and from increasing desquamation of different epithelial tissues affected by the infection. As a consequence, the structural integrity and strength of the amniotic membranes may be compromised, and pPROM may follow. Therefore, ascending infection from the maternal genital tract is one of the most important causes of chorioamnionitis, amniorrhexis, and microbial invasion of the amniotic cavity. When pathogenic organisms invade the amniotic cavity across intact membranes and overwhelm the natural defense systems, the fetal brain, lungs, liver, gastrointestinal tract, kidneys, skin, or other fetal organs may be compromised.
The brain is at risk of serious damage from intrauterine infection. Fetal and neonatal meningoencephalitis may have a benign transitory course without future consequences, or may affect brain tissue with varying degrees of damage. The process may end with simple edema and/or with varying degrees of disruption, or it may follow a chronic, progressive course with extensive compromise because of severe destruction of cerebral parenchyma, ventriculitis, necrosis, meningitis, and fluid collection. The most severe form is schizencephaly, which consists of extensive destruction of parenchymal tissue and rupture of the ventricular wall so that there is physical contact between the ventricular cavities and the meninges (Fig. 18).
There are 2 basic mechanisms by which brain lesions can occur: (1) they may occur by the effects of chronic
hypoxia, and (2) they may be caused by a direct invasion of microorganisms into brain tissues. Both events may take place simultaneously in the most severe cases. Chronic hypoxia results from atrophy of placental villi after chronic villitis or placentitis. Chronic hypoxia induces adaptive vascular reactions that are active in all living individuals, starting from fetal life. These adaptive vascular changes are useful in situations of acute asphyxia because they preserve the heart and the brain from grave and irreversible damage, as is seen, for example, in cases of acute intrapartum hypoxia or in cases of postnatal recovery from acute asphyxia after drowning. In these cases, there is a redistribution of circulating blood to the brain, the heart, and the suprarenal glands due to vasodilatation, and a reduction of blood to the lungs, kidneys, the gastrointestinal tract, the skin, and the musculoskeletal system due to vasoconstriction. When there is timely rescue from acute asphyxia, there are no permanent sequelae.
The blood redistribution that occurs in acute cases of hypoxia becomes a trap in cases of chronic hypoxia. If the conditions leading to chronic hypoxia remain for too long, there can be severe hypoxic-ischemic lesions in the organs affected by persistent vasoconstriction, and grave hypoxic-hemorrhagic lesions in organs affected by persistent vasodi-latation. In our experience, a situation of chronic fetal hypoxia can remain silent for up to 6 weeks. After this time, the risk of neonatal complications with permanent sequelae increases progressively with the duration of exposure to chronic hypoxia. Unfortunately, classical methods, such as fetal biophysical profile and fetal cardiac monitoring that are commonly used for diagnostic evaluation of fetal health, are only capable of detecting chronic hypoxia toward the end of the protective cardiovascular redistribution process, when fetal neurological and cardiac decompensation have already started. In these cases, the classical monitoring methods may prevent fetal and/or neonatal death, but they may not prevent the sequelae of chronic hypoxia because the serious damage to the brain and the heart frequently occurs during the silent
phase. At times, the manifestations of undiagnosed chronic hypoxic damage during fetal life may only emerge as late in postnatal life as adulthood.53
It is possible to diagnose atrophy of placental villi and to detect fetal adaptive vascular reactions to hypoxia with Doppler studies of the venous and arterial flows of the fetoplacental unit.62Y64 Detection of compensated chronic hypoxia will provide for earlier delivery and prevent the most serious sequelae associated with decompensated chronic hypoxia.
If one corrects for gestational age by comparing newborn neonates with evidence of chronic intrauterine hypoxia with premature neonates delivered for reasons not associated with chronic hypoxia, the risks of grave sequelae due to prematurity for neonates delivered with compensated chronic hypoxia are much lower than the risks of older neonates delivered with decompensated chronic hypoxia.65
One of the characteristic signs of encephalitis is cerebral calcification. Frequently, there is also hydrocephaly due to ventriculitis and/or brain atrophy. Doppler scanning can reveal signs of vasodilatation when there is acute encephalitis. An increase in vascular flow resistance (eleva-tion of RI) occurs when there is chronic encephalitis with fibrosis or when hydrocephaly occurs because of obstruction. If hydrocephaly occurs because of brain atrophy, the vascular flow indexes are normal. Focal tissue necrosis may produce brain cysts (Table 2).
