Delayed ripening of banana fruit by salicylic acid
Manoj K. Srivastava, Upendra N. Dwivedi*
Department of Biochemistry,Lucknow Uni6ersity,Lucknow226 007,India
Received 30 July 1999; received in revised form 26 May 2000; accepted 2 June 2000
Abstract
Salicylic acid treatment has been found to delay the ripening of banana fruits (Musa acuminata). Fruit softening, pulp:peel ratio, reducing sugar content, invertase and respiration rate have been found to decrease in salicylic acid treated fruits as compared with control ones. The activities of major cell wall degrading enzymes, viz. cellulase, polygalacturonase and xylanase were found to be decreased in presence of salicylic acid. The major enzymatic antioxidants namely, catalase and peroxidase, were also found to be decreased in presence of salicylic acid during banana fruit ripening. © 2000 Published by Elsevier Science Ireland Ltd. All rights reserved.
Keywords:Ripening; Salicylic acid; Banana (Musa acuminata); Catalase
www.elsevier.com/locate/plantsci
1. Introduction
Salicylic acid, a ubiquitous plant phenolic, has been reported to regulate a number of processes in plants [1]. One of its earliest known in vivo func-tions in plants is heat production during flower induction in some angiosperm species [2]. Later it was shown to regulate expression of pathogenesis related protein genes, which suggested its role as a signal molecule in providing resistance against pathogen attack. Salicylic acid mediated hypersen-sitive and systemic acquired resistance against pathogen attack are proposed to be mediated through the inhibition of catalase, which
subse-quently raises intracellular H2O2 concentration.
This increased intracellular H2O2 concentration
has been proposed to act as a second messenger in activation and expression of defense related genes [1]. Recently, it has been proposed to be a new kind of plant hormone [3]. Thus, salicylic acid has
been shown to interfere with the biosynthesis and/
or action of ethylene [1], abscisic acid [4] and cytokinins [5] in plants. Salicylic acid and its
derivative acetyl salicylic acid (ASA) have been reported to inhibit ethylene production in pear [6,7], carrot cell suspension cultures [8], in apple and pear discs and mung bean hypocotyls [9] suggesting the role of salicylic acid as an antago-nist to ethylene action. However, salicylic acid and ethylene have been reported to have some similar functions also. For example, both can induce cer-tain pathogenesis-related proteins and the alterna-tive pathway of respiration in many plant tissues [10]. Moreover, an increase in endogenous ethyl-ene biosynthesis at low concentrations of salicylic acid has been reported in suspension cultures of carrot [11]. However, at higher salicylic acid
con-centration (\10−4 M) an inhibition of ethylene
production was observed in this study.
The plant hormone ethylene has been reported to affect a diverse array of plant growth and developmental processes including germination, senescence and abscission of both flowers and leaves, fruit ripening as well as the response to a wide variety of stresses such as pathogen attack and drought [12]. The induction of ethylene biosynthesis by a wide variety of stimuli, including wounding, pathogen attack, various stresses, me-chanical stimulus and by hormones such as auxins,
* Corresponding author. Tel.: +91-522-389290.
E-mail address:[email protected] (U.N. Dwivedi).
cytokinins and ethylene itself during certain devel-opmental processes is well documented [12 – 14]. Among all these effects of ethylene, its role in fruit ripening has been studied in great detail including isolation and characterization of gene encoding a key enzyme of ethylene biosynthesis [15] and de-velopment of transgenic tomato having delayed ripening [16]. Treatment of green banana fruits with ethylene during the preclimacteric phase, has been shown to accelerate their ripening. All the climacteric fruits are characterized by a transient increase in both ethylene synthesis and respiration at an early stage of ripening. Recently, Vendrell et al. [17] have attributed the ethylene as a trigger for ripening in banana.
As salicylic acid has been shown to affect the biosynthesis and action of ethylene, a well known fruit ripening hormone, in the present paper, we have investigated the effects of salicylic acid on banana fruit ripening by measuring respiration rate, pulp and peel contents and their ratio, reduc-ing and non-reducreduc-ing sugars and their ratio and invertase, antioxidant enzymes such as catalase and peroxidase, cell wall degrading enzymes such as cellulase, polygalacturonase and xylanase.
2. Materials and methods
2.1. Fruit tissue
Banana fingers (Musa acuminatacv Hari chhal)
were purchased from local market.
2.2. Treatment of fruits
Banana fingers were randomized and divided into groups, containing ten fruits in each, accord-ing to the followaccord-ing treatments:
Group 1. Water
Group 2. Teepol (0.2%) [Control]
Group 3. Teepol (0.2%)+500mM salicylic acid
Group 4. Teepol (0.2%)+1000 mM salicylic
acid
Banana fingers were dipped in 2 l of distilled water (group 1) or teepol (group 2) or teepol containing salicylic acid (groups 3 and 4) under laboratory conditions for 6 h with occasional shaking. After draining the water/solution fingers were washed, air dried and kept in plastic contain-ers at room temperature and covered with poly-thene sheets containing small holes.
