Analysis of habituated embryogenic lines in
Asparagus officinalis
L.: growth characteristics, hormone content and ploidy level of
calli and regenerated plants
Anne Limanton-Grevet
a,b,c, Bruno Sotta
d, Spencer Brown
e, Marc Jullien
a,*
aUMR INRA/INA P-G Biologie des Semences,INRA Versailles,Route de Saint-Cyr,78026Versailles Cedex,France bLaboratoire‘in6itro’,J. Marionnet GFA,Route de Courmemin,41230Soings-en-Sologne,France
cAsparagus b6,Postbus6219,5960AE Horst,France
dLaboratoire de Physiologie du De´6eloppement des Plantes,UMR de Physiologie Cellulaire et Mole´culaire des Plantes, Uni6ersite´ Pierre et Marie Curie(P VI),Tour53,4place Jussieu,75252Paris Cedex05,France
eCytome´trie,Institut des Sciences Ve´ge´tales,CNRS,91198Gif-sur-Y6ette,France
Received 23 March 2000; received in revised form 26 July 2000; accepted 31 July 2000
Abstract
Habituated asparagus embryogenic lines derived from eleven genotypes were maintained on hormone-free medium and grew actively through secondary embryogenesis. Secondary embryos were of single cell origin and emerged from the transversal division of some epidermal or subepidermal cotyledonary cells of primary embryos. The intensity of secondary embryogenesis was found to be variable between embryogenic lines. Plants regenerated from three of these lines have been previously demonstrated to carry a mutation whose phenotype was the direct appearance of somatic embryos on apices or nodes cultured on hormone-free medium. Habituated lines of embryogenic calli and various tissues of embryogenic mutant and wild type plants were analysed for their hormonal content in ABA, IAA, iP, Z and their metabolites ABA-GE, iPA, iMP, ZR. No significant difference was found between different embryogenic lines, except the level of iPA, or between cladophyll or apex cultures of mutant and wild type plants. Flow cytometry analyses indicated only 34% of the embryogenic lines were diploid, most of the others being tetraploid, but 62% of regenerated plants from these lines were diploid. This indicated the process of maturation and conversion selected diploid embryos in the embryogenic lines. © 2000 Elsevier Science Ireland Ltd. All rights reserved.
Keywords:Asparagus officinalisL.; Somatic embryogenesis; Habituation; Mutation; Hormone; Flow cytometry; Ploidy level
www.elsevier.com/locate/plantsci
1. Introduction
Asparagus has a low multiplication rate using conventional methods [1,2]. In vitro clonal propa-gation using shoot multiplication from cultured meristems and shoot tips leads to higher efficiency but is very labour intensive [3]. Somatic embryos could be the most efficient method for clonal micropropagation of plants [4] and thus could also
be useful for asexual multiplication of asparagus. Different procedures for somatic embryogenesis have been described in Asparagus officinalis from different types of explants such as hypocotyls [5], terminal buds [6 – 8], stems [9,10], crowns of seedlings [10], cladophylls [11] and mesophyll cell culture [12]. In most cases, embryogenic calli were induced and maintained on a medium containing growth regulators, especially auxins and cy-tokinins. Then, they had to be transferred on a medium with reduced hormone concentration to express their embryogenic potential, allowing em-bryo development and plant conversion. Jullien
* Corresponding author. Tel.: +33-1-3083-3074/ + 33-1-4408-1831; fax: +33-1-3083-3099.
E-mail address:[email protected] (M. Jullien).
[12] and Delbreil et al. [13] described the produc-tion of long-term habituated embryogenic lines (H lines) growing through repeated secondary em-bryogenesis on hormone-free medium with a very high potential for plant multiplication which can be used for genetic transformation of asparagus [14]. Plants regenerated from H lines exhibited an increased embryogenic capacity compared to the control plants. For three of these lines, the high embryogenic capacity was transmitted to the progeny, following a Mendelian pattern, providing evidence for a dominant monogenic mutation that improved somatic embryogenesis [15,16].
An essential aspect of in vitro plant recovery is the conformity of regenerated plants. In the case of somatic embryogenesis in asparagus, little at-tention was paid to somaclonal variations with only a few exceptions concerning variations in ploidy levels [17 – 20]. As synthetic auxins have often been considered as largely responsible for polyploidisation [21], H lines maintained on hor-mone-free medium could give a material less sub-ject to ploidy level changes than embryogenic lines maintained in the presence of auxin, as usually used for asparagus [8 – 10,17 – 20]. The indication of the mutational origin of H lines cited above was another example of somaclonal variation and questioned about the function of corresponding genes. Genes implicated in the embryogenic capac-ity have been identified in alfalfa [22], in maize [23,24] and in Dactylis glomerata [25], but their function has not been defined. A relation between embryogenic capacity and hormonal metabolism has been found in alfalfa [26] and wheat [27] where the embryogenic capacity could be regulated by the ratio of abscisic acid/IAA.
The aim of this study was to characterize several H lines (including two previously demonstrated mutant lines) for future plant production. Growth modalities of the lines were compared by weight measurements, sieving of the cultures for embryo size and cytological analysis of growing tissues. Second, the intensity of secondary embryogenesis of various lines was tentatively related to their hormonal content and was compared to the hor-monal composition in vegetative tissue and culti-vated apices of the embryogenic mutant and wild type plants. Third, the ploidy level and its stability in embryogenic calli and regenerated plants were examined by flow cytometry to define long term usable H lines.
