www.elsevier.com/locate/ibmb
Identification of multiple peptides homologous to cockroach and
cricket allatostatins in the stick insect Carausius morosus
Matthias W. Lorenz
a,c,*, Roland Kellner
b, Klaus H. Hoffmann
a, Gerd Ga¨de
caDepartment of Animal Ecology 1, University of Bayreuth, Universita¨tsstrasse 30/NW I, D-95440 Bayreuth, Germany bBiomed Fo/GBT, Merck KGaA, D-64271 Darmstadt,Germany
cZoology Department, University of Cape Town, Rondebosch 7701, South Africa
Received 31 October 1999; received in revised form 31 December 1999; accepted 25 January 2000
Abstract
Eighteen peptides were isolated from brain extracts of the stick insect Carausius morosus. The peptides were purified in four steps by high-performance liquid chromatography, monitored by their ability to inhibit juvenile hormone biosynthesis by corpora allata of the cricket Gryllus bimaculatus in vitro, and chemically characterised by Edman degradation and mass spectrometry. We obtained complete primary-structure information for nine peptides, four of which belong to the peptide family characterised by a common C-terminal pentapeptide sequence –YXFGLamide. The remaining five belong to the W2W9amide peptide family,
nonapep-tides characterised by having the amino acid tryptophan in positions 2 and 9. The amino-acid sequence of two other pepnonapep-tides could not be completely resolved by means of Edman degradation; however, these peptides could be allocated to the –YXFGLamide and the W2W9amide family, respectively, by comparison of retention times, co-elution and mass spectrometry. Both classes of
neuropep-tides strongly inhibit juvenile hormone biosynthesis in crickets but show no inhibiting effect on the corpora allata of the stick insect. 2000 Elsevier Science Ltd. All rights reserved.
Keywords: Allatostatin; Peptide sequence; Juvenile hormone; Corpora allata; Stick insect; Cricket
1. Introduction
Juvenile hormones (JHs) regulate a variety of pro-cesses in insects, among which metamorphosis and reproduction are the best understood. The rate of JH biosynthesis in the corpora allata (CA) is, in turn, con-trolled by stimulatory (allatotropin) and inhibitory (allatostatin) factors. The latter have been the subject of a large number of investigations in the last decade. The first allatostatins identified were isolated from brains of the cockroach Diploptera punctata (Woodhead et al., 1989; Pratt et al., 1991). Soon thereafter structurally related neuropeptides with the C-terminal pentapeptide motif –YXFGLamide were identified in other cockroach species (Weaver et al., 1994; Belle´s et al., 1994), in flies (Duve et al., 1993), mosquitoes (Veenstra et al., 1997), bees (Kaatz, personal communication), crickets (Lorenz
* Corresponding author. Tel.:+49-921-55-2655; fax:+ 49-921-55-2784.
E-mail address: [email protected] (M.W. Lorenz).
0965-1748/00/$ - see front matter2000 Elsevier Science Ltd. All rights reserved. PII: S 0 9 6 5 - 1 7 4 8 ( 0 0 ) 0 0 0 4 2 - 4
et al., 1995a; Lorenz et al., 1999a), locusts (Veelaert et al., 1996) and moths (Davis et al., 1997; Duve et al., 1997a,b), and even in the sister group of the insects, the crustacean Carcinus maenas (Duve et al., 1997c). However, the peptides isolated from flies, mosquitoes, bees, locusts and moths are not biologically active in inhibiting JH biosynthesis in those species. In contrast, a peptide with a different structure to the –YXFGLamide peptides was identified in the tobacco hornworm
Mand-uca sexta, and it clearly inhibited JH biosynthesis
(Kramer et al., 1991). The same peptide was also found in some other moth species (Weaver et al., 1998).
Another group of neuropeptides with allatostatic activity in a homologous bioassay, which contains a common W2W9amide motif, has been characterised from
the brains of the cricket Gryllus bimaculatus (Lorenz et al., 1995b; Lorenz et al., 1999a). Peptides with structural similarities had previously been identified from Locusta
migratoria (Schoofs et al., 1991) and M. sexta
(Blackburn et al., 1995) using a bioassay that monitored their ability to inhibit contractions of smooth muscles.
physiology of the parthenogenetic stick insect Carausius
morosus. Previous studies had revealed that, although JH
is apparently not required for normal vitellogenesis (Pflugfelder, 1937; Bradley et al., 1995), the CA from adult egg-carrying stick insects do synthesise and release JH III in vitro (Lorenz et al., 1999b). In pilot studies we were also able to extract and partially purify fractions from the brain of adult stick insects, which inhibited JH biosynthesis of CA from G. bimaculatus in vitro (Lorenz and Hoffmann, 1994; Ga¨de et al., 1997). The present investigation was designed to purify and identify these factors, so that synthetic peptides could be obtained for more intensive studies of their effects on the repro-ductive cycle of the stick insect.