2.3. Determination of pulp/peel ratio
Two banana fruits from each treatment were peeled off and the pulp and peel portions of each finger were weighed separately on a laboratory balance. The ratio of pulp to peel of each finger was calculated and mean value was recorded.
2.4. Determination of respiration rate
Respiration was measured as CO2 production.
A single banana fruit was taken in a chamber and air was passed through the chamber. The effluent air was connected to an ADC 225 MK3 Infrared Gas Analyzer (IRGA) and respiratory rate was measured. The IRGA was earlier calibrated with
standard CO2. The results were expressed as ml
h−1 kg−1 fresh weight.
2.5. Determination of soluble sugars
Banana fruit pulp (1 g) was homogenized in 5.0 ml 90% ethanol by Ultra Turrax T25 for 2 min and then refluxed for 30 min. The sample was
centrifuged at 10 000×g for 30 min. The residue
was again subjected to ethanol extraction. The extracts were combined and alcohol was removed by evaporation. An aliquot was taken and total sugars were measured by phenol – sulfuric acid method [18]. Reducing sugars were measured as described [19]. Non reducing sugar content was determined by the difference between total sugar and corresponding reducing sugar value. Glucose was used as standard for sugar estimation.
2.6. Enzyme extraction and assay
A 10% homogenate was prepared by homoge-nizing 2 g of pulp in 15 ml sodium phosphate buffer (20 mM, pH 7.0) containing cysteine – HCl (20 mM), EDTA (20 mM) and Triton X-100 (0.05%) by Ultra Turrax T25 for 2 min at high speed. The homogenate was passed through two layers of muslin cloth to remove cell debris and the volume was made to 20 ml with the homoge-nization medium. The homogenate was
cen-trifuged at 15 000×g for 30 min at 4°C in a
2.7. Assay of polygalacturonase (EC 3.2.1.15)
Polygalacturonase activity was assayed by mea-suring the formation of reducing groups using the methods of Nelson [20] and Somogyi [21]. The reaction mixture contained 0.2 ml sodium acetate buffer (200 mM, pH 4.5), 0.1 ml NaCl (200 mM), 0.3 ml polygalacturonic acid (PGA) (1%, pH 4.5), and an appropriate amount of enzyme in a total volume of 1.0 ml. The reaction was initiated by the addition of substrate. The reaction mixture was incubated at 37°C for 1 h. The reaction was terminated by heating the reaction mixture in a boiling waterbath. In the control tubes, the sub-strate was added after the heat treatment. The formation of reducing group was calculated using D-galacturonic acid as a standard. One unit of
polygalacturonase activity is defined as the
amount of enzyme producing 1 mmol of reducing
groups per min at 37°C.
2.8. Assay of cellulase (EC 3.2.1.4)
Cellulase activity was measured as described by Ahmed and Labavitch [22] with slight modifica-tions. The reaction mixture consisted of sodium acetate buffer (100 mM, pH 5.0), carboxy methyl
cellulose (1.5% w/v) and enzyme in a final volume
of 1.0 ml. The reaction mixture was incubated at 37°C for 16 h. After 16 h, the substrate was added to the control tubes and colour was developed according to Miller [19] using dinitrosalicylic acid (DNS). The tubes were boiled on a waterbath for 10 min and the colour was read at 540 nm using Spectronic 20D spectrophotometer. Amount of re-ducing sugar released was calculated from a cali-bration curve drawn using glucose as standard. One unit of cellulase activity was defined as the
amount of enzyme liberating 1 mmol of reducing
sugar per min at 37°C.
2.9. Assay of xylanase (EC 3.2.1.8)
Xylanase activity was assayed as described by Singh and Singh [23] with slight modifications. The assay mixture consisted of sodium acetate buffer (100 mM, pH 5.0), xylan (0.1%) and en-zyme preparation in a total volume of 1.0 ml. The mixture was incubated for 1 h at 37°C. The re-leased reducing sugars were measured using DNS as for cellulase (described above). One unit of
xylanase activity was defined as 1 mmol of
reduc-ing sugar released per min at 37°C.
2.10. Assay of in6ertase (EC 3.2.1.26)
Invertase activity was measured as described by Moriguchi et al. [24] with slight modifications. The assay mixture contained acetate buffer (100 mM, pH 4.5), sucrose (100 mM) and enzyme prepara-tion in a total volume of 1.0 ml. The reacprepara-tion mixture was incubated for 1 h at 37°C. The sub-strate was added to control tubes after the incuba-tion and colour was developed using DNS (as described for cellulase). Amount of reducing sugar released was calculated from the calibration graph. One unit of invertase activity was defined as micromoles of reducing sugars equivalent released per min at 37°C.