2. Materials and methods
2.1. Plant material
The habituated embryogenic lines (H lines) used were derived from eleven genotypes: two female clones (CO1 and CO3) and four male clones (8, 186, DDNO5 and JMal) provided by J. Marionnet GFA (Soings en Sologne, France); two female clones (A1 and A2), two male clones (A3 and A4) provided by Asparagus bv (Horst, Holland) and 81A a F1 hybrid provided by INRA (Versailles, France). The H lines derived from these genotypes are indicated in the text and named as following: for instance A2L2 was the H line n° 2 obtained from the genotype A2. The H lines 8L1 [15], A3L3 and A4L1 [16] carry a dominant ‘high embryo-genic’ mutation that enables vegetative tissue to develop somatic embryos when cultivated on hor-mone free medium.
2.2. Cultures of habituated embryogenic lines
Isolation of H lines was described previously [13,16]. Briefly, shoot apices dissected from adult plants were cultured 1 month on MSN (basal medium) containing naphthaleneacetic acid (NAA) 10 mg l−1then were subcultured on basal medium for development of H lines. Basal medium consisted of Murashige and Skoog macronutrients [28], Nitsch micronutrients [29], Nitsch and Nitsch vitamins [30], 2% sucrose and 0.7% agar (Biomar). The pH was adjusted to 5.7 before autoclaving. Most of the H lines were maintained 2 years by subculturing every month on basal medium. For subcultures, embryogenic callus (0.1 g) containing mainly elongated and mature embryos coming from a precedent culture were plated on 3MM paper (Whatman) and cul-tured on 20 ml basal medium in a Petri dish. For recovery of H lines without an auxin treatment, shoot apices or nodes of diploid regenerated plants A4L1 and 81AL2 were cultured on basal medium. All cultures were put in a growth chamber with 16 h per day fluorescent light providing 40 – 70 mmol
mm fraction contained globular embryos, the 0.4 – 0.8 fraction globular and bipolar embryos, the 0.8 – 1.6 mm fraction bipolar elongated embryos and the 1.6 mm and over fraction mature embryos with a chlorophyllous cotyledon. Four repetitions corresponding to four Petri dishes were made.
2.3. Embryo con6ersion
For plant recovery, 0.1 g callus from H lines was plated in Petri dishes on basal medium con-taining 36 g l−1 maltose and solidified with 10 g l−1Phytagel [8]. After 1 month of culture, mature embryos were transferred to glass pots containing 30 ml of germination medium: MSN solidified with 2 g l−1 Phytagel. Later (1 month), plantlets were transferred to test tubes containing 20 ml of the same medium where they developed during 2 months. Plantlets were then transferred to the greenhouse.
2.4. Histological analysis
Embryos were fixed overnight in a 0.2% glu-taraldehyde, 0.4% formaldehyde solution, rinsed three times in water and dehydrated in successive ethanol solutions from 10 to 100%. They were embedded in Technovit 7100 and cut at 3 – 4 mm.
Sections were stained with toluidine blue.
Scanning electron microscopy was carried out using a Phillips 625M microscope. Samples were fixed by cryodesiccation in a Cryostans System CT 1500 or dehydrated by critical point method after glutaraldehyde fixation and ethanol dehydration.
2.5. Hormone analysis
2.5.1. Plant material
All plants and calli used for hormone analyses were diploid. The hormonal content of 8 H lines 1 year old was analysed in callus samples collected 2 weeks after subculture. Plants regenerated from the mutant H line A4L1 and wild type plants from the clone A4 were compared for their hormonal content in different tissues. Cladophylls of mutant and wild type plants were collected on stems at the end of growth. One to seven mutant and wild type plants were analysed. Buds of young spears (15 – 20 cm high) collected on mutant or wild type plants 1 year old, grown in greenhouses, were dissected and cultured on basal medium for 0, 7, 14 or 25 days.
Samples were frozen in liquid nitrogen, lyophilised and stored 1 month at room tempera-ture in a desiccator. Before extraction each sample was ground with a ball mill.
2.5.2. Extraction, purification and fractionation Extractions were performed at 4°C in darkness for 60 h from about 40 mg of tissue powder in 5 ml of 80% aqueous methanol supplemented with BHT (butylhydroxytoluen) 40 mg l−1 as antioxi-dant. 3H-ABA and 3H-IAA were added to the extracts to measure extraction efficiency. A prefil-ter (0.2 mm) connected to a Sep-Pak cartridge was
equilibrated with 10 ml of 80% aqueous methanol before sample loading. Eluates were reduced by rotary evaporation and taken up with 0.2% formic acid up to 500 ml and injected into a C18
(Macheray-Nagel) liquid chromatography (HPLC) column. Elution was performed at 0.8 ml min−1 with a HPLC (System gold, Beckman) with a 0.2% formic acid/methanol gradient. Retention time of ABA (abscisic acid), ABA-GE (abscisic acid glu-cose ester), IAA (indoleacetic acid), iP (isopen-tenyladenine), iMP (isopentenyladenosine monophosphate), iPA (isopentenyladenosine), Z (zeatin) and ZR (zeatin-9-riboside) were deter-mined by separate injection of pure compounds (Sigma) as standards. A total of 40 fractions of 0.8 ml were collected. They were evaporated to dry-ness in a speed-vac concentrator, methylated with 250 ml of diazomethane in ether, evaporated again to dryness and finally taken up with 1.5 ml dis-tilled water with 0.2 g l−1 NaN
3 as preservative. Aliquots (50 ml) of fractions were submitted to
scintillation counting in order to determine ABA and IAA recovery, or to enzyme-linked immuno-sorbent assay (ELISA).