2. Materials and methods
2.1. Insects
Indian stick insects, C. morosus (Brunner) (Phasmatodea, Lonchodinae), were collected in the field around Cape Town or obtained from a laboratory-bred colony that was reared on ivy leaves under short-day conditions (12 h light/12 h dark) at ambient temperature (18–22°C). Mediterranean field crickets, G. bimaculatus de Geer (Ensifera, Gryllidae), were reared at 27°C under long-day conditions as described elsewhere (Lorenz et al., 1997a).
2.2. Rapid partition assay for allatostatic activity
Release of JH III and allatostatic activity of the frac-tions purified by high-performance liquid chromato-graphy (HPLC) on single cricket CA were determined by a rapid partition assay (Feyereisen and Tobe, 1981) with some modifications (Lorenz et al., 1997a). Tests for allatostatic activity of the synthetic peptides on the CA of G. bimaculatus and C. morosus were performed in the same way, except that in the case of C. morosus a pair of CA was used.
2.3. Tissue extraction and solid-phase prepurification
Brains dissected from adult egg-laying C. morosus were transferred into 500µl of ice-cold extraction medium (methanol/water/acetic acid; 100/10/1) in batches of 50. The procedures of extraction and solid-phase prepurification have been described elsewhere (Ga¨de et al., 1997).
2.4. High-performance liquid chromatography
The first three HPLC runs were performed on a Jasco series 900 high-pressure-gradient HPLC system con-sisting of two PU-980 pumps, a DG-980-50 on-line
degasser, a UV-975 variable-wavelength ultraviolet (UV) detector set to 214 nm, a Jet-stream Peltier column thermostat (set to 25°C), and a Rheodyne 7125 injector with a 2 ml sample loop. The system was run with Bor-win Chromatography Software 1.21. The chromato-graphic conditions are shown in Table 1.
In the first HPLC run, lyophilised samples (1175 and 1100 brain equivalents) from the 40% acetonitrile (MeCN) solid-phase prepurification step were resus-pended each in 1 ml of water and injected onto the col-umn. Peak fractions that eluted between 16 and 60 min were collected and tested for allatostatic activity on cricket CA (30 brain equivalents per CA).
For the second HPLC run, active fractions pooled from the two identical first HPLC runs (equivalent to 2185 brains) were reduced in volume to approximately 500µl, diluted with 500µl of 0.13% heptafluorobutyric acid (HFBA) in 10% MeCN and injected onto the col-umn. Peak fractions were collected and tested on cricket CA (40 brain equivalents per CA).
In the third HPLC run, active fractions from the second HPLC run (equivalent to 2065 brains) were reduced in volume to approximately 500µl, diluted with 500µl of 20 mM ammonium acetate (NH4Ac) and
co-Table 1
Chromatographic conditions of the four HPLC runs employed for the isolation of stick insect allatostatins
HPLC run Column Solventsa Gradient Flow rate (ml/min)
1st LiChroCART Superspher 100 A: 0.115% TFA in water 0–5 min: 0% B 1 RP-18, 100 A˚ , 4µm, B: 0.1% TFA in MeCN 5–8 min: 0–20% B
124 mm×4 mm with guard 8–51 min: 20–33% B (linear column 4 mm×4 mm (Merck, gradient, 0.3% MeCN per min) Darmstadt, Germany)
2nd Shiseido CAPCELL PAK C18 A: 0.13% HFBA in water 0–2 min: 5% B 1
SG 300, 300 A˚ , 5µm, B: 0.13% HFBA in MeCN 2–52 min: 5–60% B (linear 250 mm×4.6 mm with guard gradient, 1.1% MeCN per min) column 10 mm×4.6 mm (Grom,
Herrenberg-Kayh, Germany)
3rd Shiseido CAPCELL PAK C8SG A: 20 mM NH4Ac in water 0–40 min: 6–63% B (linear 1
300, 300 A˚ , 5µm, (pH 7.0) gradient, 1.14% MeCN per min) 150 mm×4.6 mm with guard B: 20 mM NH4Ac in 80% MeCN
column 10 mm×4.6 mm (Grom)
4th Vydac 218TP, 300 A˚ , 5µm, A: 0.115% TFA in water 0–2 min: 5% B 0.25 250 mm×2.1 mm (MZ B: 0.1% TFA in MeCN 2–17 min: 5–20% B (linear
Analysentechnik, Mainz, gradient, 1% MeCN per min)
Germany) 17–57 min: 20–40% B (linear
gradient, 0.5% MeCN per min)
aHFBA, heptafluorobutyric acid; MeCN, acetonitrile; NH
4Ac, ammonium acetate; TFA, trifluoroacetic acid.
injected with an equal amount of the corresponding syn-thetic peptide after having run native and synsyn-thetic pep-tides separately on the same HPLC system.
2.5. Sequence analysis
The allatostatic material from the final HPLC separ-ations was loaded onto a polybrene-coated glass-fibre filter and sequenced by automated Edman degradation using a model 477A sequenator connected to a model 120A on-line phenylthiohydantoin analyser (Applied Biosystems, Weiterstadt, Germany).
