Releasing Hormone in Child Depression
Ronald E. Dahl, Boris Birmaher, Douglas E. Williamson, Lorah Dorn, James Perel,
Joan Kaufman, David A. Brent, David A. Axelson, and Neal D. Ryan
Background:
This study examined growth hormone (GH)
response to growth hormone–releasing hormone (GHRH)
in a large sample of depressed children compared with
normal control children. Within-subject comparisons were
also performed in control subjects to examine test–retest
reliability and in depressed children comparing episode
versus clinical recovery.
Methods:
The sample included depressed children (n
5
82) and normal control children (n
5
55) group-matched
for age, gender, and pubertal status; the mean ages were
11.2
6
1.7 and 11.2
6
1.8 years, respectively. We gave
GHRH (0.1 mcg/Kg) at 9
AM, and serum GH levels were
determined every 15 min from
2
30 min through
1
90 min
of the GHRH infusion. A subgroup of normal control
subjects (n
5
11) repeated the protocol for test–retest
reliability within a 2-month interval. A subgroup of
depressed children (n
5
20) were restudied off all
medi-cations following full clinical remission from depression.
Results:
The mean GH response to GHRH was
signifi-cantly lower in the depressed group (8.7 ng/mL
6
SEM
0.9) compared with normal control children [12.2 ng/
mL
6
SEM 1.3;
t(135)
5
2.59,
p
5
.01 effect size 0.44].
The test–retest reliability of GH response to GHRH was
stable (intraclass correlation
5
.93 for mean post-GH).
The GH response to GHRH remained low in subjects
restudied during clinical remission from depression.
Conclusions:
Depressed children show low GH response
to GHRH. The measure appears to be reliable, and the low
GH response continues following clinical remission.
Fur-ther studies are needed to explore the mechanism and
relative specificity of this finding.
Biol Psychiatry 2000;
48:981–988 ©
2000 Society of Biological Psychiatry
Key Words:
Depression, growth hormone, growth
hor-mone–releasing
hormone,
development,
children,
adolescents
Introduction
P
rogress in understanding affective disorders over the
past decade increasingly has focused attention on
questions of maturational influences on biological systems
of interest. There has been considerable interest in 1) the
effects of early adverse experiences on later expression of
affective dysregulation, 2) the increasing rates of
depres-sion during adolescent development, 3) the emergence of
gender differences in rates of depression during adolescent
development, and 4) changes across childhood,
adoles-cence, adulthood, and geriatrics in specific domains, such
as symptom profile, clinical course, genetic influences,
and treatment response. Broadly speaking, this work has
yielded some areas of maturational continuity, as well as
areas of developmental variation (Angold et al 1998;
Birmaher et al 1996a, 1996b; Garber et al 1993;
Har-rington et al 1994; Kovacs 1996; Puig-Antich et al 1993;
Rao et al 1995; Ryan et al 1987).
This theme of both similarities and differences across
child, adolescent, and adult depression also has been
evident in psychobiologic studies. For example, HPA-axis
alterations
and
electroencephalogram
(EEG)
sleep
changes associated with depression show considerable
maturational variance (Dahl 1996; Dahl et al 1991a,
1991b, 1992, 1994, 1996; Emslie et al 1994). In contrast,
low growth hormone (GH) response to pharmacologic
challenges seen in association with depression has shown
relative continuity across development, including
de-pressed samples ranging from prepubertal children
through old age (Jarrett et al 1990, 1994; Lesch et al 1987,
1989; Meyer et al 1991; Puig-Antich et al 1984c, 1984d;
Ryan et al 1988, 1994; Siever et al 1992).
There is also preliminary evidence that this low GH
persists after clinical recovery (in a medication-free state)
in depressed children (Puig-Antich et al 1984d) and
following treatment for depression in adults (Jarrett et al
1994).
Recently, our research group has found that low GH
response to growth hormone–releasing hormone is also
evident in children and adolescents with no personal
history of depression but with high rates of affective
From the Department of Psychiatry, University of Pittsburgh School of Medicine,Western Psychiatric Institute and Clinic, Pittsburgh, Pennsylvania (RED, BB, DEW, LD, JP, DAB, DAA, NDR) and the Department of Psychiatry, Yale University School of Medicine, New Haven, Connecticut (JK).
