Research Report
NADPH-oxidase activity is elevated in penumbral
and non-ischemic cerebral arteries following stroke
Alyson A. Miller
a,⁎
, Gregory J. Dusting
b, Carli L. Roulston
b, Christopher G. Sobey
a aDepartment of Pharmacology, Monash University, Clayton, Victoria 3800, AustraliabBernard O'Brian Institute of Microsurgery, University of Melbourne, Fitzroy, Victoria 3065, Australia
A R T I C L E I N F O A B S T R A C T
Article history:
Accepted 27 June 2006 Available online 31 July 2006
Reactive oxygen species play a role in neuronal damage following cerebral ischemia–
reperfusion. We tested whether activity of the superoxide-generating enzyme, NADPH-oxidase, is enhanced in cerebral arteries within, adjacent and distant from the ischemic core. The right middle cerebral artery (MCA) of conscious rats was temporarily occluded by perivascular injection of endothelin-1 to induce stroke (ET-1; n= 19). Control rats were injected with saline (n= 9). At 24 h or 72 h post-administration of ET-1, the MCA and its branches within the ipsilateral penumbra and infarcted core, corresponding arteries in the contralateral hemisphere, and basilar artery were excised. Anatomically similar arteries were excised from saline-injected rats. At 24 h after stroke, NADPH-stimulated superoxide production by arteries from the infarcted core did not differ from levels generated by arteries from control rats, whereas levels were significantly lower 72 h after stroke. However, at both time points after stroke, superoxide production by arteries from the ischemic penumbra was 8-fold greater than levels generated by arteries from control rats. Surprisingly, even in the non-ischemic arteries from the contralateral hemisphere and in the basilar artery, superoxide production was increased∼4- to 6-fold at 24 h, but had returned to normal
72 h after stroke. The NADPH-oxidase inhibitor, diphenyleneiodonium, virtually abolished superoxide production by all arteries. Thus, the activity of NADPH-oxidase is enhanced in cerebral arteries from the ischemic penumbra at 24 h and 72 h following cerebral ischemia. Additionally, NADPH-oxidase activity is temporarily enhanced after cerebral ischemia within arteries from non-ischemic parts of the brain.
© 2006 Elsevier B.V. All rights reserved.
Keywords:
Stroke
Cerebral ischemia NADPH-oxidase Oxidative stress Cerebral vasculature
Abbreviations:
DMSO, dimethyl sulfoxide ET-1, endothelin-1 DPI, diphenyleneiodonium H2O2, hydrogen peroxide
MCA, middle cerebral artery ANOVA, one-way analysis of variance
ROS, reactive oxygen species O2−, superoxide
SOD, O2−dismutase
1.
Introduction
A reduction in cerebral blood flow to less than 10% of normal results in irreversible death to affected neurons. Between this infarct core and unaffected regions of the brain lies an area of constrained blood flow, referred to as the penumbra. Neurons within the penumbra die over a more protracted period, which
may extend from hours to days (Dirnagl et al., 1999; Fisher and Garcia, 1996).
During reperfusion, the function and integrity of cerebral arteries are critical to support cerebral blood flow and thus minimize further neuronal injury (Fagan et al., 2004). A growing body of evidence suggests that reactive oxygen species (ROS), such as superoxide (O2
−
), contribute to neuronal
⁎Corresponding author.Fax: +613 9905 5851.
E-mail address:[email protected](A.A. Miller). 0006-8993/$–see front matter © 2006 Elsevier B.V. All rights reserved. doi:10.1016/j.brainres.2006.06.082
a v a i l a b l e a t w w w. s c i e n c e d i r e c t . c o m
damage during the early phases of reperfusion (Chan, 2001). Indeed, excessive ROS production in cerebral arteries during the first few hours of reperfusion may result in further vascular dysfunction and a decline in perfusion of the previously ischemic brain (Kontos et al., 1992; Nelson et al., 1992). It has been suggested that ROS may also participate in delayed neuronal injury in the penumbra (Dirnagl et al., 1999). However, it is unclear whether ROS production in cerebral arteries is elevated at such later time points. NADPH-oxidases are now thought to be the primary generators of O2
−
within the vasculature (Ellmark et al., 2005; Griendling et al., 1994; Miller et al., 2005). Thus, the aim of this study was to test whether NADPH-oxidase activity and ROS production within arteries of ischemic and adjacent brain regions is altered following stroke induced by temporary middle cerebral artery (MCA) occlusion.