2.5.3. ELISA procedure
The whole procedure was described by Julliard et al. [31]. ABA and ABA-GE were measured using anti-ABA monoclonal antibodies (LPDP 229), IAA using anti-IAA polyclonal antibodies (LPDP 47), iP, iPA and iMP using anti-iPA poly-clonal antibodies (LPDP 5), and ZR and Z using anti-ZR polyclonal antibodies (LPDP 17). Microt-itration plates were coated with ABA, IAA, iPA or ZR conjugated to ovalbumin. After washing the plates five times, 50ml of the fractions or 50ml
added followed by 50ml of anti-hormone antibody
solution. Plates were incubated 2 h at 4°C in darkness. After washing, anti-hormone antibodies bound to the plates were quantified by means of an anti-mouse antibody for ABA (Sigma) and anti-rabbit antibodies for IAA, iPA and ZR linked to a peroxidase system (Sigma). The peroxidase substrate (ABTS: 2,2%-azino-bis
(3-ethylbenzthaz-oline-6-sulfonic acid)), diluted in a perborate buffer, was added and optical density was mea-sured at 405 nm. The measures were repeated 5 times for each sample.
2.5.4. Statistical analysis
Hormone levels in H lines and in explants from embryogenic and wild type plants were analysed using a Fisher test atP=0.05 or a Student test at P=0.05.
2.6. Ploidy le6el analysis through flow cytometry
Ploidy level analyses were conducted with H lines calli and regenerated plants. Callus samples were generally taken from H lines 1 month after subculture, corresponding to the end of the growth phase. For asparagus plants, aerial parts including stem fragments and cladophylls were used. About 0.1 g of fresh matter was chopped with a razor blade in 600 ml Galbraith buffer [32] containing
0.5% Triton X-100 and 0.01 M sodium
metabisulfite, sieved through a 30mm filter. RNase
was added to 10mg ml−1and BET to 50 mg ml−1.
Plants from the eight genotypes were used as
controls, and tomato plants (Lycopersicon esculen-tum) as a reference. For each sample, 5000 nuclei were analysed on a cytometer (EPICS V from Coultronics France) with an Argon laser 400 mW, 514 nm [33]. Samples were considered as diploid when a peak of 2C nuclei was observed and as tetraploid when the peak corresponding to 2C nuclei was absent and a peak of 4C nuclei was observed.
3. Results
3.1. Growth of habituated embryogenic lines
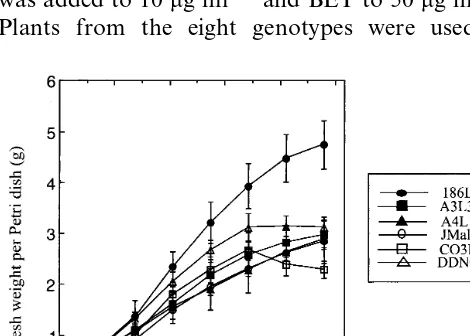
From a total of 40 H line calli isolated from eleven asparagus genotypes [15,16], eight lines from six different genotypes were chosen as repre-sentative of the variation in the intensity of sec-ondary embryogenesis. They grew actively during 20 – 30 days of subculture (Fig. 1). The growth rate then slowed and the stationary phase was reached more or less rapidly depending on the line. De-pending on the line, 2.3 – 4.8 g of fresh matter were produced in 7 weeks per Petri dish (corresponding to an increase of 2300 – 4800%). The line CO3L1 exhibited a decrease of fresh weight after 35 days.
3.2. Intensity of secondary embryogenesis
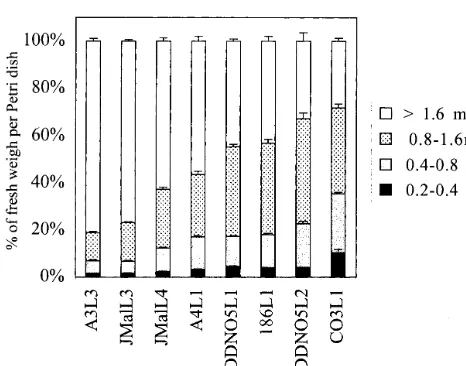
H line calli were sieved through different meshes to evaluate the development of somatic embryos 2 weeks after subculture. The distribution of embryo stages in various H lines differed greatly. The over 1.6 mm fraction containing mature embryos repre-sented 80 – 28% of the total fresh weight of the H lines (Fig. 2). The extreme H lines were A3L3-JMalL3, which were rich in mature embryos and contained few globular embryos, and DDNO5L2-CO3L1, which contained few mature embryos and numerous globular and bipolar embryos. Other lines were intermediary. Different lines derived from the same genotypes (JMal and DDNO5) were similar.
3.3. Embryo setting in habituated embryogenic lines
Mature and elongated somatic embryos begin to produce secondary embryos about 1 week after the subculture. The secondary embryos emerged
Fig. 2. Development stages of somatic embryos in different habituated embryogenic lines. H lines grown on a Petri dish for 2 weeks were sieved on different meshes (0.2, 0.4, 0.8, and 1.6 mm). Four Petri dishes were analysed for each line. \1.6 mm: mature embryos; 0.8 – 1.6 mm: bipolar embryos; 0.4 – 0.8 mm: globular+bipolar embryos; 0.2 – 0.4 mm: globular em-bryos.
mature embryos, like A4L1 (Fig. 2), secondary embryogenesis occasionally occurred on the epi-dermis (Fig. 3F).