2.6. Mass spectrometry analysis
Mass spectra were aquired using a matrix-assisted laser desorption/ionisation spectrometer (Bruker Reflex, Bruker Franzen, Bremen, Germany). The acceleration voltage was set to 30 kV for the linear mode. The matrix was a saturated solution ofα-cyano-4-hydroxycinnamic acid dissolved in water/MeCN (7:3, v/v). Peptide sol-utions (0.5µl, ca. 1 pmol) were mixed on target with the matrix solution (1:1, v/v) and left to dry. Each spectrum was the average of ca. 50–200 single-shot spectra acquired in sets of five shots.
2.7. Peptide synthesis
Peptide synthesis was performed on a model 9050 peptide synthesiser (Milligen, Eschborn, Germany) using
Fmoc/HOBt chemistry. Peptides were synthesised in the amide form using an Fmoc-peptide amide linker poly-ethylene glycol–polystyrene resin (Milligen). Syn-thesised peptides were purified after cleavage from the resin by reversed-phase HPLC and checked by mass analysis. Peptide synthesis was kindly performed by N. Weidner (Mainz).
3. Results
3.1. Peptide sequences
A previous study (Ga¨de et al., 1997) had shown that all the fractions eluting between 24.6 and 54.0 min (corresponding to 25.0–100% MeCN) from the first HPLC run exerted a clear allatostatic activity on cricket CA. In the present study, only the more polar fractions, i.e., those that eluted between 25.0 and 27.8% MeCN, were chosen for further purification because these frac-tions had shown the highest level of allatostatic activity. In total, 18 peptides were purified from these fractions of the first HPLC run. We obtained complete structural data for nine peptides of which four are members of the –YXFGLamide family and five belong to the W2W9amide family (Table 2). The peptides identified
Table 2
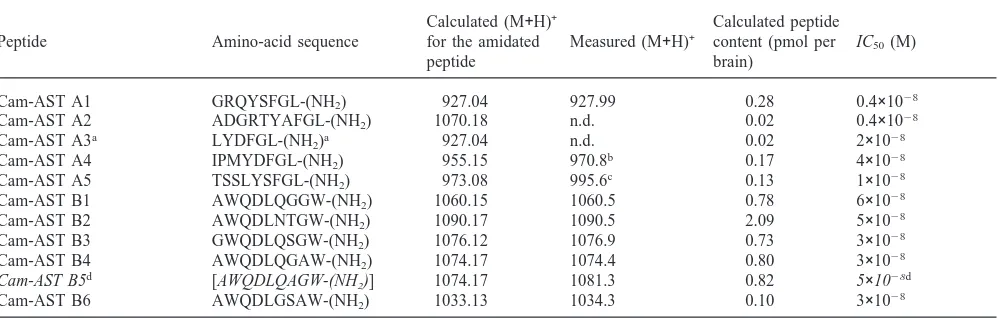
Sequence, molecular mass, estimated content per brain (losses during purification not taken into account; overall peptide recovery was around 60% as calculated from the peak areas) and IC50values (concentration required for 50% inhibition of JH III release by CA of 4–5 day old virgin female G. bimaculatus) of the isolated peptides
Calculated (M+H)+ Calculated peptide
Peptide Amino-acid sequence for the amidated Measured (M+H)+ content (pmol per IC50(M)
peptide brain)
Cam-AST A1 GRQYSFGL-(NH2) 927.04 927.99 0.28 0.4×1028
Cam-AST A2 ADGRTYAFGL-(NH2) 1070.18 n.d. 0.02 0.4×1028
Cam-AST A3a LYDFGL-(NH
2)a 927.04 n.d. 0.02 2×1028
Cam-AST A4 IPMYDFGL-(NH2) 955.15 970.8b 0.17 4×1028
Cam-AST A5 TSSLYSFGL-(NH2) 973.08 995.6c 0.13 1×1028
Cam-AST B1 AWQDLQGGW-(NH2) 1060.15 1060.5 0.78 6×1028
Cam-AST B2 AWQDLNTGW-(NH2) 1090.17 1090.5 2.09 5×1028
Cam-AST B3 GWQDLQSGW-(NH2) 1076.12 1076.9 0.73 3×1028
Cam-AST B4 AWQDLQGAW-(NH2) 1074.17 1074.4 0.80 3×1028
Cam-AST B5d [AWQDLQAGW-(NH
2)] 1074.17 1081.3 0.82 5×1028d
Cam-AST B6 AWQDLGSAW-(NH2) 1033.13 1034.3 0.10 3×1028
aSequence as deduced by HPLC coelution with synthetic LYDFGLamide. b Peptide with oxidised methionine.
cSodium adduct.
d Sequence as determined by Edman degradation; the IC
50value given here is that of the synthetic AWQDLQAGWamide.
nomenclature used for the cricket allatostatins (Lorenz et al., 1995a,b; Lorenz et al., 1999a). To further confirm the identity of the allatostatins, the synthetic peptides were run under all four chromatographic conditions used in their isolation, and their retention times were com-pared with that of the native peptide. Furthermore, the native peptides were co-injected onto the HPLC with their synthetic counterparts. Each pair of synthetic and native peptide eluted as a pure single peak (chromatograms not shown).