Fetal encephalitis may have a chronic, progressive, postnatal course, which may be clinically silent.53Different degrees of cerebral dysfunction or onset of severe mental retardation with or without microcephaly may emerge at any time in the lifetime of these individuals. Hydrocephaly and brain cysts may be found even among adult survivors of fetal encephalitis who are considered to be healthy. Neonatal neurosonography or neurosonography performed within the
first 2 years of life can identify lesions that result from congenital infection (Table 3). In our opinion, neurosono-graphy should be performed in all neonates to rule out clinically silent brain lesions that are sequelae of fetal infection. This measure is not only important because it allows prompt medical attention, but because it may also avoid charges of obstetric negligence if sequelae of fetal encephalitis become evident in later life.
The eyes are organs related to brain development and can sustain severe damage from congenital infections.66 Chorioretinitis may progress to a chronic state that ends up with fibrosis, calcifications, and retinal thickening. Cataracts and/or severe condensation of the vitreous humor may ensue. In the most severe cases, ocular atrophy, microphthalmia, and blindness may occur. On the other hand, fetal ophthalmitis may be silent and may result in gradual and progressive postnatal visual loss.
If there is fetal pneumonitis, we can observe pleural effusions and parenchymal disorganization. During the chronic phase, fibrosis, granulomas, and pulmonary and/or pleural calcifications may develop (Figs. 12, 13).
The earliest signs of fetal myocarditis are pericardial effusion, arrhythmia, and irregular ventricular contractions (Figs. 12, 21). Cardiomegaly is the most severe sign of myocarditis and is evidence of cardiac failure during the acute phase or of valvular lesions during the chronic phase
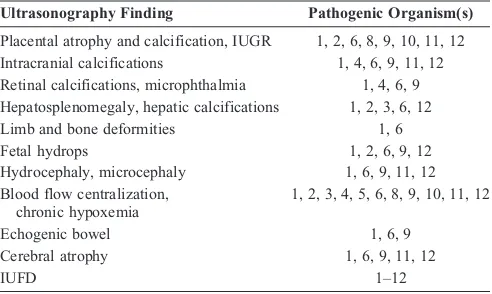
TABLE 2. Common Ultrasound Findings Associated with Fetal Infection
Ultrasonography Finding Pathogenic Organism(s)
Placental atrophy and calcification, IUGR 1, 2, 6, 8, 9, 10, 11, 12 Intracranial calcifications 1, 4, 6, 9, 11, 12 Retinal calcifications, microphthalmia 1, 4, 6, 9 Hepatosplenomegaly, hepatic calcifications 1, 2, 3, 6, 12
Limb and bone deformities 1, 6
Fetal hydrops 1, 2, 6, 9, 12
Hydrocephaly, microcephaly 1, 6, 9, 11, 12 Blood flow centralization,
chronic hypoxemia
1, 2, 3, 4, 5, 6, 8, 9, 10, 11, 12
Echogenic bowel 1, 6, 9
Cerebral atrophy 1, 6, 9, 11, 12
IUFD 1Y12
1 indicates Treponema pallidum; 2, Listeria monocytogenes; 3, Chlamydia trachomatis; 4,Neisseria gonorrhoeae; 5, Mycoplasma and Ureaplasma species; 6,
Mycobacterium tuberculosis; 7, group B streptococcus; 8, fungal infections; 9,
Toxoplasma gondii; 10, plasmodia; 11, Schistosoma species; 12,Trypanosoma cruzi; IUFD, intrauterine fetal death.
Note:Although fetal infection with any of the bacteria, fungi, or protozoa can be associated with any of the findings listed, only the most common organisms associated with a given ultrasonography finding are listed.
TABLE 3. Doppler Findings of Different Lesions that Result from Fetal Encephalitis
Condition Impedance (RI)
Acute encephalitis Normal orj
Chronic sclerosing encephalitis ,
Chronic vasculitis ,
Hydrocephaly secondary to brain atrophy Normal Hydrocephaly secondary to obstruction Normal or,
Upward arrow (j) indicates increased flow; downward arrow (,), decreased flow.
(Fig. 13). With progressive myocarditis, there will be cardiac insufficiency. When there is cardiac insufficiency, fetal hydrops eventually follows (Fig. 11).