Address reprint requests to Ronald E. Dahl, M.D., University of Pittsburgh Medical Center, Western Psychiatric Institute and Clinic, 3811 O’Hara Street, Pitts-burgh PA 15213.
Received January 8, 2000; revised May 1, 2000; accepted May 18, 2000.
© 2000 Society of Biological Psychiatry 0006-3223/00/$20.00
illness in their families (Birmaher et al 2000). These
results have been considered within an emerging model
based on the hypothesis that low GH response to GHRH
represents a stable
trait
marker for affective illness—a
marker evident before the onset of depression, persisting
across episode and recovery, and independent of age and
maturational changes.
The studies reported in this paper address the basic
foundation for further investigations of this model,
focus-ing on three specific aims: 1) to extend earlier findfocus-ings of
low GH response to GHRH by comparing a large sample
of depressed children and well-matched control subjects
with careful assessment of psychiatric and family history
data, 2) to examine the
reliability
of GH responses to
physiologic stimuli (within-subject test–retest reliability),
and 3) to examine GH responses after clinical recovery
within a subset of depressed children studied initially
during a depressive episode.
Methods and Materials
Phase 1 and Phase 2 Studies
The data described in this paper include GH data collected in two similar studies. The research facility, most of the personnel, GH assay procedures, and design of the study up to and including the GHRH test were identical. Phase 1 of the study included a larger number of neuroendocrine tests after the GHRH test; a later phase of the study had a shorter neuroendocrine protocol. The GHRH data from n 5 36 children with major depressive disorder (MDD) n5 18 normal control subjects from phase 1 studies were included in a preliminary report (Ryan et al 1994). All of the phase 2 data are new, and there is no overlap with any previously reported GH data. The methods for the GHRH test measures and protocol were identical across studies, and analyses were performed showing that there was no effect of phase on any of the GH measures being examined, thus the data were com-bined across phase 1 and phase 2 studies to maximize the power to examine covariates and subgroup effects.
Recruitment and Inclusion–Exclusion Criteria
Depressed children were recruited from the inpatient and outpa-tient clinics at Western Psychiatric Institute and Clinic, Univer-sity of Pittsburgh Medical Center. Normal control children were recruited through advertisement and personal contacts. Informed consent to participate in the study was obtained in accordance with the University of Pittsburgh Institutional Review Board guidelines. Inclusion criteria common for all subjects were that they be 7 to 15 years of age and medically healthy. Children in the depressed cohort were required to meet DSM-III-R criteria for MDD. Additional exclusion criteria for the MDD cohorts only included 1) concurrent DSM-III-R diagnosis of anorexia nervosa, bulimia nervosa, autism, schizoaffective disorder, or schizophrenia and 2) MDD chronologically secondary to conduct disorder.
For the normal control subjects only, additional inclusion
criteria included 1) no lifetime history of any psychiatric disorder and 2) low familial risk for affective illness. In this study, low familial risk for affective disorder was operationally defined as having no first-degree relative with a lifetime episode of any affective disorder or schizophrenia spectrum illness; no second-degree relative with a lifetime history of recurrent, bipolar, or psychotic depression, schizoaffective disorder, or schizophrenia; and no more than 20% of second-degree relatives with a single episode of MDD.
Exclusion criteria for both groups were 1) significant medical illnesses, 2) medications (except acetaminophen) within 2 weeks of the study, 3) inordinate fear of needles, 4) obesity (weight greater than 150% of ideal body weight) or severe growth failure (weight or height less than 3% of the National Health Statistic Curve), and 5) mental retardation or the presence of a specific learning disability.
DIAGNOSTIC ASSIGNMENT. Diagnostic assessments of the
depressed cohorts were completed by research assistants who administered the Present Episode (Chambers et al 1985) and Epidemiologic (Orvaschel and Puig-Antich 1987) versions of the semistructured diagnostic interview, the Schedule for Affective Disorders and Schizophrenia for School-Aged Children (K-SADS). The diagnosis of MDD was made using DSM-III-R criteria, with the presence of all positive symptoms confirmed by a child psychiatrist or psychologist. For normal control subjects, if an initial telephone screen of the family indicated preliminary eligibility and interest, the child’s lifetime history of psychiatric symptomatology was assessed using only the K-SADS-E (Or-vaschel and Puig-Antich 1987). All symptom ratings were made by interviewing the parent(s) first, then interviewing the child. All children who participated in the study were reinterviewed using a short version of the K-SADS at the time of the psychobiological studies to confirm presence of depressive symptomatology.