Two additional peptides (Cam-AST A3 and B6) could not be completely identified by means of automated Edman degradation sequencing and mass determination, but each could be allocated to one of the two allatostatin families (Table 2). For the peptide Cam-AST A3, Edman degradation revealed the sequence LYD but no further amino acids were detected. Also, mass spectrometry revealed no clear-cut results. In the cockroach Blattella
germanica, the allatostatin LYDFGLamide has been
iso-lated from brain extracts (Belle´s et al., 1994), and from the allatostatin precursor of the cricket G. bimaculatus, the peptide LYDFGVamide was identified (unpublished results). Therefore, it seemed to be likely that Cam-AST A3 would be identical to one of these two peptides. This assumption was proved by comparing the retention times of the native Cam-AST 3 with synthetic LYDFGLamide (a generous gift of Dr. X. Belle´s) and LYDFGVamide. Co-injection of native and synthetic peptides was not possible since all the native peptide had been used up for Edman degradation. The native Cam-AST A3 had the same retention times as the peptide LYDFGLamide under all four chromatographic systems used for the pep-tide purification whereas the peppep-tide LYDFGVamide eluted at different retention times. These results suggest
that Cam-AST A3 is identical to the cockroach peptide (chromatograms not shown).
For the peptide Cam-AST B6, Edman degradation revealed the sequence AWQDLgXaw, giving faint sig-nals for the positions 6, 8 and 9 and no definite signal for position 7. With a measured (M+H)+ of 1034.3 for the amidated peptide, the mass of the unidentified amino acid in position 7 was calculated to be 88 Da, indicating that it might be serine. The C-terminal sequence –SAW has been found in the lepidopteran myoinhibiting pep-tides Mas-MIP I and II (Blackburn et al., 1995). There-fore, the peptide AWQDLGSAWamide was synthesised and, upon co-injection with the native Cam-AST B6, the peptide eluted as a single pure peak, confirming the identity of the peptide (chromatograms not shown).
For the peptide Cam-AST B5 we unequivocally obtained the amino-acid sequence AWQDLQAGW; however, mass spectrometry revealed a (M+H)+ of 1081.3 which does not correspond to the theoretical (M+H)+ of 1074.17. In addition, the synthetic AWQDLQAGWamide did not co-elute with its native counterpart. These results indicate that this peptide may be modified.
Seven additional peptides with allatostatic activity have been isolated from the brain extracts. However, due to the low amount of the substances and partial impurities, we did not obtain complete sequence and mass data.
The estimated content of the isolated –YXFGLamide allatostatins ranges from 0.02 to 0.28 pmol per brain and that of the W2W9amide allatostatins from 0.10 to
3.2. Heterologous bioassays
Synthetic peptides were tested on cricket CA in con-centrations ranging from 10210to 1026M. All the
pep-tides gave the typical sigmoid dose–response curves with a maximum inhibition of JH III release at an allatostatin concentration of 10-7–1026M. The dose–response curves
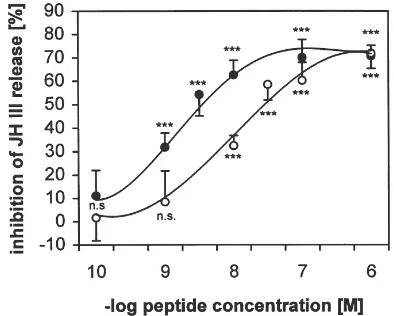
of the peptides Cam-AST A1 and B4 are shown in Fig. 1. Fifty percent inhibition of JH III release (IC50) was
caused by 0.4–6×1028M allatostatin (Table 2). The
glands showed a complete recovery from inhibited rates of synthesis after having been transferred to fresh medium without allatostatin (not shown).
3.3. Homologous bioassays
When tested on paired CA from adult egg-laying C.
morosus (older than 30 days) at 1025and 1027M,
allato-statins of both peptide families failed to inhibit JH III biosynthesis (not shown). Glands from egg-laying C.
morosus were chosen since only in those females had a
measurable JH biosynthesis been observed (Lorenz et al., 1999b). The peptides Cam-AST A1, A2, B1 and B4 were also tested on CA from 20–25 day old females and on CA from penultimate larval instars (activated by the addition of 100µM farnesol into the incubation medium, because only under such “stimulated” conditions we were able to measure in vitro JH biosynthesis in larval stick insects; Lorenz et al., 1999b). Again, no inhibition of JH III biosynthesis occurred, indicating that the iso-lated peptides do not act as allatostatins in C. morosus.