2.
Results
2.1. Outcome of ET-1 or saline administration
All rats displayed no signs of neurological deficit pre-surgery, but had low deficit scores prior to ET-1/saline administration
(0 h,Figs. 1A, B). This slight deficit was presumably as a result of unavoidable neuronal damage during cannula implanta-tion. Control rats showed no signs of stroke. In contrast, rats displayed neurological deficits, indicative of stroke, within 10 min of injection of ET-1 (data not shown). 24 h and 72 h after administration of ET-1 rats exhibited a greater deficit score than prior to ET-1 injection indicating successful induction of stroke (P< 0.05,Figs. 1A, B). Since the maximum deficit score that a rat could receive was 9, the rats used in this study were deemed to have a mild stroke.
2.2. NADPH-stimulated O2−production by cerebral arteries
Preliminary data demonstrated that NADPH-stimulated O2
−
generation by cerebral arteries (MCA and basilar) from control rats (i.e., following surgical implantation of cannula) were not
Fig. 1– Neurological deficit scoring for rats injected with saline or ET-1 7 days post-implantation of cannula.
Assessment data are shown for before (0 h) and, 24 h (A) and 72 h (B) after administration of ET-1 (stroke) or saline (control) Values are given as means ± SEM (n= 6–11). *P< 0.05 vs. 0 h.
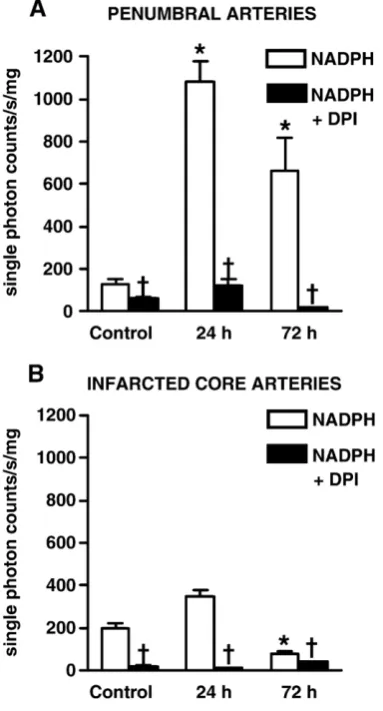
Fig. 2 –Effect of NADPH (100μmol/l) ± DPI (5μmol/l) on O−2
production by penumbral (A) and infarcted core (B) cerebral arteries from rats 24 h or 72 h after stroke, and by
corresponding arteries from control rats 24 h after injection of saline. Experiments were carried out in the presence of DETCA (3 mmol/l). Values are expressed as single photon counts/s/mg dry tissue weight; means ± SEM (n= 6). *P< 0.05 vs. corresponding arteries from control rats;
higher than O2−levels in arteries from naïve hooded Wistar rats
(n= 3, data not shown). 24 h after ET-1-induced stroke, NADPH-stimulated O2
−
generation by arteries from the penumbra was 8-fold higher than in anatomically similar arteries from control rats (24 h, 1080 ± 100; control, 130 ± 20 single photon counts/s/mg of dry tissue,P< 0.05,Fig. 2A). In comparison, O2−
generation in penumbral arteries was∼40% lower 72 h after
stroke (660 ± 160 single photon counts/s/mg of dry tissue) but remained greater than corresponding arteries from control rats (P< 0.05, Fig. 2A). NADPH-stimulated O2− production in
arteries from the infarcted core was much lower than in the penumbra, and at 24 h did not differ from anatomically similar
arteries from control rats (Fig. 2B). In contrast, at 72 h after stroke induction O2−production by arteries from the infarcted
core was lower than in anatomically similar arteries from control rats (P< 0.05,Fig. 2B). Surprisingly, in arteries from the contralateral (non-ischemic) hemisphere of stroke rats corre-sponding to both ipsilateral penumbral and infarcted core arteries, NADPH-stimulated O2− generation 24 h after stroke
was greater than in arteries from control rats (P< 0.05,Fig. 3A, B). This increase in O2
−
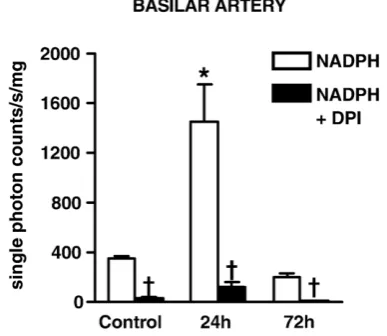
production by non-ischemic cerebral arteries had resolved to control levels by 72 h (Figs. 3A, B). Similarly, in basilar arteries of stroke rats, O2−production was
4-fold greater than in basilar arteries after 24 h (24 h, 1450± 300; control, 350 ± 20 single photon counts/s/mg of dry tissue,
P< 0.05,Fig. 4) but returned to the control level by 72 h (200 ± 30 single photon/s/mg of dry tissue, Fig. 4). DPI virtually abolished O2
−
generation in all arteries (P< 0.05,Figs. 2 and 3).