3.4. Hormonal content of H lines calli and regenerated plants
The eight H lines examined above have been cultured on hormone-free medium for more than 1 year. The hormonal content of these H line calli exhibiting contrasting intensities of secondary em-bryogenesis was examined 2 weeks after subcul-ture, during active growth (Table 1). The ABA level was highly variable from one line to another and ranged from 220 to 5850 pmol g dry weight−1. IAA levels were more homogenous from one line to another ranging from 207 to 1032 pmol g dry weight−1. The cytokinins iP, iPA and iMP were in most of the samples below the detec-tion level. No significant difference in Z and ZR content was observed. CO3L1 was the only H line presenting a significant difference in hormone (iPA) content with the other lines. This line showed extremely active secondary embryogenesis as illustrated in Fig. 3G and contained a very high proportion of globular embryos (Fig. 2).
The mutant H line A4L1 was selected from the genotype A4. Analyses were conducted on adult mutant plants and adult plants of the male clone A4 as the wild type control. Freshly harvested cladophylls and meristems dissected from young spears and cultured on basal medium for 0, 7, 14 or 25 days were analysed. Hormone levels in the cladophylls varied widely between plants, but hor-mone levels in cladophylls from mutant and wild type plants were not significantly different accord-ing to Fisher’s test (not shown). No significant differences in hormone content appeared between shoot meristems of wild-type and mutant plants cultivated on hormone-free medium for 0, 14 or 25 days, (not shown) a time corresponding with the emergence of somatic embryos on the basal part of the cultured apices of the high embryogenic mu-tant [16].
3.5. Ploidy le6el of H lines and regenerated plants
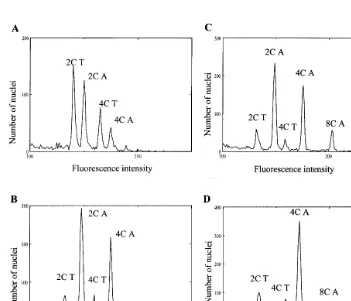
We analysed 32 H lines coming from eight different genotypes maintained on hormone-free medium. The lines were about 12 months old. Fig. 5 illustrates the distribution of fluorescence of BET-stained nuclei in a diploid control plant (A) from epidermal and subepidermal cotyledonary
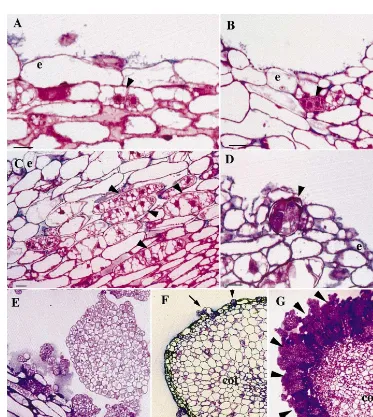
layers, where large cells presented a transversal division (Fig. 3A, Fig. 4A), which appeared to be the first step of secondary embryogenesis. Sec-ondary embryos were therefore of single cell origin. A second division of one or of the two cells led to the formation of a three-celled or a four-celled pro-embryo (Fig. 3B, Fig. 4A). After
subse-quent divisions, globular embryos were
recognisable (Fig. 3D – E, Fig. 4B – C). Not all cells presenting divisions produced a somatic embryo. In particular, numerous cells in deeper cotyle-donary cell layers presented transversal divisions (Fig. 3C), while globular embryos were only visi-ble in the superficial layers (Fig. 3E – F). The emer-gence of secondary embryos was responsible for the disorganisation of the epidermis when sec-ondary embryogenesis was very intensive (Fig. 3G, Fig. 4D). Later, secondary globular embryos were detached from the primary embryos (Fig. 3E, G). No tissue connection related secondary embryos to the original embryos (Fig. 3E). Secondary em-bryogenesis was not synchronised (Fig. 4C).
and in the calli of diploid H lines (B, C), and a tetraploid H line (D). Among 32 lines, 35% were diploid, 59% tetraploid, and 3% hexaploid or ane-uploid (Table 2). To evaluate the possibility of ploidy level changes during subculture on hor-mone-free medium, ten diploid H lines maintained on hormone-free medium were analysed at differ-ent ages during another year. After 9 months, one diploid line had become tetraploid and after 20 months, two others had become tetraploid (that is
30% of tetraploid to compare to the 59% of te-traploid detected 1 year earlier).