4. Discussion
We have isolated and identified 11 peptides from methanolic brain extracts of the stick insect C. morosus,
Fig. 1. Dose response for inhibition of JH III release by Cam-AST A1 (closed circles) and B4 (open circles). For the tests, single CA were taken from 4–5 day old virgin female G. bimaculatus. Means±SEM of 10–20 determinations. n.s., not significantly different; ***, P,0.001 (Mann–Whitney U-test). The other allatostatins gave similar dose– response curves.
each of which inhibit JH III release by cricket CA. The use of CA taken from G. bimaculatus as an in vitro test system for peptides with allatostatic activity allowed the detection of two different classes of allatostatins, the cockroach (–YXFGLamide) allatostatin family (A-AST) and the cricket (W2W9amide) allatostatin family
(B-AST). Five of the isolated peptides belong to the – YXFGLamide family and six to the W2W9amide family.
With the exception of Cam-AST A3, which is identical to the cockroach (B. germanica, D. punctata,
Per-iplaneta americana) allatostatin 1 (for a survey see
Belle´s et al., 1999), all the other isolated Carausius-alla-tostatins are novel structures that have not yet been found in any other species. Some of the structures, how-ever, closely resemble those of known peptides. The peptide Cam-AST A1 might be a homologue of the locust peptide Scg-AST 5 (Veelaert et al., 1996) from which it differs only at position 3 (Gln instead of Leu), an amino-acid exchange that can be explained by a point mutation, and Cam-AST A5 could be a homologue of the cockroach peptide Dip-AST 8 (Dip-AST III; Wood-head et al., 1989; Donly et al., 1993) from which it dif-fers only at the amino-acid positions 1 and 2 (Thr instead of Gly and Ser instead of Gly, respectively). The peptide Cam-AST A2 seems to be a homologue of the cockroach peptide Pea-AST 9 (Weaver et al., 1994), from which it differs at position 5 (Thr instead of Leu), while Cam-AST A4 may be a homologue of the cockroach peptides Dip-AST 13 and Pea-AST 14 (Stay et al., 1994). It dif-fers from these peptides only at position 8 where Leu is present in C. morosus instead of Ile as in the cockroach peptides; this can again be explained by a point mutation. The amino acids Gln and Thr in the pre-tyrosyl position of the peptides Cam-AST A1 and A2, respect-ively, occur in only four of the known type A allatostat-ins, namely Gln in Grb-AST A1 and A2 (Lorenz et al., 1995a) and in carcinustatin 11 (Duve et al., 1997c), and Thr in carcinustatin 20 (Duve et al., 1997c).
The six isolated allatostatins of the B-type have the N-terminal sequence (A/G)WQDL in common, which has also been found in the peptides Lom-MIP (Schoofs et al., 1991), Mas-MIP 1 and 2 (Blackburn et al., 1995), and Grb-AST B1 (Lorenz et al., 1995b). The peptide Cam-AST B2 differs from Lom-MIP only in position 7 (Thr instead of Ala), whereas Cam-AST B6 closely resembles Mas-MIP 1 from which it differs in position 6 (Gly instead of Asn). One of the peptides, Cam-AST B5, may be modified, but the nature of this modification remains to be elucidated. The finding of W2W9amide
peptides in an insect order other than Lepidoptera, Caeli-fera and EnsiCaeli-fera suggests that these peptides are wide-spread among insects, as is the case for the –YXFGLam-ide pept–YXFGLam-ide family.
JH III biosynthesis by cricket CA in vitro, causing maxi-mal inhibition at a concentration of 1027–1026M. As
with the allatostatins isolated from the cricket brain, the type B allatostatins from C. morosus have an IC50value
that is almost one order of magnitude higher than that of the type A allatostatins. On the other hand, the brain contains larger amounts of the type B than of the type A allatostatins (Table 2).
Allatostatic activity of either class of allatostatins could not be found when tested on the CA of C.
morosus. Furthermore, partially purified brain extracts of C. morosus did not inhibit JH biosynthesis by stick
insect CA but exerted an allatotropic effect (Lorenz et al., 1999b), indicating that JH biosynthesis may be posi-tively regulated in C. morosus as it seems to be the case in L. migratoria (Girardie, 1966; Ferenz and Diehl, 1983; Gadot and Applebaum, 1985). Under “stimulated” conditions (medium supplemented with farnesol), we could show that both types of allatostatin inhibit the far-nesol-induced accumulation of methyl farnesoate within the CA of crickets (Lorenz et al., 1999a) but this effect was not observed in stick insects. The failure of the iso-lated allatostatins to inhibit JH biosynthesis in C.