3.
Discussion
The major new finding of this study is that NADPH-oxidase activity in cerebral arteries from the ischemic penumbra is elevated for up to 3 days after mild stroke induced by temporary MCA occlusion in rats. A second, unexpected finding is that for the first 24 h after stroke, the increase in vascular NADPH-oxidase activity was not confined to penum-bral arteries but also occurred in arteries from the contral-ateral (non-ischemic) hemisphere and in the basilar artery.
In the present study, we investigated the activity of the O2−
generating enzyme, NADPH-oxidase, in branches of the MCA from the ischemic hemisphere at 24 h and 72 h after ET-1-induced MCA occlusion or saline injection. This is the first study to examine NADPH-oxidase activity in cerebral arteries following stroke. Importantly we found that for at least the
Fig. 3 –Effect of NADPH (100μmol/l) ± DPI (5μmol/l) on O−2
production in contralateral cerebral arteries corresponding to ipsilateral penumbral arteries (A) and ipsilateral infarcted core arteries (B) from rats 24 h or 72 h after stroke, and by corresponding arteries from control rats 24 h after injection of saline. Experiments were carried out in the presence of DETCA (3 mmol/l). Values are expressed as single photon counts/s/mg dry tissue weight, means ± SEM (n= 6). *P< 0.05 vs. corresponding arteries from control rats;
†P< 0.05 vs. NADPH-treated arteries.
Fig. 4–Effect of NADPH (100μmol/l) ± DPI (5μmol/l) on O2−
production in basilar arteries from rats 24 h or 72 h after stroke, and from control rats 24 h after injection of saline. Experiments were carried out in the presence of DETCA (3 mmol/l). Values are expressed as single photon counts/s/mg dry tissue weight, means ± SEM (n= 6). *P< 0.05 vs. corresponding arteries from control rats;
†P
first 72 h after cerebral ischemia, O2−production by
NADPH-oxidase in cerebral arteries from the ischemic penumbra is greater than in corresponding arteries from saline-injected control rats. In contrast, 24 h after ischemia, O2
−
production by NADPH-oxidase in cerebral arteries from the infarct core was comparable to that of arteries from control animals, however, by 72 h the O−2had significantly decreased. A loss or reduction
in blood flow to brain tissue within the immediate area of the occlusion (infarcted core) results in irreversible death of neurons within minutes (Callaway, 2001). Thus, vascular cell death or dysfunction could account for this finding.