2.11. Assay of peroxidase (EC 1.11.1.7)
The peroxidase activity was assayed as de-scribed by Pu¨tter [25] with slight modifications. The assay mixture consisted of sodium phosphate
buffer (50 mM, pH 7.0), H2O2 (4mM), guaiacol
(3.33 mM) and 0.1 ml enzyme in a final volume of 3.0 ml. The increase in absorbance at 470 nm was measured using a Spectronic-2000 spectrophoto-meter. One unit of enzyme activity was defined as the amount of enzyme catalyzing the production
of 1 mmol tetraguaiacol per min at 30°C.
2.12. Assay of catalase (EC 1.11.1.6)
The catalase activity was assayed as described by Aebi [26] with some modifications. The assay medium consisted of sodium phosphate buffer (50 mM, pH 7.0), (20 mM) and 0.1 ml enzyme in a final volume of 3.0 ml. The disappearance of H2O2 was measured as decrease in absorbance at 240 nm using a Spectronic-2000 spectrophotometer. One unit of enzyme activity was defined as the amount of the enzyme catalyzing the decomposition of 1 mmol H2O2 per min at 30°C.
3. Results
3.1. Effect of salicylic acid on the rate of
respiration during banana fruit ripening
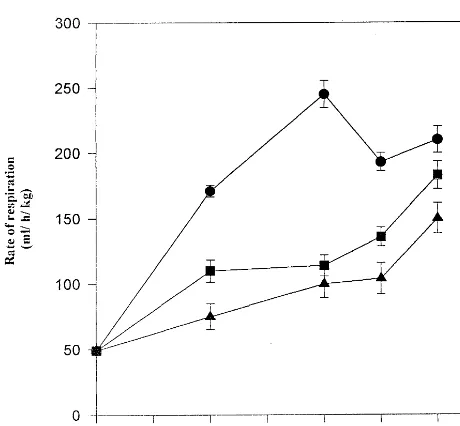
respi-Fig. 1. Effect of salicylic acid on rate of respiration during ripening of banana fruit in the absence ( — ) and presence of 500 (—) and 1000 (—) mM salicylic acid. Each value represents a mean of three independent replicates.
3.2. Effect of salicylic acid on softening of banana
fruits during its ripening
Softening of fruits is one of the most common physical parameter to assess the progress of ripen-ing. Therefore, the effect of salicylic acid on soft-ening of banana fruits, as a physical parameter to assess the extent of ripening, has been investigated. It was observed that salicylic acid treatment inhib-ited the process of banana fruit softening during ripening. Another physical parameter, yellowing of peel was also found to be less in salicylic acid treated than those of control fruits. Thus, the control fruits were soft enough at day 8 of treat-ment while the salicylic acid treated fruits were still hard.
3.3. Effect of salicylic acid on pulp and peel
contents of banana fruit during its ripening
Effect of salicylic acid on pulp and peel contents of banana fruits have been investigated during its ripening. Pulp content increased throughout the experimental period both in control and salicylic acid treated banana fruits. However, salicylic acid treatment resulted in decreased pulp content of fruits in a concentration dependent manner. On the other hand, peel content decreased throughout the ripening both in the absence as well as pres-ence of salicylic acid. However, the decrease in peel content was less in salicylic acid treated fruits as compared with that of control. Because the pulp to peel ratio of fruits is taken as an important parameter for fruit ripening, we have presented the data in the form of their ratio. (Table 1). It is noteworthy that pulp to peel ratio increased with ration during banana fruit ripening have been
investigated. Results are shown in Fig. 1. In control fruits (in the absence of salicylic acid) the rate of respiration was found to increase with ripening exhibiting a peak on day 4 (climacteric peak). Rate of respiration has been reported to increase with ripening of all climacteric fruits. Salicylic acid treatment resulted in a decrease in the rate of respiration as well as a delay in appearance of climacteric peak, in a concentration dependent manner, as compared to control. There was a gradual increase in respiration rate of salicylic acid treated fruits up to 6th day of
treatment suggesting a delay in ripening
(respiratory burst).