All interviews were carried out by trained research clinicians blind to the subject’s clinical status under the supervision of child psychiatrists (DA, BB) or a child psychologist (JK). Interrater reliability for all diagnoses for our studies has beenk$ .70. Socioeconomic status (SES) was measured using the Hollings-head four-factor index (HollingsHollings-head 1975).
GHRH TEST. Following a visit and orientation to the
labo-ratory, children and family members arrived in the lab to begin the psychobiological study at 5 PM. The sleep–neuroendocrine laboratory is furnished with many age-appropriate materials, including books, art supplies, board games, entertainment videos, and computer games. Children are given considerable individual attention from staff with years of experience conducting biolog-ical studies with children. On a rating scale completed immedi-ately after finishing the psychobiological studies, the majority of children who participated in this and other studies conducted in the lab rated the experience as “very positive” (Dahl and Ryan 1999).
following morning, baseline blood samples for GH were ob-tained through the indwelling catheter at 8:30, 8:45, and 9:00AM. Immediately following the 9:00AMdraw, children received an
infusion of 1.0 mcg/kg of GHRH over 2 min. There were no significant side effects of any sort related to the GHRH test. Children generally showed no signs of behavioral reaction of any kind. A physician was in attendance throughout the procedure. Following GHRH administration, blood samples for GH were obtained every 15 min for a total of 90 min. The child remained fasting from bedtime (of the previous evening) until completion of the GHRH test.
BLOOD SAMPLES AND GH ASSAYS. Blood samples were collected in plastic tubes containing edetic acid (EDTA) then centrifuged immediately in a refrigerated centrifuge. Plasma was separated and stored at 280°C until assayed. Human growth hormone levels were measured on a 50mL plasma sample, run in duplicate. A modified version of the double antibody 125I
radioimmunoassay procedure developed by Diagnostic Products Corporation (Los Angeles) was used for these determinations. To increase the sensitivity of the assay to 0.5 ng/mL of growth hormone from that of the straight kit procedure, the amount of antibody was decreased, and a longer, sequential incubation of the antibody and radiolabeled antigen was used. Subjects’ duplicates with a coefficient of variation exceeding 5.0% were retested. The range for the intraassay variation of subjects’ sample duplicates was 0.00 to 4.98% CV, with a mean of 0.87% CV. Interassay variation ranged from 20.15% CV (mean of 2.47 ng/mL) to 13.68% CV (mean of 12.31 ng/mL).
Statistical Analyses
Sample characteristics were compared using analyses of vari-ance,x2, and Fisher’s exact tests as appropriate. Problem with missing hormonal data were minimal, with only a few subjects missing one or two data points. Analyzing the data with and without linear interpolation to fill in the occasional missing values yielded similar results, therefore only the interpolated data are reported. Before conducting any analyses, summary variables for the hormonal measures and clinical rating scales were examined for normality using the Shapiro and Wilks’W statistic. The data were subject to log or square root transformation where significantly nonnormal distributions were found. In cases where no transformation normalized the data, nonparametric tests were used. The following summary values were used to characterize children’s GH responses: baseline, peak, and mean post-GH. The baseline values were computed by determining the mean of the three GH specimens taken at230,215, and 0 min preinfusion. The peak value represents the highest value postinfusion, and the mean post-GH values were the average of samples from115 through190 min. (The GH levels by 90 min were back to a level that was not significantly different from baseline.) A comparison using total GH, as computed by determining the area under the curve with all GH values obtained from 0 to 90 min post-GH using the trapezoidal rule revealed identical group comparisons; only the mean values are reported here.
Reliability Study
To assess the test–retest reliability of the GHRH test, the protocol was repeated in 11 normal control subjects within 1 to 2 months after the initial study.