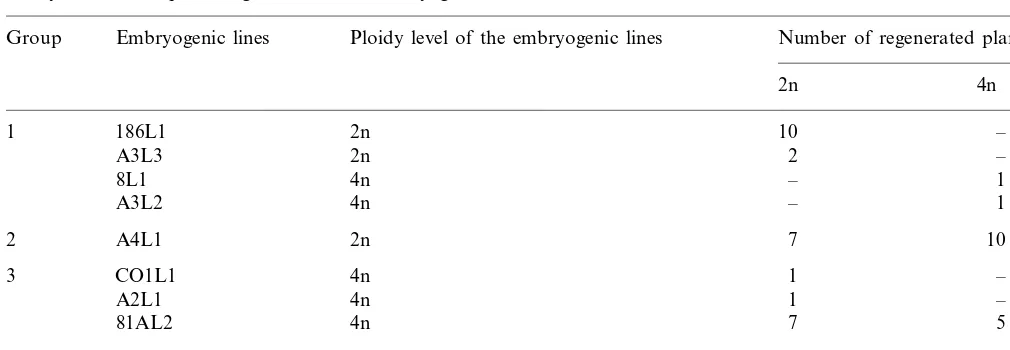
A total of 45 plants regenerated from eight of the previously examined 1-year-old H lines were analysed and classified into three groups (Table 3) depending on the ploidy level of regenerated plants. In group 1 the plants showed the same ploidy level as the corresponding H line. In partic-ular, the diploid H line 186L1 produced only diploid plants. In group 2 some of the plants had
Fig. 3. Cytological analysis of secondary embryogenesis. Longitudinal sections of somatic embryos from the H lines A4L1 (A – F) and CO3L1 (G) 7 (A – D) and 18 days (E – G) after subculture on basal medium. (A) First transversal division of a subepidermal cell in the cotyledon of a primary embryo (arrow). e: epidermis. Bar: 10 mm. (B) Four-celled pro-embryo that arise from
transversal divisions of a subepidermal cell (arrow). e: epidermis. Bar: 10mm. (C) Reactivated cells in the 7th – 12th cell layers from
the epidermis of the cotyledon (arrows). e epidermis. Bar: 10mm. (D) Globular embryo emerging from the disrupted epidermis
of a primary embryo (arrow). e: epidermis. Bar: 10mm. (E) A group of globular embryos emerging from the primary embryo. Bar:
10mm. (F) Secondary embryos (arrows) emerging from the superficial layer of the cotyledon (cot) of a somatic embryo in A4L1
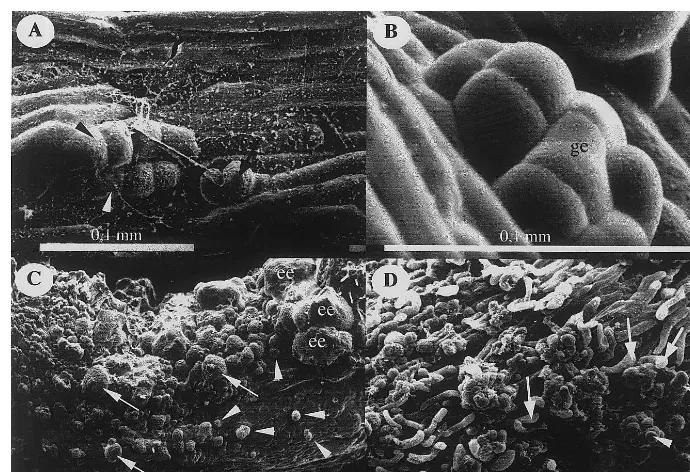
Fig. 4. Scanning electron microscopy of secondary embryogenesis. (A) Two (arrow) and four celled proembryos (black and white arrowheads) appeared on epidermis of a primary embryo from the H line A4L1 7 days after subculture. (B) Globular embryo (ge) emerging through the epidermis of a primary embryo from the H line A4L1, 7 days after subculture. (C) Intensive emergence of secondary embryos on a primary embryo from the H line CO3L1, 12 days after subculture. The development of secondary embryos was nonsynchronous. Very young globular embryos (arrows), post globular embryos (arrowheads) and elongated embryos (ee) can be distinguished. (D) Complete disorganisation of epidermis following proliferation of superficial cells of a primary embryo from the embryogenic line CO3L1, 12 days after subculture. Clusters of globular somatic embryos (arrowheads) emerged through dislocated epidermis cells (arrows).
Table 1
Hormone content in embryogenic lines derived from six asparagus genotypesa
ABA IAA iMP iP iPA Z ZR
Embryogenic line ABA-GE
3165 950 0
A3L3 65 0 1.5 37 25
116 207 9
A4L1 6 1 4 24 5
5850 407 10 0
91 0
JMalL3 33 82
487 414 11 0
JMalL4 24 0 54 8
2015 332 50 3
60 0.5
186L1 31 13
2700 338 93 0.5
DDNO5L1 0 5 32 3
2761 442 67 0
27 2
DDNO5L2 10 6
220
CO3L1 23 1032 55 2 21b 20 1
aTwo callus samples were analysed for each H line; values are given in pmol g−1DW. ABA, abscisic acid; ABA-GE, abscisic
acid glucose ester; IAA, indoleacetic acid; iP, isopentenyladenine; iMP, isopentenyladenosine monophosphate; iPA, isopenteny-ladenosine; Z, zeatin; and ZR, zeatin-9-riboside.
bA significant difference with the other embryogenic lines was detected atP=0.05.
a higher ploidy level than the corresponding H line. Typically, the H line A4L1 was diploid, and the plants regenerated were diploid or tetraploid. In group 3 some of the plants showed a lower ploidy level than the H line, for instance the tetraploid H line 81AL2. Existence of group 2 and 3 suggests some H lines contained a mixture of diploid and tetraploid cells.
3.6. Ploidy le6el of H lines re-isolated from the
regenerated plants without auxin treatment
apices of diploid regenerated plants. We used A4L1 and 81AL2 regenerants. A total of 91% of the new H lines were found to be diploid, and 9% were found to be tetraploid (Table 4). The propor-tion of diploid H lines obtained here was very much higher than after induction in presence of auxin (34%) (Table 1). This confirmed the strong implication of the auxin in polyploidisation. How-ever, it also proved that ploidy changes could occur in the absence of exogenous hormone, al-though they were less frequent.