Table 1
Effect of salicylic acid on ratio of pulp to peel of banana fruits during its ripeninga
Concentration of salicylic acid (mM) Pulp to peel ratio
Days
5th 4th
2nd
0 6th
1.4090.22 1.5490.13
0 (control) 1.7090.12 1.9090.17 2.0090.18
1.5090.13 1.5890.18 1.6090.20 1.6490.14
500 1.4090.22
1.4690.08 1.5290.07 1.5690.07
1000 1.4090.22 1.6390.15
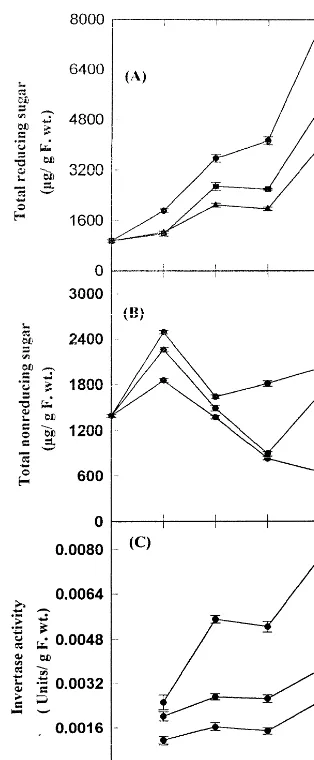
Fig. 2. Effect of salicylic acid on reducing (A), nonreducing (B) sugars and invertase (C) activity during ripening of ba-nana fruit in the absence ( — ) and presence of 500 (—) and 1000 (—) mM salicylic acid. Each value represents a mean of three independent replicates.
treatment resulted in, a concentration dependent manner, decreased levels of reducing sugar than those in the absence of salicylic acid (control) throughout the ripening period of banana fruits. On the other hand, the measurement of nonreduc-ing sugar content of banana fruit revealed an opposite trend to that of reducing sugar, during ripening (Fig. 2B). Thus, on day 4 of ripening, salicylic acid treatment resulted in a decrease of total reducing sugar by 25 and 42% at 500 and 1000 mM salicylic acid concentration, respectively, while it resulted in an increase of nonreducing
sugar by 9 and 20% at 500 and 1000 mM salicylic
acid, respectively.
3.5. Effect of salicylic acid on in6ertase during
banana fruit ripening
Effect of salicylic acid on invertase activity has been investigated during ripening of banana fruit. The results are shown in Fig. 2C. Invertase activ-ity increased throughout the ripening both in con-trol and in salicylic acid treated fruits. However, salicylic acid treatment resulted in decreased levels of invertase than those of controls, in a concentra-tion dependent manner, during banana ripening. Thus, on day 4, salicylic acid treatment resulted in a decrease of invertase activity by 51 and 71% of the control value at 500 and 1000mM salicylic acid concentrations, respectively, during the banana fruit ripening.
3.6. Effect of salicylic acid on cell wall degrading
enzymes during banana fruit ripening
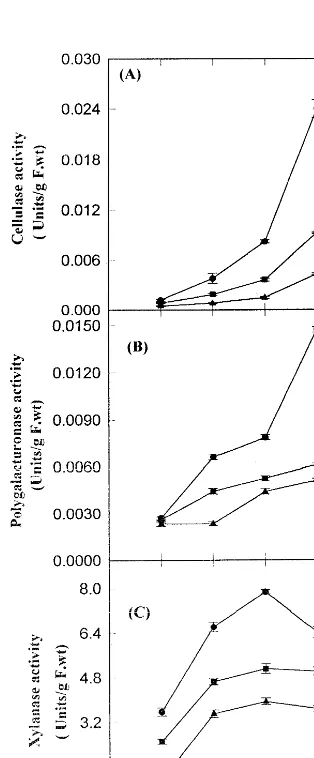
Effect of salicylic acid on the developmental profiles of major cell wall degrading enzymes namely, cellulase, polygalacturonase (PG) and xy-lanase in the absence and presence of salicylic acid have been investigated during ripening of banana fruits. Results are shown in Fig. 3. It is notewor-thy that the activity of all the three major cell wall degrading enzymes increased gradually both in the absence and presence of salicylic acid during the ripening of banana fruits. However, the salicylic acid treatment resulted in decreased levels of all these cell wall degrading enzymes in a concentra-tion dependent manner, during the ripening of banana. Thus, on day 6, the activities of cellulase, polygalacturonase and xylanase decreased by 55 and 82, 33 and 45, 35 and 50% of their respective the progress of ripening both in the absence and
presence of salicylic acid. However, salicylic acid treatment decreased the pulp to peel ratio in a concentration dependent manner. Our data of pulp to peel ratio suggested a delay in ripening of banana fruit in the presence of salicylic acid.
3.4. Effect of salicylic acid on soluble sugar
content of banana fruit during ripening
controls at 500 and 1000mM salicylic acid, respec-tively during banana fruit ripening. Thus, out of the three cell wall degrading enzymes studied, cellulase seems to be most sensitive to salicylic acid inhibition. Furthermore, the two cell wall degrad-ing enzymes namely, cellulase and polygalactur-onase seems to play a major role in banana fruit ripening as their activities increased several folds during ripening in comparison to that of xylanase. Similarly, salicylic acid inhibited cellulase and polygalacturonase activity more strongly than that of xylanase.