Recovery Study
In the clinical follow-up of the depressed sample, subjects were invited to participate in a repeat study when they had recovered from the depressive episode. To be considered recovered, de-pressed children were required to be fully remitted for 8 consecutive weeks (Frank et al 1991) and medication free for at least 8 weeks. At the time of these analyses,n520 subjects had been restudied in the same protocol following remission and had their data compared with the findings from GH data obtained in the depressed state.
Results
There were no significant group differences in age,
gen-der, body mass index, or Tanner stage of sexual
matura-tion (Table 1). The groups were significantly different in
SES [39.2
6
SD 13.9 vs. 47.8
6
SD 11.1;
t(127)
5
3.68,
p
5
.0003].
Because SES was significantly different between MDD
and control groups, this variable was examined in relation
to the summary GH measures and was not found to be a
significant covariate for any of the GH measures. There
were also no significant differences in GH responses
between male and female subjects in any of these
analy-ses; thus, male and females subjects were combined for all
comparisons. Analyses examining pubertal status (Tanner
stage) also showed no significant effect on GH response to
GHRH.
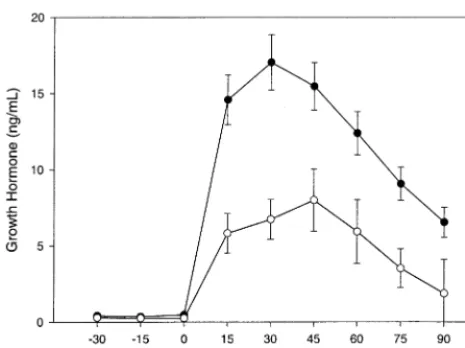
Depressed versus Normal Control Subjects
Baseline GH measures (
2
30
2
15, and 0 min before
GHRH infusion) were not different between groups (0.4
ng/mL
6
SEM 0.05 vs. 0.4
6
0.05). Following infusion of
GHRH, the depressed subjects (n
5
82) secreted
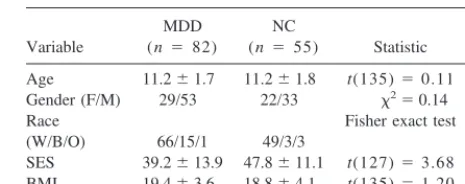
signif-Table 1. Demographic Characteristics in Depressed and Low-Risk Children
Variable
MDD (n 5 82)
NC
(n 5 55) Statistic
p value
Age 11.261.7 11.261.8 t(135)50.11 ns Gender (F/M) 29/53 22/33 x250.14 ns Race Fisher exact test (W/B/O) 66/15/1 49/3/3 .0314 SES 39.2613.9 47.8611.1 t(127)53.68 .0003 BMI 19.463.6 18.864.1 t(135)51.20 ns
icantly less GH than control subjects (n
5
55) as
measured by peak levels [13.9 ng/mL
6
SEM 1.4 vs. 19.5
ng/mL
6
SEM 2.1;
t
(135)
5
2.6,
p
5
.02], and mean
levels postinfusion [8.7 ng/mL
6
SEM 0.9 vs. 12.2
6
1.3;
t(135)
5
2.6,
p
5
.01; Figure 1 and Table 2]. The
effect size for the between-group differences in GH
response based on the entire sample (n
5
82 MDD
subjects;
n
5
55 control subjects) was 0.44; the effect
size when examining only the independent replication
sample (
n
5
46 MDD subjects;
n
5
37 control subjects)
was 0.39.
GH Results in Relation to Severity and Other
Clinical Symptoms
We examined GH response to GHRH in relation to clinical
symptoms, including prior episodes of depression (15% of
the sample had a history of a past episode of depression),
duration of depression (mean duration was 42.4
6
36.8
weeks), severity of depression (mean depression 12 score
from the K-SADS was 31.4
6
5.7), and comorbid
symp-toms of anxiety sympsymp-toms (53% of the depressed sample
had current or past anxiety). None of these analyses
showed any significant effect on GH response to GHRH
within the MDD sample.
Test–Retest Reliability
The GH response to GHRH showed an intraclass
correla-tion (ICC)
5
.89 (peak) and
r
5
.93 (mean post) within
the 11 subjects who underwent repeat testing within 2
months (Figure 2).