morosus CA is not surprising. So far, only the CA of
cockroaches and crickets have been shown to react to type A allatostatins in vitro by reduced rates of JH biosynthesis, whereas the type B allatostatins seem to be active as allatostatins only in crickets (Lorenz et al., 1997b). The results obtained after injecting allatostatins into cockroaches (Woodhead et al., 1993; Weaver et al. 1994, 1995; Piulachs et al., 1997) and crickets (Lorenz et al., 1998) suggest that, in these species, allatostatins may be the principal regulators of JH biosynthesis also in vivo. In the stick insect, the allatostatins may have other functions. A myoinhibiting activity has been estab-lished in various insect species for both types of peptide (Lange et al., 1993; Duve and Thorpe, 1994; Duve et al., 1997a; Veelaert et al., 1996; Schoofs et al., 1991; Blackburn et al., 1995). The allatostatin Blg-AST 2 (BLAST-2) inhibits vitellogenin release in B. germanica (Martı´n et al., 1996) and a possible neuromodulatory role for the –YXFGLamide allatostatins in insects has been discussed (Stay et al., 1992). Furthermore, the type B allatostatins exert an ecdysiostatic effect on cricket ovar-ies (Lorenz et al., 1997a). The finding of allatostatins or allatostatin-like peptides as well as their immunocyto-chemical distribution in insect tissues (Duve and Thorpe, 1994; Lange et al., 1993; Veenstra et al., 1995; Maestro et al., 1998) and in other invertebrates (Duve et al., 1997c; Skiebe and Schneider, 1994; Smart et al. 1994, 1995) suggests that the basic function of these peptides may indeed be a neuro-/myomodulatory one, mainly associated with feeding/digestion, gut motility and repro-duction. Since we have no data on the distribution of allatostatins in the nervous system of C. morosus, we can only speculate on their possible functions in this
species. The apparently JH-independent vitellogenesis in
C. morosus (Pflugfelder, 1937; Bradley et al., 1995)
might suggest that ecdysteroids are the main regulators of egg development as was found in dipteran insects (Hagedorn et al., 1975; Huybrechts and de Loof, 1982). As in crickets, the allatostatins could be involved in the regulation of ecdysteroid biosynthesis.
Immunocytochemical studies as well as bioassays that are directed to monitor functions other than the regu-lation of JH biosynthesis will help to shed some light on the physiological role of these peptides in C. morosus.
Acknowledgements
We thank Dr. Xavier Belle´s (Barcelona) for the syn-thetic LYDFGLamide, Frank Ramming (Bayreuth) for technical assistance, Nina Weidner (Mainz) for technical assistance and peptide synthesis, and Dr. Joseph Wood-ring (Baton Rouge) for critically reading the manuscript. This work was financially supported by a grant from the Foundation for Research Development (Pretoria, RSA), a UCT staff award (both to G.G.), and a grant by the Volkswagen Stiftung (I/73024 to G.G. and R.K.). M.W.L. acknowledges partial support by FRD, the UCT visitors fund and the Deutsche Forschungsgemeinschaft (Ho 631/15-2).
References
Belle´s, X., Maestro, J.L., Piulachs, M.D., Johnson, A.H., Duve, H., Thorpe, A., 1994. Allatostatic neuropeptides from the cockroach
Blattella germanica (L) (Dictyoptera, Blattellidae). Identification,
immunolocalization and activity. Regul. Pept. 53, 237–248. Belle´s, X., Graham, L.A., Bendena, W.G., Ding, Q., Edwards, J.P.,
Weaver, R.J., Tobe, S.S., 1999. The molecular evolution of the allatostatin precursor in cockroaches. Peptides 20, 11–22. Blackburn, M.B., Wagner, R.M., Kochansky, J.P., Harrison, D.J.,
Thomas-Laemont, P., Raina, A.K., 1995. The identification of two myoinhibitory peptides, with sequence similarities to the galanins, isolated from the ventral nerve cord of Manduca sexta. Regul. Pept. 57, 213–219.
Bradley, J.T., Masetti, M., Cecchettini, A., Giorgi, F., 1995. Vitellog-enesis in the allatectomized stick insect Carausius morosus (Br.) (Phasmatodea: Lonchodinae). Comp. Biochem. Physiol. 110B, 255–266.
Davis, N.T., Veenstra, J.A., Feyereisen, R., Hildebrand, J.G., 1997. Allatostatin-like-immunoreactive neurons of the tobacco hornworm, Manduca sexta, and isolation and identification of a new neuropeptide related to cockroach allatostatins. J. Comp. Neu-rol. 385, 265–284.
Donly, B.C., Ding, Q., Tobe, S.S., Bendena, W.G., 1993. Molecular cloning of the gene for the allatostatin family of neuropeptides from the cockroach Diploptera punctata. Proc. Nat. Acad. Sci., USA 90, 8807–8811.
Duve, H., Thorpe, A., 1994. Distribution and functional significance of Leu-callatostatins in the blowfly Calliphora vomitoria. Cell Tiss. Res. 276, 367–379.
Calliphora vomitoria with sequence homology to cockroach
allato-statins. Proc. Nat. Acad. Sci., USA 90, 2456–2460.
Duve, H., Johnsen, A.H., Maestro, J.L., Scott, A.G., Crook, N., Win-stanley, D., Thorpe, A., 1997a. Identification, tissue localisation and physiological effect in vitro of a neuroendocrine peptide ident-ical to a dipteran Leu-callatostatin in the codling moth Cydia
pomonella (Tortricidae: Lepidoptera). Cell Tiss. Res. 289, 73–83.
Duve, H., Johnsen, A.H., Maestro, J.L., Scott, A.G., Winstanley, D., Davey, M., East, P.D., Thorpe, A., 1997b. Lepidopteran peptides of the allatostatin superfamily. Peptides 18, 1301–1309.