Intriguingly, we found that NADPH-oxidase activity was enhanced in cerebral arteries distant from the ischemic insult. Indeed, O2
−
production by branches of the MCA in the non-ischemic hemisphere and the basilar artery, were substan-tially elevated at 24 h after ischemia. Vallet et al. recently reported the analogous finding that neuronal expression of Nox4 was elevated in the non-ischemic hemisphere following MCA occlusion (Vallet et al., 2005). It is well documented that humoral factors, such as angiotensin II and inflammatory cytokines, can increase NADPH-oxidase activity (Cheranov and Jaggar, 2006; De Keulenaer et al., 1998; Griendling et al., 1994; Miller et al., 2005). Furthermore, there is a rise in inflammatory cytokines following the onset of ischemia, which can persist for up to two days following the insult (Becker, 2001). In a rat model of cerebral ischemia, TNF-α, Il-1β
and IL-6, are similarly elevated for at least 24 h in areas far removed from the primary site of ischemic infarct, such as the lumbosacral spinal cord and bladder (Fu et al., 2004). Such observations may, therefore, be indicative of a systemic inflammatory response occurring during the early stages following cerebral ischemia, leading to an upregulation of vascular and non-vascular NADPH-oxidase activity. We acknowledge that at 1 nmol/l ET-1 is itself reported to increase the activity of vascular NADPH-oxidase by∼2-fold (Li et al.,
2003). Thus, we cannot exclude the possibility that diffusion of ET-1 throughout the cerebrospinal fluid might have increased NADPH-oxidase activity in sites distant from the infarct core. However, we believe that this is unlikely to account for our findings because we observed up to an 8-fold increase in O−2
production and we estimate that steady-state levels of ET-1 throughout the cerebrospinal fluid were likely to have been less than 1 nmol/l.
In the systemic circulation, enhanced O2− generation by
NADPH-oxidase increases the inactivation of endothelium-derived nitric oxide and causes a subsequent rise in vascular tone. Furthermore, the metabolism of O2
−
generates, hydrogen peroxide (H2O2), which along with its Fenton reaction product,
the hydroxyl radical, has been reported to have numerous other deleterious effects on vascular function (Miller et al., in press). Thus, the generation of large quantities of O2− by
NADPH-oxidase in cerebral arteries following ischemia could have detrimental effects on the perfusion of the penumbra and thus contribute to delayed neuronal damage. However, as discussed, the generation of O2− by NADPH-oxidase is also
enhanced in cerebral arteries from areas of the brain that do not have neuronal damage (Callaway et al., 2000, 2003; Macrae et al., 1993), suggesting that enhanced O2
−
generation by NADPH-oxidase does not necessarily lead to neuronal damage following cerebral ischemia. Intriguingly, we and others have
reported that activation of cerebral vascular NADPH-oxidase results in the generation of O−2 and/or H2O2, and elicits
profound cerebral vasodilatation (Didion and Faraci, 2002; Miller et al., 2005; Paravicini et al., 2004, 2006; Park et al., 2004). Furthermore, this vasodilator mechanism is enhanced in spontaneously hypertensive rats, raising the possibility that NADPH-oxidase could paradoxically serve a beneficial role in maintaining cerebral blood flow during disease (Paravicini et al., 2004).
It is noteworthy that although there is recent evidence that the activity of NADPH-oxidase expressed in phagocytes (Green et al., 2001; Sankarapandi et al., 1998; Walder et al., 1997) and neurons (Vallet et al., 2005) may be increased and/or involved in worsening the outcome of cerebral ischemia, this is the first study to specifically examine vascular NADPH-oxidase ac-tivity following stroke.
In summary, we report that the activity of NADPH-oxidase is elevated in rat cerebral arteries from the ischemic hemi-sphere and that this increase persists for up to three days following the ischemic insult. Surprisingly, NADPH-oxidase activity is also temporarily elevated in cerebral arteries from non-ischemic areas of the brain following stroke. The func-tional consequences of this increased NADPH-oxidase activity remain to be established.
4.
Experimental procedures
4.1. Surgical preparation
All procedures were approved by the institutional animal ethics committee. Male hooded Wistar rats (n= 31; weight, 280–320 g) were anesthetized with sodium pentobarbital (60 mg/kg, i.p.). A guide cannula was stereotaxically implanted into the piriform cortex 2 mm dorsal to the ipsilateral MCA as previously described (Sharkey et al., 1993). The stereotaxic coordinates were modified for this rat strain (0.2 mm anterior,
−5.2 mm lateral and−6.1 ventral, according to a stereotaxic
atlas) (Paxinos and Watson, 1986). The cannula was secured with dental acrylate cement and two small screws inserted into the skull, to assist the cement to adhere to the skull. The scalp was closed with sutures. Animals were housed indivi-dually and allowed to recover for 7 days before induction of stroke. Occlusion of the right MCA was attained in conscious rats by administration of endothelin-1 (ET-1; 20 pmol in 2μl
over 30 min; n= 19) through a 30-gauge injector which protruded 2 mm beyond the end of the previously implanted guide cannula. Stroke was confirmed by contralateral forepaw clenching or anti-clockwise circling as previously described (Callaway et al., 1999). Rats which did not show such behavioral signs were deemed not to have had a stroke and were excluded from the study (n= 3). Control rats underwent implantation of guide cannula but were injected with saline instead of ET-1 (n= 9).