GH Responses following Clinical Recovery
On restudy in a medication-free state, GH responses were
not significantly different than their previous values
ob-tained during the episode of MDD; mean GH post-GHRH
was 9.21
6
10.82 versus 8.10
6
7.61 [t
(19)
5
0.36, ns]
and peak GH post-GHRH was 13.40
6
14.78 versus
13.72
6
12.82 [t(19)
5
0.07, ns] in depressed and
remitted states respectively. As shown in Figure 3, the GH
response in the MDD-recovered group (n
5
20)
re-mained significantly lower than the GH response in the
control group.
GH Responses in Relation to
Hypothalmic–Pituitary–Adrenal Axis Measures
The subjects in this study had also undergone
measure-ments of cortisol and corticotropin (baseline and following
corticotropin-releasing
hormone
challenge);
analyses
comparing summary hypothalmic–pituitary–adrenal axis
Figure 1. Growth hormone response to growthhormone–releas-ing hormone (GHRH) in children and adolescents with major depressive disorder (F;n5 82) compared to low-risk normal control subjects (E; n 5 55). Time 0 corresponds to the infusion of GHRH. Values are in ng/mL and represent the means6SEMs.
Figure 2. Growth hormone response to growth hormone–releas-ing hormone in low-risk normal control subjects restudied for test–retest reliability. Values are in ng/mL and represent the means6SEMs.F, time 1 (n 5 11);E, time 2 (n 5 11).
Table 2. Demographic Characteristics and Growth Hormone Response to GHRH and during Baseline in Depressed and Low-Risk Children
Variable
MDD (n 582)
NC
(n 5 55) Statistic p value
GHRH
Baseline 0.460.05 0.460.05 t13550.32 ns
Postinfusion 8.760.9 12.261.3 t13552.59 .0107
Peak postinfusion 13.961.4 19.562.1 t13552.55 .0119
All between-group comparisons on growth hormone were done using log-transformed growth hormone values; reported growth hormone values are means6
measures and GH measures revealed no significant
corre-lations within the MDD or control samples.
Discussion
Because of the challenges in recruitment and performing
biological studies in children, psychobiological studies in
child psychiatry typically entail small clinical and control
samples. There is also an inherent bias toward publishing
studies that have found group differences, compared with
small-sample “negative” studies that are less likely to be
written-up and published. This classic “file-drawer
prob-lem” is particularly concerning in the arena of child
psychiatry.
Large controlled studies and independent replications
are infrequent but essential to moving the field forward.
This article establishes a large, independent replication of
low GH response to pharmacologic stimulation associated
with child depression, which was first reported in
Puig-Antich’s seminal papers (Puig-Antich et al 1984a, 1984b,
1984c, 1984d).
By examining a large sample of depressed children
(n
5
82) selected over a 10-year interval in a
well-controlled study, the data in this article establish strong
support for the hypothesis that low GH response to GHRH
represents a neuroendocrine marker of affective illness in
children. Our study also establishes good test-retest
reli-ability for the measure, and demonstrates continuity of low
GH response to GHRH in depressed children in clinical
remission.
There are also several limitations to these data to be
considered. One issue that has not been addressed is the
specificity
of the finding to depression. It is possible that
low GH is associated more generally with psychiatric or
emotional difficulties in childhood. Few studies have
examined GH responses to pharmacologic challenge in
other child psychiatric disorders. A recent study in
chil-dren with anxiety using a clonidine challenge (Sallee et al
1998) reported
increased
GH responses in the anxious
children compared with control subjects. The sample in
that study also included children with
obsessive-compul-sive disorder, as well as other anxiety disorders.
Further-more, studies in adult samples have generally reported low
GH responses to several types of pharmacologic
chal-lenge, in depression
and
anxiety disorders (Coplan et al
1997; Holsboer 1995; Uhde et al 1986) including
gener-alized anxiety disorder (Abelson et al 1991) social phobia
(Tancer et al 1993), and panic disorder (Charney et al
1987; Coplan et al 1997, in press; Nutt 1989; Rapaport et
al 1989). Many of the pharmacologic challenges in adult
studies, however, (particularly clonidine and yohimbe
challenges) were based on the assumption that researchers
were evaluating the integrity of noradrenergic sensitivity
and that GH was simply a marker.