3.7. Effect of salicylic acid on catalase and
peroxidase during banana fruit ripening
As catalase and peroxidase have also been re-ported to be affected during fruit ripening, the effect of salicylic acid on these two enzymes has been investigated during banana fruit ripening. Results are shown in Table 2. Salicylic acid treat-ment resulted in decreased levels of catalase and peroxidase, than their respective controls, in a
concentration dependent manner, during the
ripening of banana fruits. Thus, the effect of sali-cylic acid on these two enzymes, during banana fruit ripening, revealed a similar response as ob-served for that of the pea during seedling growth [27].
4. Discussion
The data presented in this paper suggest that salicylic acid delays banana fruit ripening. Thus, the rapid rise in respiration during ripening of banana fruits, an observation typical of climacteric fruits, was found to be delayed by salicylic acid in a concentration dependent manner.
The ripening of banana fruit was accompanied by increase in pulp to peel ratio. Similar observa-tion has been reported by others [28,29]. Rise in pulp to peel ratio during fruit ripening was sug-gested to be due to change in sugar concentration in the two tissues. A rapid increase in sugar con-tents in the pulp than those in the peel leads to a change in osmotic pressure, as a result of which water is withdrawn from the peel and hence pulp to peel ratio increases accordingly. Salicylic acid treatment reduced this increase in pulp to peel ratio, in a concentration dependent manner, lead-ing to a delay in banana fruit ripenlead-ing. Delay in ripening of banana is reported by ABA [30] and GA3[31]. GA3is also reported to delay ripening in tomato pericarp disc [31] and mango [32]. Post harvest dips of mature unripe Alphonso (variety
of mango) fruits in solution of GA3 (10−6 M),
IAA (10−6M) and kinetin (10−5M) are reported
to delay ripening [33].
The data on soluble sugar content and invertase suggested that banana ripening was accompanied by an increase in the reducing sugar content and invertase activity concomitant with decrease in nonreducing sugar content. Salicylic acid treat-Fig. 3. Effect of salicylic acid on cellulase (A),
K
.
Sri
6
asta
6
a
,
U
.
N
.
Dwi
6
edi
/
Plant
Science
158
(2000)
87
–
96
93
Table 2
Effect of salicylic acid on catalase and peroxidase activities of banana fruits during ripeninga
Catalase activity (U/ml) Peroxidase activity (U/ml) Concentration of
salicylic acid (mM)
Number of days Number of days
2nd 4th 6th
2nd 4th 6th 8th 0 8th
0.12990.006 0.02790.007 0.00990.004 0.01290.005 0.01490.003 0.03690.004 0 (control) 0.09190.015 0.04790.011 0.02790.006
0.00490.002 0.00690.001 0.01190.003 0.01690.006 0.02790.007
0.07590.012 500 0.09190.015 0.03790.007 0.01590.006
1000 0.09190.015 0.02990.011 0.01290.004 0.06790.010 0.02790.007 0.00290.001 0.00390.001 0.00890.003 0.01290.004
ment resulted in decreased levels of invertase and reducing sugar contents while an opposite effect on nonreducing sugar content, suggesting that salicylic acid delays banana fruit ripening. This accumulation of reducing sugars may be due to increased breakdown of starch during ripening as reported by Beaudry et al. [34]. Increased starch phosphorylase activity has been reported during
development and ripening of banana fruits
[35,36]. Recently, an increase in sucrose phos-phate synthase and decrease in sucrose synthase activity have been reported during ripening of banana fruits [37]. Increase in invertase activity has been shown during papaya fruit ripening [38]. The data on cell wall degrading enzymes sug-gested that banana ripening was accompanied by an increase in all the three cell wall degrading enzymes namely, cellulase, polygalacturonase and xylanase. Levels of these cell wall degrading en-zymes were found to be decreased, in a
concen-tration dependent manner, suggesting that
salicylic acid delays ripening of banana. Poly-galacturonase is reported to be primarily respon-sible for ripening associated pectin degradation and fruit softening [39]. Level of polygalactur-onase activity has been positively correlated with fruit ripening and softening in banana [29]. Poly-galacturonase activity [40], mRNA [41] and gene transcription [42,43] are reported to increase at the onset of ripening of tomato fruits. Further-more, tomato fruit ripening mutants, with
de-layed ripening exhibited decreased levels of
polygalacturonase activity [44], mRNA [45] and gene transcription [43], while the activities of sev-eral other cell wall enzymes appeared to be af-fected to a lesser extent. Also, physical and chemical treatments which suppress ripening, in-hibit polygalacturonase gene expression [46 – 48].