4.2. Assessment of stroke induction
Neurological abnormalities 24 h or 72 h following ET-1/saline administration were evaluated using a neurological deficit score based on detection of abnormal posture and hemiplegia as previously described (De Ryck et al., 1989; Moyanova et al., 2003). Briefly, abnormal postures were assessed by suspending rats by the tail above a platform and then slowly lowered towards it. Extension of both forelimbs toward the platform was scored as no deficit (0 point) and forearm flexion towards the body as slight deficit (1 point). Absence of thoracic twisting was scored as no deficit (0 point), twisting of the thorax at a 45° angle away from the platform (i.e., to the handlers hand) as slight deficit (1 point), 90° as moderate deficit (2 point) and 180° as severe deficit (3 point). Hemiplegia was evaluated by placing the rats on a raised platform. Limb placements following contralateral forelimb and/or the contralateral hindlimb slips were scored as follows: no deficit (0 point), immediate and complete placement; slight deficit (1 point), incomplete and/or delayed placement (>2 s, <6 s); moderate deficit (2 point), incomplete and/or delayed placement (>6 s); or severe deficit (3 point), no placing. All behaviors were scored in a blind fashion. Thus, when scores for the three tests were summed, the maximum potential score was 9. It has been demonstrated previously that neurological deficit scores positively correlate with cerebral infarct size (Callaway et al., 2003).
4.3. O2−production by NADPH-oxidase in intact
cerebral arteries
24 h or 72 h post-administration of ET-1/saline, rats were euthanased with Lethobarb (sodium pentobarbitone, 500 mg/ kg, i.p.). Brains were removed and placed in ice-cold Krebs-Hepes solution (pH 7.4). In the ischemic (ipsilateral) hemi-sphere, the MCA and/or its branches were excised from the penumbra and infarct core. Infarcted tissue was visible as clearly defined opaque areas (Callaway et al., 2000). In general,
‘penumbral arteries’(i.e. those in the region surrounding the infarct core) corresponded to the ipsilateral MCA and its second order branches, and ‘infarct core arteries’ corre-sponded to the first order branch of the ipsilateral MCA. For each animal, anatomically similar arteries were also excised from the non-ischemic contralateral hemisphere. In control rats, the MCA and its first and second order branches were excised from both hemispheres. Basilar arteries from both stroke and control rats were excised. Each artery was cut into 5-mm ring segments. NADPH (100 μmol/l)-stimulated O2
−
production was measured by 5 μmol/l lucigenin-enhanced
chemiluminescence as previously described (Paravicini et al., 2002). Briefly, rings were incubated with NADPH (100μmol/l)
and diethyldithiocarbamate (DETCA; 3 mmol/l) to inhibit Cu2+/
Zn2+–O 2
−
dismutase (SOD), for 30 min at 37 °C, in the absence and presence of the NADPH-oxidase inhibitor, diphenyleneio-donium (DPI; 5 μmol/l). Arteries were then transferred into individual wells of a 96-well Optiplate (Packard Bioscience), containing lucigenin (5 μmol/l), NADPH (100 μmol/l) and
DETCA (3 mmol/l), in the absence and presence of DPI (5μmol/l). Photon emission was measured using a TopCount
single photon counter. Background single photon counts were subtracted, O2− production normalized for dry tissue weight
and expressed as single photon counts/s/mg of dry tissue.
4.4. Drugs
All drugs were purchased from Sigma. ET-1 was dissolved in 0.05 M acetic acid and diluted in saline. DPI was dissolved in dimethyl sulfoxide (DMSO) and diluted in Krebs-Hepes (pH 7.4) to give a final concentration of ≤0.05% for DMSO. All other
drugs were dissolved and diluted in Krebs-Hepes (pH 7.4) solution.