Focusing more specifically on GHRH stimulation
stud-ies in psychiatry, a critical review by (Skare et al 1994)
discussed four replications showing low GH response to
GHRH in adult depression, as well as two failures to
replicate. These authors summarized the promising results
but raised several concerns, including 1) the variability of
GH response among control subjects, 2) the need to
consider the age and gender of the subjects, 3) the lack of
uniformity of procedures and measures, and 4) absence of
data in adult studies regarding the test–retest reliability
within subjects. It is important to note that among the
controlled studies reviewed in that paper, the sample sizes
ranged from
n
5
7 to
n
5
19 subjects with depression
and the total number of depressed adults is only
n
5
80
(combining all studies). In contrast, our study contained
n
5
82 depressed children and
n
5
55 control subjects.
In addition, we performed reliability tests, examined the
influences of age and gender carefully, and used uniform
procedures based on previous studies.
One study of GHRH in depressed adults (Gann et al
1995) published after the Skare review reported no
differ-ences in GH response to GHRH; however, the sample
contained only
n
5
12 MDD subjects and
n
5
12 control
subjects and reported the GH was 645
6
770 (area under
the curve) in the MDD subjects and 1031
6
279 in the
control subjects. Thus, that “negative” paper actually
found lower GH response to GHRH in the MDD group
with an effect size of 0.67 (comparable to our results) and
likely failed to find significant group differences because
of the small samples.
Effects on Growth
The functional significance of lower GH response
regard-ing growth processes is unclear. There were no significant
differences in height or weight between groups, and
preliminary measures of height velocity were not different
between groups. It is possible that any difference in GH
regulation in response to GHRH challenge is not reflected
in overall GH production or IGF-1 levels, and that it does
not have any functional impact on growth. On the other
hand, these effects may be subtle and below the threshold
of detection over short intervals of time because Pine et al
reported a slight but statistically significant reduction in
expected adult height in the follow-up of children with
anxiety disorders, but only in the females (Pine et al 1996).
Functional Significance and Mechanism of Action
Since our finding is stable following clinical recovery, one
possibility is that this represents some type of early “scar”
marker related to having had a depressive episode;
how-ever, other work by our research group (Birmaher et al
2000) shows evidence of low GH in children at high
familial risk of depression but without any clinical signs of
depression. In that study, the mean GH response in the
nondepressed “high-risk” sample (n
5
64) was 8.6
ng/mL
6
1.1, similar to the MDD sample (
n
5
82) 8.7
ng/mL
6
0.9 with both groups significantly different from
the normal control subjects without family loading for
depression (n
5
55) at 12.2 ng/mL
6
1.3. Taken
together, these data are consistent with a model that low
GH response to GHRH represents a “trait” marker
asso-ciated with the risk or vulnerability toward depression.
Further, the absence of any significant relationship of GH
response to severity of depression or duration of
depres-sion is also consistent with the concept of a trait marker.
Another possibility is that early stress or adverse social
experiences early in life may alter GH regulation
and
confer greater risk of depression. Some animal studies
have found low GH response in monkeys and rodents that
were exposed to maternal separation early in life.
Cham-poux et al 1989 reported low GH responses to
pharmaco-logic challenge in nursery-reared infant rhesus macaques
at 37 days of age compared with mother-reared control
subjects (the nursery-reared monkeys also showed higher
rates of depressive like behaviors later in life (Champoux
et al 1989). Coplan et al (1998), studying bonnet macaques
raised in variable foraging demand (VFD) conditions
(Rosenblum et al 1994), found low GH response to
clonidine infusion in the VFD offspring that showed high
rates of anxious social behaviors and low exploration.
These VFD offspring were also found to have elevated
cerebrospinal fluid somatostatin (GH-inhibiting peptide)
(Coplan et al 1998). Most recently Plotsky’s research
group (Mason et al 1998) found blunted GH response to
clonidine infusion in rodents that had experienced
180-min maternal separations early in life compared with
handled control rats. Taken together, the animal data are
consistent with the concept that low GH responses are
associated with early social stress, anxious behavioral
patterns, or both across several species.