With chronic myocarditis, we may find isolated ventri-cular dilatation, severe valvular lesions, myocardial fibrosis, and pericardial, endocardial, or valvular calcifications. Doppler scanning is of great help for the evaluation of cardiac arrhythmias, valvular and vascular functional integ-rity, anomalous ventricular contraction, and early signs of cardiac insufficiency. In our experience, the so-calledBgolf ball sign^ is related more to congenital infection than to chromosomal anomalies (Fig. 22). We realize that this con-clusion is not widely accepted in the literature. We are awaiting results from other investigators to determine whether their results support or are in conflict with our observations.
The liver is frequently affected by transplacental fetal infections acquired by the hematogenous route (Figs. 9, 15). The fetus has 2 protective barriers against hematogenous infections. The first and most efficient are the placental villi. Invading pathogens must destroy the affected villi before being able to invade the fetal compartment. The second is the liver, which offers partial protection against generalized microbial dissemination to fetal tissues. When fetal blood returns from the placenta through the umbilical cord vein, it must flow through the liver. The umbilical vein serves functionally as part of the portal-hepatic system because 55% to 65% of the blood transported from the placenta is distributed through the liver parenchyma across the portal vessels, and the remainder flows toward the heart by way of the ductus venosus. Because of this phenomenon, the liver is the first point of fetal contact with pathogens coming from the placenta and serves as a protective filter that interferes temporarily with microbial invasion of other fetal organs. As a result, the liver usually develops lesions
from bloodborne pathogens before any other fetal organ. This knowledge can be useful to establish route and timing of infection in neonates. For example, in the case of a neonate diagnosed with tuberculosis, if the primary infection is in the liver, it is most likely caused by a congenital infection. If it is in the lungs, the initial infection most likely occurred postnatally.
During the acute phase of an infection, the fetal liver presents with an increase in volume, edema, parenchymal disruption, and abscesses. During the chronic phase, we may find irregular fibrosis, calcifications, cysts, granulomata, and, rarely, atrophy of the biliary ducts. Congenital chronic liver infections are the cause of many postnatal, lifelong complications. There may be difficult-to-control neonatal jaundice, postnatal hepatic insufficiency, recurrent infections, cirrhosis, thrombosis, and tumors presenting as complications of chronic fetal liver infection. For example, 80% of fetuses who develop hepatitis B infection will become lifelong chronic carriers, and 25% of these will develop cirrhosis and/ or hepatocellular carcinoma between the ages of 15 and 60 years. Ultrasound scanning of the fetal liver is an excellent method to detect lesions caused by various pathogens acquired hematogenously.
Because of their important filtering role, the fetal kidneys are targets of congenital infection. They may develop edema, calyceal dilatation, abscesses, fibrosis, and, even-tually, atrophy (Fig. 23). Nephritis and vasoconstriction secondary to placental atrophy may provoke renal insuffi-ciency and oliguria. Renal insuffiinsuffi-ciency is an important cause for third-trimester oligohydramnios. Doppler scanning can detect hypoxic vasoconstriction of renal arteries after the 32nd gestational week.
The skin can become infected either by the hemato-genous route or by ascending infection of the amniotic cavity, either across intact amniotic membranes or after amnior-rhexis. The fetal skin may become edematous or may develop rashes, petechiae, vesicles, bullous lesions, abscesses, or
FIGURE 22. Golf ball calcification. In our experience, this finding is related to an inflammatory process. We have detected them in several cases of fetal infections with viruses (ie, Cytomegalovirus species) and bacteria (ie, group B streptococcus).
granulomas. In cases of fetal hydrops, there will be diffuse subcutaneous edema (Fig. 24). With the exception of edema, diagnosis of skin lesions is usually made postnatally, as is the case with bone lesions.
SUMMARY
Intrauterine bacterial, fungal, and parasitic infections commonly cause placental abnormalities that lead to chronic hypoxia and IUGR. Villitis can lead to placental calcifica-tions, infarccalcifica-tions, and atrophyVconditions that interfere with fetomaternal exchange of blood gases and nutrients. Organic and physiological compensatory changes and alterations of amniotic fluid volume occur in response to chronic fetal hypoxia. Many of these changes and alterations are readily detectable with ultrasound scanning.