Carboxymethyl cellulase (Cm– cellulase) activity
increases during ripening of tomato [49,50], strawberry, pear, peach [51] and avocado [52]. Modifications of hemicellulose structure of cell wall, during fruit ripening have been reported in tomato, pepper, strawberry and melons. The sizes of hemicellulose polymers are found to decrease during ripening of these fruits [39,53,54]. Ripen-ing associated changes in hemicellulose structure are proposed to be responsible for the textural change in fruit.
Data on catalase and peroxidase revealed that ripening of banana fruit was accompanied by an
increase in the activities of catalase and perox-idase while levels of these enzymes were found to be decreased in presence of salicylic acid, in a concentration dependent manner. Ripening of pa-paya fruit has been reported to be accompanied by an increase in the catalase and peroxidase activity [38]. Salicylic acid has been demonstrated to inhibit catalase and peroxidase in pea [26]. The inhibition of catalase by salicylic acid is sug-gested to be one of modes of action of salicylic acid in providing defense against pathogen attack [27,55].
Thus, the data presented here unequivocally suggest that salicylic acid delays banana fruit ripening. Based on these observations salicylic acid may be considered as antagonist of ethylene, a major fruit ripening hormone. Salicylic acid is reported to inhibit biosynthesis of ethylene [7,8]. Recently, salicylic acid has also been shown to inhibit ACC oxidase activity [56]. Even in the studies of Nissen [11] in which salicylic acid (at low concentration) has been shown to stimulate ethylene biosynthesis, an inhibition of ethylene production occurs at higher concentrations of
salicylic acid (\10−4 M). Therefore, in the
present study since we have taken a much higher concentration of salicylic acid than those of Nis-sen [11] (\10−4 M), only the inhibition of ethyl-ene biosynthesis is expected to occur in our case. Ethylene synthesis is normally limited by the sup-ply of the immediate precursor amino cyclo-propane-1-carboxylic acid (ACC). There is a sharp rise in ACC synthase activity and the con-tent of ACC during ripening [57]. Thus, present studies on the effect of salicylic acid in delaying the ripening of banana fruits may be thought of
due to inhibition of ethylene biosynthesis and/or
action. However, this needs further investiga-tions.
Acknowledgements
References
[1] I. Raskin, Role of salicylic acid in plants, Ann. Rev. Plant Physiol. Mol. Biol. 43 (1992) 439 – 463.
[2] I. Raskin, A. Ehmann, W.R. Melander, B.J.D. Meeuse, Salicylic acid: a natural inducer of heat production in
Arum lillies, Science 237 (1987) 1601 – 1602.
[3] I. Raskin, Salicylate, a new plant hormone, Plant Phys-iol. 99 (1992) 799 – 803.
[4] P.V. Apte, M.M. Laloraya, Inhibitory action of phenolic compounds on abscisic acid-induced abscission, J. Exp. Bot. 33 (1982) 826 – 830.
[5] H. Sano, S. Seo, E. Orudgev, S. Youssefian, K. Ishizuka, Y. Ohashi, Expression of the gene for a small GTP binding protein in transgenic tobacco elevates endoge-nous cytokinin levels, abnormally induces salicylic acid acid in response to wounding and increases resistance to tobacco mosaic virus infection, Proc. Natl. Acad. Sci. USA 91 (1994) 10556 – 10560.
[6] C.A. Leslie, R.J. Romani, Salicylic acid: a new inhibitor of ethylene biosynthesis, Plant Cell Rep. 5 (1986) 144 – 146.
[7] C.A. Leslie, R.J. Romani, Inhibition of ethylene biosyn-thesis by salicylic acid, Plant Physiol. 88 (1988) 833 – 837. [8] J.P. Roustan, A. Latche´, J. Fallot, Inhibition of ethylene production and stimulation of carrot somatic embryoge-nesis by salicylic acid, Biol. Plant. 32 (1990) 273 – 276. [9] R.J. Romani, B.M. Hess, C.A. Leslie, Salicylic acid
inhibition of ethylene production by apple discs and other plant tissues, J. Plant Growth Regul. 8 (1989) 63 – 69.
[10] N. Marissen, L.H.W. Vander Plas, J.G. Duys, Influence of temperature, ethylene and cyanide on the occurrence of alternative respiration in mitochondria from iris bulbs, Plant Sci. 45 (1986) 19 – 25.
[11] P. Nissen, Stimulation of somatic embryogenesis in car-rot by ethylene: effects of modulators of ethylene biosyn-thesis and action, Physiol. Plant. 92 (1994) 397 – 403. [12] F.B. Abeles, P.W. Morgan, M.E. Saltveit Jr, Ethylene in
Plant Biology, Academic Press, San Diego, 1992. [13] S.F. Yang, N.E. Hoffman, Ethylene biosynthesis and its
regulation in higher plants, Annu. Rev. Plant Physiol. 35 (1984) 155 – 189.