Duve, H., Johnsen, A.H., Maestro, J.L., Scott, A.G., Jaros, P.P., Thorpe, A., 1997c. Isolation and identification of multiple neuro-peptides of the allatostatin superfamily in the shore crab Carcinus
maenas. Eur. J. Biochem. 250, 727–734.
Ferenz, H.-J., Diehl, I., 1983. Stimulation of juvenile hormone biosynthesis in vitro by locust allatotropin. Z. Naturforsch. 38C, 856–858.
Feyereisen, R., Tobe, S.S., 1981. A rapid partition assay for routine analysis of juvenile hormone released by insect corpora allata. Anal. Biochem. 111, 401–409.
Gadot, M., Applebaum, S.W., 1985. Rapid in vitro activation of corpora allata by extracted locust brain allatotropic factor. Arch. Insect Biochem. Physiol. 2, 117–129.
Ga¨de, G., Lorenz, M.W., Hoffmann, K.H., 1997. Stick insect (Carausius morosus; Phasmatodea: Lonchodidae) brain extract contains multiple fractions with allatostatic activity. Eur. J. Ento-mol. 94, 361–386.
Girardie, A., 1966. Controˆle de l’activite´ ge´nitale chez Locusta
migratoria. Mise en e´vidence d’un facteur gonadotrope et d’un
facteur allatotrope dans la pars intercerebralis. Bull. Soc. Zool. Fr. 91, 423–439.
Hagedorn, H.H., O’Connor, J.D., Fuchs, M.S., Sage, B., Schlaeger, D.A., Bohm, M.K., 1975. The ovary as a source ofα-ecdysone in an adult mosquito. Proc. Nat. Acad. Sci., USA 72, 3255–3259. Huybrechts, R., De Loof, A., 1982. Similarities in vitellogenin and
control of vitellogenin synthesis within the genera Sarcophaga,
Calliphora, Phormia and Lucilia (Diptera). Comp. Biochem.
Phy-siol. 72B, 339–344.
Kramer, S.J., Toschi, A., Miller, C.A., Kataoka, H., Quistad, G.B., Li, J.P., Carney, R.L., Schooley, D.A., 1991. Identification of an allatostatin from the tobacco hornworm Manduca sexta. Proc. Nat. Acad. Sci., USA 88, 9458–9462.
Lange, A.B., Chan, K.K., Stay, B., 1993. Effect of allatostatin and proctolin on antennal pulsatile organ and hindgut muscle in the cockroach, Diploptera punctata. Arch. Insect Biochem. Physiol. 24, 79–92.
Lorenz, J.I., Lorenz, M.W., Hoffmann, K.H., 1997a. Factors regulating juvenile hormone and ecdysteroid biosynthesis in Gryllus
bimac-ulatus (Ensifera: Gryllidae). Eur. J. Entomol. 94, 369–379.
Lorenz, M.W., Hoffmann, K.H., 1994. Interspecific actions of allatos-tatins. Verh. Dtsch. Zool. Ges. Jena 87 (1), 179.
Lorenz, M.W., Kellner, R., Hoffmann, K.H., 1995a. Identification of two allatostatins from a cricket, Gryllus bimaculatus de Geer (Ensifera, Gryllidae): additional members of a family of neuropep-tides inhibiting juvenile hormone biosynthesis. Regul. Pept. 57, 227–236.
Lorenz, M.W., Kellner, R., Hoffmann, K.H., 1995b. A family of neuro-peptides that inhibit juvenile hormone biosynthesis in the cricket,
Gryllus bimaculatus. J. Biol. Chem. 270, 21103–21108.
Lorenz, M.W., Ga¨de, G., Hoffmann, K.H., 1997b. Interspecific actions of allatostatins. Mttg. Dtsch. Ges. allg. angew. Ent. 11, 549–553. Lorenz, M.W., Lorenz, J.I., Treiblmayr, K., Hoffmann, K.H., 1998. In
vivo effects of allatostatins in crickets, Gryllus bimaculatus (Ensifera: Gryllidae). Arch. Insect Biochem. Physiol. 38, 32–43. Lorenz, M.W., Kellner, R., Hoffmann, K.H., 1999a. Cricket
allatostat-ins: new structures and physiological properties. Eur. J. Entomol. 96, 267–274.
Lorenz, M.W., Hoffmann, K.H., Ga¨de, G., 1999b. Juvenile hormone biosynthesis in larval and adult stick insects, Carausius morosus. J. Insect Physiol. 45, 443–452.
Maestro, J.L., Belle´s, X., Piulachs, M.D., Thorpe, A., Duve, H., 1998. Localization of allatostatin-immunoreactive material in the central nervous system, stomatogastric nervous system, and gut of the cockroach Blattella germanica. Arch. Insect Biochem. Physiol. 37, 269–282.
Martı´n, D., Piulachs, M.D., Belle´s, X., 1996. Inhibition of vitellogenin production by allatostatin in the German cockroach. Mol. Cell. Endocrinol. 121, 191–196.