Fetal central nervous system infections can cause brain tissue destruction and initiate changes that lead to cyst formation, intracerebral calcification, hydrocephaly, and/or microcephaly. If the fetal eyes are involved, cataracts, fibrosis, or retinal calcifications may develop. Fetal myocarditis can result in anatomical alterations, such as cardiomegaly, and provoke lesions, such as cardiac calcifications, valvular lesions, and cardiac failure. Infections of the fetal liver and spleen can cause hepatosplenomegaly and provoke the development of granulomas, calcifications, and atrophy. Infections that induce anemia cause fetal hydrops and placental edema. Several other fetal structures may be adversely affected by infection. Among these are the lungs, kidneys, and intestines. These changes are detectable with ultrasonography. Doppler scanning can be used to evaluate changes in vascular flow that result from fetal infection. Notably, Doppler scanning can detect flow alterations in the uterine and umbilical cord arteries, the umbilical vein, the fetal heart and aorta, the fetal cerebral and renal arteries, the inferior vena cava, and the ductus venosus (Figs. 2Y5). Unexplained alter-ations and abnormalities of the fetal and placental circulation require careful ultrasound examination and Doppler scanning to rule out intrauterine infection.
Ultrasound evidences of inappropriate calcification in fetal tissues and placental villi are strongly suggestive of congenital infection. These findings require laboratory follow-up to detect or rule out specific infections that may have long-lasting adverse effects. Some of these infections are treatable in utero. We propose a minimum of 4 ultrasound examinations during pregnancy (in the 13th, 22nd, 30th, and 36th week of gestation) for the detection and, in some cases, treatment of silent fetal infections.
REFERENCES
1. Schlievert P, Larsen P, Johnson W, et al. Bacterial growth inhibition by amniotic fluid.Am J Obstet Gynecol. 1975;122:809Y819.
2. Baila˜o LA, Osborne NG, Rizzi MCS, et al. Ultrasound markers of fetal infection part 1.Ultrasound Q. 2005;21:295Y308.
3. Baila˜o LA, Rizzi MCS, Bonilla-Musoles F, et al. Ultrasound markers of fetal infection.Ultrasound Q. 2000;16:221Y233.
4. Tafari N, Ross SM, Naeye RL, et al. Failure of bacterial growth inhibitor by amniotic fluid.Am J Obstet Gynecol. 1977;128:187Y189.
5. Christensen KK. Infection as a predominant cause of perinatal mortality.
Obstet Gynecol. 1982;59:499Y507.
6. Naeye RL, Peters EO. Amniotic fluid infections with intact membranes leading to perinatal death: a prospective study.Pediatrics. 1978;61: 171Y177.
7. Labarrere C, Altnabe O, Telenta M. Chorionic villitis of unknown etiology in placentae of idiopathic small for gestational age infants.
Placenta. 1982;3:309Y318.
8. Centers for Disease Control and Prevention. Sexually Transmitted Diseases Treatment Guidelines.MMWR. 2002;51(RR-6):25. 9. Boyer KM, Gotoff SP. Prevention of early-onset neonatal group B
streptococcal disease with selective intrapartum chemoprophylaxis.
N Engl J Med. 1986;314:1665Y1669.
10. Gotoff SP, Boyer KM. Prevention of group B streptococcal early onset sepsis.Pediatr Infect Dis J. 1989;8:268Y270.
11. Judge DM. Congenital syphilis. In: Judge DM, ed.Transplacental Effects on Fetal Health. New York, NY: Liss; 1988:87Y106.
12. Oppenheimer EH, Dahms BB. Congenital syphilis in the fetus and neonate.Perspect Pediatr Pathol. 1981;6:115Y138.
13. Russel P, Altshuler G. Placental abnormalities of congenital syphilis. A neglected aid to diagnosis.Am J Dis Child. 1974;128:160Y163. 14. Bortolussi R, Schlech WF III, Albritton WL. Listeria. In: Lennette EH,
Balows A, Hausler WJ Jr, Shadomy HJ, eds.Manual of Clinical Microbiology. Washington, DC: American Society for Microbiology; 1985:205.
15. Barresi JA.Listeria monocytogenes: a case of premature labor and neonatal sepsis.Am J Obstet Gynecol. 1980;136:410.
16. Romero R, Winn HN, Wan M, et al. Listeria monocytogenes chorioamnionitis and premature labor.Am J Perinatol. 1988;5:286. 17. Schlech WF, Lavigne PM, Bortolussi RA, et al. Epidemic
listeriosisVevidence of transmission by food.N Engl J Med. 1983;308:203.