Holsboer, drawing from findings in adult depression
and from animal work, has presented a model whereby the
mechanism of GH changes seen in depression may be
mediated by alterations in central corticotropin-releasing
factor (Holsboer 1995). Coplan et al (in press) reported
results consistent with this link by showing that GH
response to clonidine in the VFD monkeys was
signifi-cantly correlated with corticotropin-releasing factor in the
cerebrospinal fluid of these monkeys.
Clearly, there are several important mechanistic
ques-tions regarding the changes in GH response associated
with depression and possibly with the risk for depression
early in life. Promising preliminary results from a
nonhu-man primate model showing stable individual differences
in GH response to GHRH and clonidine in young monkeys
and a correlation between low GH response and low
behavioral reactivity in a stranger-approach condition (K.
Coleman et al, unpublished data) present one promising
approach to explore the underlying mechanisms.
Summary
Low GH response to GHRH appears to be a trait marker of
depression that is evident and stable early in development.
The mechanism and functional significance of this marker
requires further investigation. Progress in understanding
these findings will likely require both clinical and basic
investigations into the neurobehavioral systems
underpin-ning these alterations in GH regulation, including
devel-opmental and genetic influences and the possible effects of
early adverse experiences.
This work was supported by National Institute of Health Grant Nos. PO1-MH41712 (NDR and colleagues) and KO2-MH01362 (RED). This line of investigation was originally initiated under the direction of the late Joaquim Puig-Antich, to whom this article is dedicated. The authors wish to extend our appreciation to Laura Trubnick and her staff in the Sleep Laboratory, Stacy Stull and the staff of the Neuroendocrine Laboratory, the clinical interviewers, and the children and families who made this study possible.
References
Angold A, Costello EJ, Worthman CM (1998): Puberty and depression: The roles of age, pubertal status and pubertal timing.Psychol Med28:51– 61.
Birmaher B, Dahl RE, Williamson DE, Perel JM, Brent DA, Axelson DA, et al (2000): Growth hormone secretion in children and adolescents at high risk for major depressive disorder.Arch Gen Psychiatry57:867– 872.
Birmaher B, Ryan ND, Williamson DE, Brent DA, Kaufman J (1996a): Childhood and adolescent depression: A review of the past 10 years. Part II.J Am Acad Child Adolesc Psychiatry
35:1575–1583.
Birmaher B, Ryan ND, Williamson DE, Brent DA, Kaufman J, Dahl RE, et al (1996b): Childhood and adolescent depression: A review of the past 10 years. Part I. J Am Acad Child Adolesc Psychiatry35:1427–1439.
Chambers WJ, Puig-Antich J, Hirsch M, Paez P, Ambrosini PJ, Tabrizi MA, Davies M (1985): The assessment of affective disorders in children and adolescents by semistructured inter-view. Test-retest reliability of the schedule for affective disorders and schizophrenia for school-age children, present episode version.Arch Gen Psychiatry42:696 –702.
Champoux MCC, Schanber SM, Kuhn CM, Suomi SJ (1989): Hormonal effects of early rearing conditions in the infant rhesus monkey.Am J Primatol19:111–117.
Charney DS, Woods SW, Goodman WK, Heninger GR (1987): Neurobiological mechanisms of panic anxiety: Biochemical and behavioral correlates of yohimbine-induced panic attacks.
Am J Psychiatry144:1030 –1036.
Coplan JD, Pine DS, Papp LA, Gorman JM (1997): A view on noradrenergic, hypothalamic-pituitary-adrenal axis and extra-hypothalamic corticotrophin-releasing factor function in anx-iety and affective disorders: The reduced growth hormone response to clonidine.Psychopharmacol Bull33:193–204. Coplan JD, Owens MJ, Trost RC, Nemeroff CB, Smith ELP,
Rosenblum LA, et al (in press): Growth hormone response to clonidine in adversely reared primates and inhibitory role of corticotropin-releasing factor.Psychiatry Res.