[14] A.K. Mattoo, J.C. Suttle, The Plant Hormone Ethylene, CRC Press, Boca Raton, FL, 1991.
[15] D. van der Straeten, L. van Wiemeersch, H.M. Good-man, M. van Montague, Cloning and sequence of two different cDNAs encoding 1-aminocyclopropane-1-car-boxylic acid synthase in tomato, Proc. Natl. Acad. Sci. USA 87 (1990) 4859 – 4863.
[16] A.J. Hamilton, G.W. Lycett, D. Grierson, Antisense gene that inhibits synthesis of hormone ethylene in transgenic plants, Nature 346 (1990) 284 – 287.
[17] M. Vendrell, M. Domningnez, E. Dominguez-Puigjaner, A. Aitoubahou, Ethylene biosynthesis in bananas: effect of inhibitors, in: M. Elotmani (Ed.), Post-Harvest Physi-ology, Pathology and Technologies for Horticultural Commodities: Recent Advances Proceedings of Interna-tional Symposium. Agadir, Morocco, 16 – 21 January, 1994.
[18] M.K.A. Dubois, J.K. Hamilton, P.A. Rebers, F. Smith, Colorimetric method for determination of sugars and related substances, Anal. Chem. 28 (1956) 350 – 356. [19] G.L. Miller, Use of dinitro salicylic acid reagent for
determination of reducing sugar, Ann. Chem. 31 (1959) 426 – 428.
[20] N. Nelson, A photometric adaptation of the Somogyi method for determination of glucose, J. Biol. Chem. 153 (1944) 378 – 380.
[21] M. Somogyi, Notes on sugar determination, J. Biol. Chem. 195 (1952) 19 – 23.
[22] A.E.R. Ahmed, J.M. Labavitch, Cell-wall metabolism in ripening fruit. II. Changes in carbohydrate degrading enzymes in ripening bartlett pears, Plant Physiol. 65 (1980) 1014 – 1016.
[23] A. Singh, M. Singh, Cell wall degrading enzymes in
Orobanche aegyptiaca and its host Brassica campestris, Physiol. Plant. 89 (1993) 177 – 181.
[24] T. Moriguchi, T. Sanada, S. Yamaki, Properties of acid invertase purified from peach fruits, Phytochemistry 30 (1991) 95 – 97.
[25] J. Pu¨tter, Peroxidases, in: H.U. Bergmeyer (Ed.), Meth-ods of Enzymatic Analysis, Varley Chemie/Academic Press, Weinheim/New York, 1974, p. 685.
[26] H. Aebi, Catalase, in: H.U. Bergmeyer (Ed.), Methods of Enzymatic Analysis, Varley Chemie/Academic Press, Weinheim/New York, 1974, p. 680.
[27] M.K. Srivastava, U.N. Dwivedi, Salicylic acid modu-lates glutathione metabolism in pea seedlings, J. Plant Physiol. 153 (1998) 409 – 414.
[28] S. Singh, H.B. Ram, U.K. Tripathi, Biochemical studies on the developing and ripening of banana, Prog. Hort. 12 (1980) 51 – 57.
[29] N. Pathak, Biochemistry of ripening in banana fruits. PhD thesis, Lucknow University, Lucknow, India, 1997. [30] H. Yunovitz, K.C. Gross, Delay of tomato fruit ripening by oligosaccharide N-glycan interactions with IAA, galactose and lectins, Physiol. Plant. 90 (1994) 152 – 156. [31] R. Ben-Arie, I. Mignani, L.C. Greve, M. Huysamer, J.M. Labavitch, Regulation of the ripening of tomato pericarp discs by GA3 and divalent cations, Physiol. Plant. 93 (1995) 99 – 107.
[32] S.E.S.A. Khader, Effect of gibberellic acid and vapor guard on ripening, amylase and peroxidase activities and quality of mango fruits during storage, J. Hort. Sci. 67 (1992) 855 – 860.
[33] G. Majumdar, V.V. Modi, V.A. Palejwala, Effect of plant growth regulators on mango ripening, Indian J. Exp. Biol. 19 (1981) 885 – 886.
[34] R.M. Beaudry, F.S. Ray, C.B. Clanton, J.K. Stanley, Banana ripening: implications of changes in glycolytic intermediate concentration, glycolytic and gluconeogenic carbon flux and fructose 2,6-bisphosphate concentration, Plant Physiol. 91 (1989) 1436 – 1444.
[35] S. Singh, G.G. Sanwal, Characterization of multiple forms of glucan phosphorylase from Musa paradisiaca
fruits, Phytochemistry 14 (1975) 113 – 118.