18. Centers for Disease Control and Prevention. Sexually Transmitted Diseases Treatment Guidelines.MMWR. 2002;51(RR-6):32Y36. 19. Pao CC, Kao SM, Wang HC, et al. Intraamniotic detection of
Chlamydia trachomatisdeoxyribonucleic acid sequences by polymerase chain reaction.Am J Obstet Gynecol. 1991;164: 1295Y1299.
20. Martin DH, Koutsky L, Eschenbach DA, et al. Prematurity and perinatal mortality in pregnancies complicated by maternalChlamydia trachomatisinfection.JAMA. 1982;250:1585Y1588.
21. Osborne NG, Hecht Y, Gorsline J, et al. A comparison of culture, direct fluorescent antibody test, and a quantitative indirect immunoperoxidase assay for detection ofChlamydia trachomatisin pregnant women.Obstet Gynecol. 1988;79:412Y415.
22. Harrison HR, Alexander ER, Weinstein L, et al. CervicalChlamydia trachomatisand mycoplasma infections in pregnancy. Epidemiology and outcome.JAMA. 1983;250:1721Y1727.
23. Kundsin RB, Driscoll SG. Mycoplasmas and human reproductive failure.
Surg Gynecol Obstet. 1970;131:89Y92. FIGURE 24. Subcutaneous edema (arrows) in hydropic,
24. Embree JE, Krause VW, Embil JA, et al. Placental reinfection with
Mycoplasma hominisandUreaplasma urealyticum: clinical correlation.
Obstet Gynecol. 1980;56:475Y481.
25. Osborne NG, Kundsin RB. The role of mycoplasmas in obstetric and gynecologic infections. In: Monif GRG, ed.Infectious Diseases in Obstetrics and Gynecology. Omaha, NE: IDI Publications; 1993:294Y300. 26. Centers for Disease Control and Prevention. Sexually transmitted
diseases treatment guidelines.MMWR. 2002;51(RR-6):36Y42. 27. Smith LG Jr, Summers PR, Miles RW, et al. Gonococcal
chorioamnionitis associated with sepsis: a case report.Am J Obstet Gynecol. 1989;160:573Y574.
28. Boyer KM, Gotoff SP. Prevention of early-onset neonatal group B streptococcal disease with selective intrapartum chemoprophylaxis.
N Engl J Med. 1986;314:1665Y1669.
29. Centers for Disease Control and Prevention. Prevention of perinatal group B streptococcal disease: a public health perspective.MMWR. 1996;45(RR-7):1Y24.
30. Desa DJ, Trevenen CL. Intrauterine infections with group B beta-hemolytic streptococci.Br J Obstet Gynaecol. 1984;91:237Y239. 31. Creatsas GC, Charalambidis VM, Zagotzidou E, et al. Untreated cervical
infections, chorioamnionitis, and prematurity.Int J Gynaecol Obstet. 1995;49:1Y7.
32. Bate TW, Sinclair RE, Robinson MJ. Neonatal tuberculosis.Arch Dis Child. 1986;61:512Y514.
33. Jacobs RF, Abernathy RS. Management of tuberculosis in pregnancy and the newborn.Clin Perinatol. 1988;15:305Y319.
34. Reece EA, Hobbins JC, Mahoney MJ, et al. Infec0o˜es fetais de origem maternal e tratamento. Chapter 15. In: Reece EA, Hobbins JC, Mahoney MJ, Petrie RH, eds.Compendio de Medicina Fetal e Materna. Porto Alegre, Brazil: Editora Artes Medicas; 1996:96.
35. Namir RL, O’Hare D. Congenital tuberculosis. Review and diagnostic guidelines.Am J Dis Child. 1985;139:284Y287.
36. Whyte RK, Hussain Z, deSa D. Antenatal infections with Candida.Arch Dis Child. 1982;57:528.
37. Lopez E, Alterman K. Intrauterine infection by Candida.Am J Dis Child. 1968;115:663.
38. Albarracin NS, Pattereson WS, Haust MD.Candida albicansinfection of the placenta and fetus.Obstet Gynecol. 1967;30:838.
39. Osborne NG, Bonilla-Musoles F, Raga F, et al. Umbilical cord cysts: color Doppler and three-dimensional ultrasound evaluation.Ultrasound Q. 2000;16:133Y139.
40. Kingdom JCP, Burrell SJ, Kaufmann P. Pathology and clinical implications of abnormal umbilical artery Doppler waveforms.