4. Discussion
We examined the main characteristics of habitu-ated embryogenic lines isolhabitu-ated from eleven geno-types of asparagus. Cytological analyses done on some of the H lines revealed that they grew through secondary embryogenesis. Secondary em-bryogenesis was direct and began with two succes-sive transversal divisions of an epidermal or subepidermal cotyledonary cell leading to the for-mation of a linear four-celled proembryo (Figs. 3
Fig. 5. Typical DNA histograms of BET-stained nuclei extracted from embryogenic lines. Nuclei from a tomato leaf were used as a DNA standard. (A) DNA histogram from cladophylls of a diploid A4 asparagus plant. (B) DNA histogram of the diploid H line 186L1. (C) DNA histogram of the diploid H line A4L1. (D) DNA histogram of the tetraploid H line 81AL2. 2C T, 4C T: peak of 2C and 4C tomato nuclei. 2C A, 4C A, 8C A: peak of 2C, 4C and 8C asparagus nuclei.
Table 2
Ploidy level of embryogenic lines determined by flow cytometrya
Ploidy level Total
4n
2n 6n Aneuploid
Number of lines 11 (34%) 19 (59%) 1 (3%) 1 (3%) 32
Table 3
Ploidy level of the plants regenerated from embryogenic linesa
Ploidy level of the embryogenic lines
Group Embryogenic lines Number of regenerated plants
2n 4n
aH lines are classified in three groups. Group 1: regenerated plants showed the same ploidy level as the callus line. Group 2:
some of the regenerated plants showed a higher ploidy level than the callus line. Group 3: some of the regenerated plants showed a smaller ploidy level than the callus line.
and 4). Similar linear four-celled proembryos were also observed in Freesia refracta embryos cultures after two successive periclinal divisions of subepi-dermal cells [34]. Globular embryos developed then rapidly from the primary embryo, disrupting the epidermis. In some of the H lines, the emergence of secondary embryos was only occasional on the surface of primary embryos (Fig. 3F). In other H lines (CO3L1 for example), which exhibited an extreme intensity of secondary embryogenesis, sub-cultured embryos became rapidly covered by a palisade of globular embryos, resulting in the com-plete disorganisation of the epidermis (Fig. 3G, Fig. 4D). In this case H lines presented a high ratio of globular and bipolar embryos to mature embryos (Fig. 2). These lines were generally difficult to convert into whole plants contrary to the less active H lines. Whatever the intensity of secondary em-bryogenesis of the lines, their growth kinetics were about the same but the extreme H line CO3L1 displayed hastened senescence (Fig. 1).
Most of the habituated embryogenic lines here depicted can regenerate whole plants through so-matic embryo conversion and the derived plants showed a high embryogenic character, i.e. apices cultures regenerated somatic embryos then H lines without any auxin treatment [15]. For the H lines 8L1, A3L3 and A4L1, this character was governed by a dominant mutation [15,16]. Consequently, habituation which is a remarkable characteristic of the embryogenic lines described here is closely related to the embryogenic mutation. Habituation
has been defined as a heritable loss in the require-ments of cultured plant cells for exogenous growth hormones, which can originate from epigenetic as well as genetic changes [35]. It could be attributed to an increased biosynthesis of the growth sub-stances, a decrease in their rate of degradation, an altered sensitivity of the cells to the growth sub-stances or an interaction of some or all of these [35,36]. Comparing the hormonal composition of H line calli, no correlation between intensity of sec-ondary embryogenesis and hormone levels could be established, except in term of cytokinin because the level of iPA was significantly increased in the extreme H line CO3L1. The ribosides of cytokinin are very active in bioassays [37]. The abundance of
Table 4
Ploidy level of habituated embryogenic lines reisolated from diploid high embryogenic plants, without hormone treatmenta
Number of embryogenic lines
aNodes or apices from diploid plants regenerated from the
iPA could therefore explain the very high mitotic activity of line CO3L1 illustrated in Fig. 3G. The high level of iPA was correlated with normal level of cytokinin bases and ribotides (Table 1). Refer-ring to cytokinin pathways [37], this could indicate a normal interconversion metabolism and allows postulating a defect in the oxidation side chain cleavage by cytokinin oxidase.
No significant differences in hormone content between cladophylls of wild type and cladophylls of mutant plants could be identified. This is not very surprising, as mutant plants were morpholog-ically indistinguishable from wild type plant. In the same way, no significant hormonal differences appeared between mutant and wild type meristems cultured for 0, 7, 14 or 25 days when somatic embryos began to appear on the A4L1 mutant explants. Consequently, the expression of the mu-tation was not correlated with a notable modifica-tion of the hormonal economy of the explanted apices. Because of difficulties in embryo conver-sion the mutant status of the H line CO3L1 has not been confirmed even if probable. Because of the different hormonal status of this H line in comparison with the others we can infer that the habituated and embryogenic character have prob-ably different origin (and could be controlled by different mutations?) affecting either hormonal metabolism or sensitivity. Rare attempts have been made to associate the embryogenic compe-tence with endogenous hormone levels. In Medicago falcata the embryogenic capacity was positively correlated with IAA level and negatively with ABA level but not with cytokinin level [26]. In wheat, the embryogenic capacity was related to a low IAA and a low ABA content and increased cytokinin level [27]. In Dactylis glomerata no sig-nificant differences were found in the endogenous IAA levels of embryogenic and non-embryogenic genotypes but cytokinin decreased in the latter [38]. Therefore the relation between embryogenic capacity and hormone content is very dependent on the species. In asparagus, the embryogenic mutation A4L1 seemed not to be related to hor-mone metabolism.