[37] R.R. Cordenunsi, F.M. Lajolo, Starch breakdown dur-ing banana ripendur-ing. Sucrose synthase and sucrose phos-phate synthase, J. Agric. Food Chem. 43 (1995) 347 – 351.
[38] D.K. Pal, Y. Selvaraj, Biochemistry of papaya (Carica papaya L.) fruit ripening: changes in RNA, DNA, protein and enzymes of mitochondrial, carbohydrate, respiratory and phosphate metabolism, J. Hortic. Sci. 62 (1987) 117 – 124.
[39] D.J. Huber, The role of cell wall hydrolases in fruit softening, Hortic. Rev. 5 (1983) 169 – 219.
[40] G.E. Hobson, The firmness of tomato fruit in relation to polygalacturonase activity, J. Hortic. Sci. 40 (1965) 66 – 72.
[41] D. Della Penna, D.C. Alexander, A.B. Bennett, Molecu-lar cloning of tomato fruit polygalacturonase analysis of polygalacturonase mRNA levels during ripening, Proc. Natl. Acad. Sci. USA 83 (1986) 6420 – 6424.
[42] R.E. Sheehy, M.K. Kramer, W.R. Hiatt, Reduction of polygalacturonase activity in tomato fruit by antisense RNA, Proc. Natl. Acad. Sci. USA 85 (1988) 8805 – 8809. [43] D. DellaPenna, J.E. Lincoln, R.L. Fischer, A.B. Ben-nett, Transcription analysis of polygalacturonase and other ripening associated genes in Rutgers, rin, nor and Nr tomato fruit, Plant Physiol. 90 (1989) 1372 – 1377. [44] E.C. Tigchelaar, W.B. McGlasson, R.W. Buescher,
Ge-netic regulation of tomato fruit ripening, Hortic. Sci. 13 (1978) 508 – 513.
[45] D. DellaPenna, D.S. Kates, A.B. Bennett, Polygalactur-onase gene expression in Rutgers, rin, nor and Nr tomato fruits, Plant Physiol. 85 (1987) 502 – 507. [46] N. Ogura, H. Nakagawa, H. Takenhana, Effect of
stor-age temperature of tomato fruits on changes of their polygalacturonase and pectinesterase activities accompa-nied with ripening, J. Agric. Chem. Soc. Japan 49 (1975) 271 – 274.
[47] K. Davies, G.E. Hobson, D. Grierson, Silver ions inhibit the ethylene stimulated production of ripening related
mRNAs in tomato, Plant Cell Environ. 11 (1988) 729 – 738.
[48] S. Picton, S.L. Barton, M. Bouzayen, A.J. Hamilton, D. Grierson, Altered fruit ripening and leaf senescence in tomatoes expressing an antisense ethylene forming en-zyme transgene, Plant J. 3 (1993) 469 – 481.
[49] G.E. Hobson, Cellulase activity during the maturation and ripening of tomato fruit, J. Food Sci. 33 (1968) 588 – 592.
[50] D.M. Pharr, D.B. Dickinson, Partial characterization of Cx-cellulase and cellobiase from ripening tomato fruits, Plant Physiol. 52 (1973) 577 – 583.
[51] D.M. Hinton, R. Pressey, Cellulase activity in peaches during ripening, J. Food Sci. 39 (1974) 783 – 785. [52] L.G. Cass, K.A. Kirven, R.E. Christofferson, Isolation
and characterization of a cellulase gene family member expressed during avocado fruit ripening, Mol. Gen. Genet. 223 (1990) 76 – 86.
[53] D.J. Huber, Strawberry fruit softening: the potential roles of polyuronides and hemicelluloses, J. Food Sci. 49 (1984) 1310 – 1315.
[54] K.C. Gross, A.E. Watada, M.S. Kang, S.D. Kim, K.S. Kim, S.W. Lee, Biochemical changes associated with the ripening of hot pepper, Physiol. Plant. 66 (1986) 31 – 36. [55] Z. Chen, J. Malamy, J. Henning, U. Conrath, P. Sanchez-Casas, H. Silva, J. Ricigliano, D.F. Klessig, Induction, modification and transduction of the salicylic acid signal in plant defense responses, Proc. Natl. Acad. Sci. USA 92 (1995) 4134 – 4137.
[56] X. Fan, J.P. Mattheis, J.K. Fellman, Inhibition of apple fruit 1-amino cyclopropane-1-carboxylic acid oxidase ac-tivity and respiration by acetyl salicylic acid, J. Plant Physiol. 149 (1998) 469 – 471.
[57] N.E. Hoffman, S.F. Yang, Changes of 1-amino cyclo-propane 1-carboxylic acid content in ripening fruits in relation to ethylene production rate, J. Am. Soc. Hortic. Sci. 105 (1980) 492 – 495.