Ultrasound Obstet Gynecol. 1997;9:271.
41. Harris RE. Coccidioidomycosis complicating pregnancy: report of three cases and review of the literature.Obstet Gynecol. 1966;28:401. 42. Drutz DJ, Huppert M, Sun SH, et al. Human sex hormones stimulate
the growth and maturation ofCoccidioides immitis.Infect Immun. 1981;32:897.
43. Desmonts G, Couvreur J. Toxoplasmosis in pregnancy and its transmission to the fetus.Bull N Y Acad Med. 1974;50:146Y159. 44. Bader TJ, Macones GA, Asch DA. Prenatal screening for toxoplasmosis.
Obstet Gynecol. 1997;90:457Y464.
45. Daffos F, Forestier F, Capella-Pavlovsky M, et al. Prenatal management of 746 pregnancies at risk for congenital toxoplasmosis.N Engl J Med. 1988;318:271Y275.
46. Desmonts G, Couvreur J. Congenital toxoplasmosis: a prospective study of the offspring of 542 women who acquired toxoplasmosis during pregnancy. Pathophysiology of congenital disease. In: Thalhammer O, Baumgarten K, Pollack A, eds.Proceedings of the Sixth European Congress on Perinatal Medicine, Vienna, 1978. Stuttgart, Germany: Thieme; 1979:51Y60.
47. Arvin A, Ruskin J, Yeager A. Protozoon and helminth infections (includingPneumocystis carinii). In: Remington JS, Klein JO, eds.
Infectious Diseases of the Fetus and Newborn Infant, 3rd ed. Philadelphia, PA: Saunders; 1990:528Y573.
48. Bray RS, Andersen MJ. Falciparum malaria and pregnancy.Trans R Soc Trop Med Hyg. 1979;73:427Y431.
49. Gilles HM, Lawson JB, Sibelas M, et al. Malaria, anemia, and pregnancy.
Ann Trop Med Parasitol. 1969;63:245.
50. Bittencourt AL, Cardoso MA, Fonseca MA, et al. Placental
involvement with schistosomiasis mansoni.Am J Trop Med Hyg. 1980; 29:571Y575.
51. Bullough CHW. Infertility and bilharziasis of the female genital tract.
Br J Obstet Gynaecol. 1976;83:819Y822.
52. Bittencourt AL. Congenital Chagas’ disease.J Pediatr. 1976;130: 97Y103.
53. Menser MA, Forrest JM. High incidence of defects in children considered normal at birth.Med J Aust. 1974;1:123Y126.
54. Bryan EM. IgG deficiency in association with placental edema.Early Hum Dev. 1977;112:133Y143.
55. Bryan EM, Nicholson E. Hydrops fetalis in South Korea.Ann Trop Paediatr. 1981;1:181Y187.
56. Boyd PA, Keeling JW. Fetal hydrops.Clin Genet. 1992;29:91Y97. 57. Baila˜o LA, Osborne NG, Rizzi MCS, et al. Ultrasound markers of
fetal infection part 1: viral infections.Ultrasound Q. 2005;21: 295Y308.
58. Tyndall VR, Scott JS. Placental calcification. A study of 3025 singleton and multiple pregnancies.J Obstet Gynaecol Br Commonw. 1965;72: 356Y373.
59. Fujikura T. Placental calcification and maternal age.Am J Obstet Gynecol. 1963;87:41Y45.
60. Fujikura T. Placental calcification and seasonal difference.Am J Obstet Gynecol. 1963;87:46Y47.
61. Boyd JD, Hamilton WJ.The Human Placenta. Cambridge, MA: W Heffer & Sons; 1970.
62. Zeichner SL, Plotkin SA. Mechanisms and pathways of congenital infections.Clin Perinatol. 1988;15:163.
63. Ley D, Laurin J, Bjerre I, et al. Abnormal fetal aortic velocity waveform and minor neurological dysfunction at 7 years of age.
Ultrasound Obstet Gynecol. 1996;8:152Y159.
64. Ley D, Tideman E, Laurin J, et al. Abnormal fetal aortic velocity waveform and intellectual function at 7 years of age.Ultrasound Obstet Gynecol. 1996;8:160Y165.
65. Verma V, Tejani N, Klein S, et al. Obstetric antecedents of intraventricular hemorrhage and periventricular leukomalacia in the low-birth-weight neonate.Am J Obstet Gynecol. 1997;176: 275Y281.