4.5. Data analysis
All results are expressed as mean ± standard error of mean (SEM). Comparisons were performed using one-way analysis of variance (ANOVA) with Dunn's multiple comparisons post hoc test.P< 0.05 was considered statistically significant.
Acknowledgments
These studies were supported by an Institute Block Grant (No. 983001) from the National Health and Medical Research Council of Australia. C.G.S. is a Senior Research Fellow of the National Health and Medical Research Council of Australia.
R E F E R E N C E S
Becker, K.J., 2001. Targeting the central nervous system inflammatory response in ischemic stroke. Curr. Opin. Neurol. 14, 349–353.
Callaway, J.K., 2001. Investigation of AM-36: a novel neuroprotective agent. Clin. Exp. Pharmacol. Physiol. 28, 913–918.
Callaway, J.K., Knight, M.J., Watkins, D.J., Beart, P.M., Jarrott, B., 1999. Delayed treatment with AM-36, a novel neuroprotective agent, reduces neuronal damage after endothelin-1-induced middle cerebral artery occlusion in conscious rats. Stroke 30, 2704–2712 (discussion 2712).
Callaway, J.K., Knight, M.J., Watkins, D.J., Beart, P.M., Jarrott, B., Delaney, P.M., 2000. A novel, rapid, computerized method for quantitation of neuronal damage in a rat model of stroke. J. Neurosci. Methods 102, 53–60.
Callaway, J.K., Lawrence, A.J., Jarrott, B., 2003. AM-36, a novel neuroprotective agent, profoundly reduces reactive oxygen species formation and dopamine release in the striatum of conscious rats after endothelin-1-induced middle cerebral artery occlusion. Neuropharmacology 44, 787–800.
Chan, P.H., 2001. Reactive oxygen radicals in signaling and damage in the ischemic brain. J. Cereb. Blood Flow Metab. 21, 2–14. Cheranov, S.Y., Jaggar, J.H., 2006. TNF-αdilates cerebral arteries via
NAD(P)H oxidase-dependent Ca2+spark activation.
Am. J. Physiol.: Cell Physiol. 290, C964–C971.
De Keulenaer, G.W., Alexander, R.W., Ushio-Fukai, M., Ishizaka, N., Griendling, K.K., 1998. Tumour necrosis factor alpha activates a p22phox-based NADH oxidase in vascular smooth muscle. Biochem. J. 329 (Pt. 3), 653–657.
De Ryck, M., Van Reempts, J., Borgers, M., Wauquier, A., Janssen, P.A., 1989. Photochemical stroke model: flunarizine prevents sensorimotor deficits after neocortical infarcts in rats. Stroke 20, 1383–1390.
Didion, S.P., Faraci, F.M., 2002. Effects of NADH and NADPH on superoxide levels and cerebral vascular tone. Am. J. Physiol.: Heart Circ. Physiol. 282, H688–H695.
ischaemic stroke: an integrated view. Trends Neurosci. 22, 391–397.
Ellmark, S.H., Dusting, G.J., Fui, M.N., Guzzo-Pernell, N., Drummond, G.R., 2005. The contribution of Nox4 to NADPH oxidase activity in mouse vascular smooth muscle. Cardiovasc. Res. 65, 495–504.
Fagan, S.C., Hess, D.C., Hohnadel, E.J., Pollock, D.M., Ergul, A., 2004. Targets for vascular protection after acute ischemic stroke. Stroke 35, 2220–2225.
Fisher, M., Garcia, J.H., 1996. Evolving stroke and the ischemic penumbra. Neurology 47, 884–888.
Fu, D., Ng, Y.K., Gan, P., Ling, E.A., 2004. Permanent occlusion of the middle cerebral artery upregulates expression of cytokines and neuronal nitric oxide synthase in the spinal cord and urinary bladder in the adult rat. Neuroscience 125, 819–831.
Green, S.P., Cairns, B., Rae, J., Errett-Baroncini, C., Hongo, J.A., Erickson, R.W., Curnutte, J.T., 2001. Induction of gp91-phox, a component of the phagocyte NADPH oxidase, in microglial cells during central nervous system inflammation.