Pflugfelder, O., 1937. Bau, Entwicklung und Funktion der Corpora allata und cardiaca von Dixippus morosus Br. Z. Wiss. Zool. 149, 477–512.
Piulachs, M.-D., Vilaplana, L., Bartholome´, J.-M., Carren˜o, C., Martı´n, D., Gonza´les-Mun˜iz, R., Herrenz, R., Garcı´a-Lo´pez, M.-T., And-reu, D., Belle´s, X., 1997. Ketomethylene and methyleneamino pseudopeptide analogues of insect allatostatins inhibit juvenile hor-mone and vitellogenin production in the cockroach Blattella
germ-anica. Insect Biochem. Mol. Biol. 27, 851–858.
Pratt, G.E., Farnsworth, D.E., Fok, K.F., Siegel, N.R., McCormack, A.L., Shabanowitz, J., Hunt, D.F., Feyereisen, R., 1991. Identity of a second type of allatostatin from cockroach brains: an octadeca-peptide amide with a tyrosine-rich address sequence. Proc. Nat. Acad. Sci., USA 88, 2412–2416.
Raina, A.K., Ga¨de, G., 1988. Insect peptide nomenclature. Insect Biochem. 18, 785–787.
Schoofs, L., Holman, G.M., Hayes, T.K., Nachman, R.J., De Loof, A., 1991. Isolation, identification and synthesis of locustamyoinhibit-ing peptide (Lom-MIP), a novel biologically active neuropeptide from Locusta migratoria. Regul. Pept. 36, 111–119.
Skiebe, P., Schneider, H., 1994. Allatostatin peptides in the crab stoma-togastric nervous system: inhibition of the pyloric motor pattern and distribution of allatostatin-like immunoreactivity. J. Exp. Biol. 194, 195–208.
Smart, D., Johnston, C.F., Curry, W.J., Williamson, R., Maule, A.G., Skuce, P.J., Shaw, C., Halton, D.W., Buchanan, K.D., 1994. Pep-tides related to the Diploptera punctata allatostatins in nonarthro-pod invertebrates: an immunocytochemical survey. J. Comp. Neu-rol. 347, 426–432.
Smart, D., Johnston, C.F., Maule, A.G., Halton, D.W., Hrckova´, G., Shaw, C., Buchanan, K.D., 1995. Localization of Diploptera
punctata allatostatin-like immunoreactivity in helminths: an
immu-nocytochemical study. Parasitology 110, 87–96.
Stay, B., Woodhead, A.P., Chan, K.K., 1992. Immunocytochemical distribution of allatostatin in the brain and retrocerebral complex of Diploptera punctata. In: Mauchamp, B., Couillaud, F., Baehr, J.C. (Eds.), Insect Juvenile Hormone Research: Fundamental and Applied Approaches. Chemistry, Biochemistry and Mode of Action. INRA Edition, Paris, pp. 257–263.
Stay, B., Tobe, S.S., Bendena, W.G., 1994. Allatostatins: identification, primary structures, functions and distribution. Adv. Insect Physiol. 25, 267–337.
Veelaert, D., Devreese, B., Schoofs, L., Van Beeumen, J., Vanden Broeck, J., Tobe, S.S., De Loof, A., 1996. Isolation and characteriz-ation of eight myoinhibiting peptides from the desert locust
Schisto-cerca gregaria: new members of the cockroach allatostatin family.
Mol. Cell. Endocrinol. 122, 183–190.
Veenstra, J.A., Lau, G.W., Agricola, H.-J., Petzel, D.H., 1995. Immu-nohistochemical localization of regulatory peptides in the midgut of the female mosquito Aedes aegypti. Histochem. Cell. Biol. 104, 337–347.
Veenstra, J.A., Noriega, F.G., Graf, R., Feyereisen, R., 1997. Identifi-cation of three allatostatins and their cDNA from the mosquito
Aedes aegypti. Peptides 18, 937–942.
Periplaneta americana: novel members of a family of neuropeptide
inhibitors of insect juvenile hormone biosynthesis. Comp. Biochem. Physiol. 107C, 119–127.
Weaver, R.J., Paterson, Z.A., Short, J.E., Edwards, J.P., 1995. Effects of Diploptera punctata allatostatins on juvenile hormone biosynth-esis and endogenous juvenile hormone III levels in virgin and mated female Periplaneta americana. J. Insect Physiol. 41, 117– 125.
Weaver, R.J., Edwards, J.P., Bendena, W.P., Tobe, S.S., 1998. Struc-tures, functions and occurrence of insect allatostatic peptides. In:
Coast, G.M., Webster, S.G. (Eds.), Recent Advances in Arthropod Endocrinology. Cambridge University Press, Cambridge, pp. 3–32. Woodhead, A.P., Stay, B., Seidel, S.L., Khan, M.A., Tobe, S.S., 1989. Primary structure of four allatostatins: neuropeptide inhibitors of juvenile hormone synthesis. Proc. Nat. Acad. Sci., USA 86, 5997–6001.