Habituated embryogenic lines isolated after NAA treatment were only 34% diploid 1 year later, after 11 subcultures on basal medium, the others being tetraploid. The polyploidisation pro-cess clearly slow down during the following year of subculture. The ploidy level of plants
regener-ated from the 1 year old H lines was found variable and not always correlated with the ploidy level of the corresponding H lines (Table 3). In case of the diploid A4L1 line which regenerated a high proportion of tetraploid plant it is clear from its DNA histogram of BET-stained nuclei (Fig. 5C) that the tissues contained a significant ratio of tetraploid nuclei. That tetraploid lines regenerated frequently diploid plants (Table 3) was more sur-prising, because generally no peak corresponding to the 2C ploidy level could be detected on the DNA histogram of BET-stained nuclei of the lines (Fig. 5D). Probably a few diploid cells are present in H lines which appeared favoured through the regeneration process as described before in em-bryogenic culture of peal millet [39]. However, the ploidy level of regenerated plants was generally unpredictable only considering the ploidy level of the corresponding H line, except for the H line 186L1, which seemed homogeneously diploid. One way to stabilise the diploid level of H lines could be to reinitiate them from apices or nodes of regenerated plants on auxin free medium. In this last case, more than 90% of the new H lines were diploid (Table 4) in comparison with the 34% obtained after NAA induction of the H lines. Reports on ploidy level in asparagus plants regen-erated from somatic embryos are conflicting in literature, varying from diploid [17,19], tetraploid [18] to a mixture of diploid and tetraploid plants [20]. All the regenerated plants studied came from embryogenic lines generally obtained through a visual selection on an auxin-containing medium. It has been shown [16] this selection process allowed in fact the obtaining of H lines and of auxin-de-pendent embryogenic lines. The diversity of the ploidy status of the H lines here presented proba-bly explained the diversity of results reported in literature. Obtaining exclusively diploid plants through somatic embryogenesis in asparagus need probably the use of stable diploid embryogenic lines, which appear rather rare.
Acknowledgements
References
[1] J.H. Ellison, Asparagus breeding, in: M.J. Bassett (Ed.), Breeding Vegetable Crops, Avi Publishing Company, Westport, CT, 1986, pp. 521 – 568.
[2] H.J. Yang, M.J. Clore, Rapid vegetative propagation of asparagus through lateral bud culture, HortScience 41 (1973) 141 – 143.
[3] C. Dore´, La multiplication clonale de l’asperge (Aspara
-gus officinalisL.) par culture in vitro: son utilisation en se´lection, Ann. Ame´lior. Plantes 25 (1975) 201 – 204. [4] C.J.J.M. Raemakers, E. Jacobsen, R.G.F. Visser,
Sec-ondary somatic embryogenesis and applications in plant breeding, Euphytica 81 (1995) 93 – 107.
[5] C. Wilmar, M. Hellendoorn, Growth and morphogene-sis of asparagus cells cultured in vitro, Nature 217 (1968) 369 – 371.
[6] A. Levi, K.C. Sink, Somatic embryogenesis in aspara-gus: the role of explants and growth regulators, Plant Cell Rep. 10 (1991) 71 – 75.
[7] H. Kohmura, S. Chokyu, T. Harada, An effective mi-cropropagation system using embryogenic calli induced from bud clusters inAsparagus officinalisL., J. Jpn. Soc. Hort. Sci. 63 (1994) 51 – 59.
[8] H. Kunitake, T. Nakashima, K. Mori, M. Tanaka, Normalization of asparagus somatic embryogenesis us-ing a maltose-containus-ing medium, J. Plant Physiol. 150 (1997) 458 – 461.
[9] G. Reuther, Adventitious organ formation and somatic embryogenesis in callus of asparagus and iris and its possible application, Acta Hort. 78 (1977) 217 – 224. [10] T. Saito, S. Nishizawa, S. Nishimura, Improved culture
conditions for somatic embryogenesis from Asparagus officinalisL. using an aseptic ventilative filter, Plant Cell Rep. 10 (1991) 230 – 234.
[11] H. Harada, Differentiation of shoots, roots and somatic embryos in asparagus tissue culture, 4e`me re´union sur la se´lection de l’asperge, Versailles (1973) 163 – 170. [12] M. Jullien, La culture in vitro de cellules du tissu foliaire
d’Asparagus officinalis L.: obtention de souches a` em-bryogene`se permanente et re´ge´ne´ration de plantes en-tie`res, C. R. Acad. Sci. D 279 (1974) 747 – 750.
[13] B. Delbreil, I. Goebel-Tourand, D. Lefranc¸ois, M. Jul-lien, Isolation and characterization of long-term em-bryogenic lines in Asparagus officinalis L., J. Plant Physiol. 144 (1994) 194 – 200.
[14] B. Delbreil, P. Guerche, M. Jullien,Agrobacterium -me-diated transformation of Asparagus officinalis L. long-term embryogenic callus and regeneration of transgenic plants, Plant Cell Rep. 12 (1993) 129 – 132.