J. Cereb. Blood Flow Metab. 21, 374–384.
Griendling, K.K., Minieri, C.A., Ollerenshaw, J.D., Alexander, R.W., 1994. Angiotensin II stimulates NADH and NADPH oxidase activity in cultured vascular smooth muscle cells. Circ. Res. 74, 1141–1148.
Kontos, C.D., Wei, E.P., Williams, J.I., Kontos, H.A., Povlishock, J.T., 1992. Cytochemical detection of superoxide in cerebral inflammation and ischemia in vivo. Am. J. Physiol. 263, H1234–H1242.
Li, L., Fink, G.D., Watts, S.W., Northcott, C.A., Galligan, J.J., Pagano, P.J., Chen, A.F., 2003. Endothelin-1 increases vascular superoxide via endothelin(A)-NADPH oxidase pathway in low-renin hypertension. Circulation 107, 1053–1058.
Macrae, I.M., Robinson, M.J., Graham, D.I., Reid, J.L., McCulloch, J., 1993. Endothelin-1-induced reductions in cerebral blood flow: dose dependency, time course, and neuropathological consequences. J. Cereb. Blood Flow Metab. 13, 276–284. Miller, A.A., Drummond, G.R., Schmidt, H.H.H.W., Sobey, C.G.,
2005. NADPH-oxidase activity and function are profoundly greater in cerebral versus systemic arteries. Circ. Res. 97, 1055–1062.
Miller, A.A., Drummond, G.R., Sobey, C.G., in press.
Novel isoforms of NADPH-oxidase in cerebral vascular control, Pharmacol. Ther.
Moyanova, S., Kirov, R., Kortenska, L., 2003. Multi-unit activity suppression and sensorimotor deficits after
endothelin-1-induced middle cerebral artery occlusion in conscious rats. J. Neurol. Sci. 212, 59–67.
Nelson, C.W., Wei, E.P., Povlishock, J.T., Kontos, H.A.,
Moskowitz, M.A., 1992. Oxygen radicals in cerebral ischemia. Am. J. Physiol. 263, H1356–H1362.
Paravicini, T.M., Gulluyan, L.M., Dusting, G.J., Drummond, G.R., 2002. Increased NADPH oxidase activity, gp91phox expression, and endothelium-dependent vasorelaxation during neointima formation in rabbits. Circ. Res. 91, 54–61.
Paravicini, T.M., Chrissobolis, S., Drummond, G.R., Sobey, C.G., 2004. Increased NADPH-oxidase activity and Nox4 expression during chronic hypertension is associated with enhanced cerebral vasodilatation to NADPH in vivo. Stroke 35, 584–589. Paravicini, T.M., Miller, A.A., Drummond, G.R., Sobey, C.G., 2006.
Flow-induced cerebral vasodilatation in vivo involves activation of phosphatidylinositol 3-kinase (PI3-K), NADPH-oxidase, and nitric oxide synthase. J. Cereb. Blood Flow Metab. 26, 836–845.
Park, L., Anrather, J., Zhou, P., Frys, K., Wang, G., Iadecola, C., 2004. Exogenous NADPH increases cerebral blood flow through NADPH oxidase-dependent and -independent mechanisms. Arterioscler., Thromb., Vasc. Biol. 24, 1860–1865.
Paxinos, G., Watson, C., 1986. The Rat Brain in Stereotaxic Coordinates, 2nd ed. Academic Press Inc.
Sankarapandi, S., Zweier, J.L., Mukherjee, G., Quinn, M.T., Huso, D.L., 1998. Measurement and characterization of superoxide generation in microglial cells: evidence for an NADPH oxidase-dependent pathway. Arch. Biochem. Biophys. 353, 312–321.
Sharkey, J., Ritchie, I.M., Kelly, P.A., 1993. Perivascular microapplication of endothelin-1: a new model of focal cerebral ischaemia in the rat. J. Cereb. Blood Flow Metab. 13, 865–871.
Vallet, P., Charnay, Y., Steger, K., Ogier-Denis, E., Kovari, E., Herrmann, F., Michel, J.P., Szanto, I., 2005. Neuronal expression of the NADPH oxidase NOX4, and its regulation in mouse experimental brain ischemia. Neuroscience 132, 233–238. Walder, C.E., Green, S.P., Darbonne, W.C., Mathias, J., Rae, J.,