[15] B. Delbreil, M. Jullien, Evidence for in vitro induced mutation which improves somatic embryogenesis inAs
-paragus officinalisL., Plant Cell Rep. 13 (1994) 372 – 376. [16] A. Limanton-Grevet, M. Jullien, Somatic embryogenesis in Asparagus officinalis can be an in vitro selection process leading to habituated and 2,4-D dependent em-bryogenic lines, Plant Physiol. Biochem. 38 (2000) 1 – 11. [17] H. Kunitake, M. Mii, Somatic embryogenesis and plant regeneration from protoplasts of asparagus (Asparagus officinalisL.), Plant Cell Rep. 8 (1990) 706 – 710.
[18] Y. Odake, A. Udagawa, H. Saga, M. Mii, Somatic embryogenesis of tetraploid plants from internodal seg-ments of a diploid cultivar of Asparagus officinalis L. grown in liquid culture, Plant Sci. 94 (1993) 173 – 177. [19] S. Mukhopadhyay, Y. Desjardins, Plant regeneration
from protoplast-derived somatic embryos of Asparagus officinalis L., J. Plant Physiol. 144 (1994) 94 – 99. [20] R.A. May, K.C. Sink, Genotype and auxin influence
direct somatic embryogenesis from protoplasts derived from embryogenic cell suspensions of Asparagus offici
-nalisL., Plant Sci. 108 (1995) 71 – 84.
[21] H.J. Swartz, Post culture behaviour: genetic and epige-netic effects and related problems, in: P.C. Debergh, R.H. Zimmerman (Eds.), Micropropagation Techniques and Applications, Kluwer Academic Publishers, The Netherlands, 1991, pp. 95 – 121.
[22] G.A. Kielly, S.R. Bowley, Genetic control of somatic embryogenesis in alfalfa, Genome 35 (1992) 475 – 477. [23] T.K. Hodges, K.K. Kamo, C.W. Imbrie, M.R. Becwar,
Genotype specificity of somatic embryogenesis and re-generation in maize, Bio/Technology 4 (1986) 219 – 223. [24] C.L. Armstrong, J. Romere-Severson, T.K. Hodges, Im-proved tissue culture response of an elite maize inbred through backcross breeding, and identification of chro-mosomal regions important for regeneration by RFLP analysis, Theor. Appl. Genet. 84 (1992) 755 – 762. [25] A.L. Gavin, B.V. Conger, R.N. Trigiano, Sexual
trans-mission of somatic embryogenesis inDactylis glomerata, Plant Breed. 103 (1989) 251 – 254.
[26] A. Ivanovna, M. Velcheva, P. Denchev, A. Atanassov, H.A. Van Onckelen, Endogenous hormone levels during direct somatic embryogenesis inMedicago falcata, Phys-iol. Plant. 92 (1994) 85 – 89.
[27] J.R. Hess, J.G. Carman, Embryogenic competence of immature wheat embryos: genotype, donor plant, envi-ronment, and endogenous hormone levels, Crop Sci. 38 (1998) 249 – 253.
[28] T. Murashige, F. Skoog, A revised medium for rapid growth and bioassays with tobacco tissue culture, Phys-iol. Plant. 15 (1962) 473 – 497.
[29] J.P. Nitsch, Induction in vitro de la floraison chez une plante de jours courts: Plumbago indica L., Ann. Soc. Bot. 9 (1968) 1 – 92.
[30] J.P. Nitsch, C. Nitsch, Ne´oformation de fleurs in vitro chez une espe`ce de jours courts: Plumbago indica L., Ann. Physiol. Ve´g. 7 (1965) 251 – 256.
[31] J. Julliard, R. Maldiney, B. Sotta, E. Miginiac, L. Ker-hoas, J. Einhorn, HPLC-ELISA and MS as complemen-tary techniques to study plant hormone metabolites, Analysis 22 (1994) 483 – 489.
[32] D.W. Galbraith, K.R. Harkins, J.M. Maddox, N.M. Ayres, D.P. Sharma, E. Firoozabady, Rapid cytometric analysis of the cell cycle in intact plant tissues, Science 220 (1983) 1049 – 1051.
[33] S.C. Brown, C. Bergounioux, S. Tallet, D. Marie, Flow cytometry of nuclei for ploidy and cell cycle analysis, in: I. Negrutiu, G. Gharti-Chhetri (Eds.), A Laboratory Guide for Cellular and Molecular Plant Biotechnology, Basel, 1991.
Seeds, Springer-Verlag, Berlin Heidelberg, 1995, pp. 294 – 305.
[35] F.J. Meins, Habituation: heritable variation in the re-quirement of cultured plant cells for hormones, Ann. Rev. Gen. 23 (1989) 395 – 408.
[36] J.A. Jackson, R.F. Lyndon, Habituation: cultural cu-riosity or developmental determinant?, Physiol. Plant. 79 (1990) 579 – 583.
[37] P.E. Jameson, Cytokinin metabolism and compartmen-tation, in: D.W.S. Mok, M.C. Mok (Eds.), Cytokinins:
Chemistry, Activity and Function, CRC Press, Boca Raton, 1994, p. 113.
[38] A.R. Wenck, B.V. Conger, R.N. Trigiano, C.E. Sams, Inhibition of somatic embryogenesis in orchardgrass by endogenous cytokinins, Plant Physiol. 88 (1988) 990 – 992.
[39] B. Swedlund, I.K. Vasil, Cytogenetic characterisation of embryogenic callus and regenerated plants of Pen
-nisetum americanum, Theor. Appl. Genet. 69 (1985) 575 – 583.