Coplan JD, Trost RC, Owens MJ, Cooper TB, Gorman JM, Nemeroff CB, Rosenblum LA (1998): Cerebrospinal fluid concentrations of somatostatin and biogenic amines in grown primates reared by mothers exposed to manipulated foraging conditions.Arch Gen Psychiatry55:473– 477.
Dahl R (1996): The regulation of sleep and arousal: Develop-ment and psychopathology.Dev Psychopathol8:3–27. Dahl RE, Kaufman J, Ryan ND, Perel J, al-Shabbout M,
Birmaher B (1992): The dexamethasone suppression test in children and adolescents: A review and a controlled study.
Biol Psychiatry32:109 –26.
Dahl RE, Ryan ND (1999): Methodologic issues in sleep and neuroendocrine measures in assessment. In Shaffer D, Richters J, Christopher P, editors.Assessment in Child and Adolescent Psychopathology.New York: Guilford.
Dahl RE, Ryan ND, Birmaher B, al-Shabbout M, Williamson DE, Neidig M, et al (1991a): Electroencephalographic sleep measures in prepubertal depression.Psychiatry Res38:201– 214.
Dahl RE, Ryan ND, Matty MK, Birmaher B, al-Shabbout M, Williamson DE, Kupfer DJ (1996): Sleep onset abnormalities in depressed adolescents.Biol Psychiatry39:400 – 410. Dahl RE, Ryan ND, Perel J, Birmaher B, al-Shabbout M, Nelson
B, Puig-Antich J (1994): Cholinergic REM induction test with arecoline in depressed children.Psychiatry Res51:269 – 282.
Dahl RE, Ryan ND, Puig-Antich J, Nguyen NA, al-Shabbout M, Meyer VA, Perel J (1991b): 24-Hour cortisol measures in adolescents with major depression: A controlled study.Biol Psychiatry30:25–36.
Emslie GJ, Rush AJ, Weinberg WA, Rintelmann JW, Roffwarg HP (1994): Sleep EEG features of adolescents with major depression.Biol Psychiatry36:573–581.
Frank E, Jarrett RB, Keller MB, Martin B, Prien RF (1991): Conceptualization and rationale for consensus definitions of terms in major depressive disorder: Remission, recovery, relapse, and recurrence.Arch Gen Psychiatry48:851– 855. Gann H, Riemann D, Stoll S, Berger M, Muller WE (1995):
Growth hormone response to growth hormone-releasing hor-mone and clonidine in depression.Biol Psychiatry38:325– 329.
Garber J, Weiss B, Shanley N (1993): Cognitions, depressive symptoms, and development in adolescents.J Abnorm Psy-chol102:47–57.
Harrington R, Bredenkamp D, Groothues C, Rutter M, Fudge H, Pickles A (1994): Adult outcomes of childhood and adoles-cent depression. III. Links with suicidal behaviours.J Child Psychol Psychiatry35:1309 –1319.
Hollingshead A (1975):Four-Factor Index of Social Status.New Haven, CT: Yale University, Department of Sociology. Holsboer F (1995): Neuroendocrinology of mood disorders. In:
Bloom F, Kupfer D, editors.Neuropsychopharmacology: The Fourth Generation of Progress.New York: Raven, 957–970. Jarrett DB, Kupfer DJ, Miewald JM, Grochocinski VJ, Franz B (1994): Sleep-related growth hormone secretion is persis-tently suppressed in women with recurrent depression: A preliminary longitudinal analysis. J Psychiatr Res 28:211– 223.
Jarrett DB, Miewald JM, Kupfer DJ (1990): Recurrent depres-sion is associated with a persistent reduction in sleep-related growth hormone secretion.Arch Gen Psychiatry47:113–118. Kovacs M (1996): Presentation and course of major depressive disorder during childhood and later years of the life span.
J Am Acad Child Adolesc Psychiatry35:705–715.
Lesch KP, Laux G, Pfuller H, Erb A, Beckmann H (1987): Growth hormone (GH) response to GH-releasing hormone in depression.J Clin Endocrinol Metab65:1278 –1281. Lesch KP, Muller U, Rupprecht R, Kruse K, Schulte HM (1989):
Endocrine responses to growth hormone-releasing hormone, thyrotropin-releasing hormone and corticotropin-releasing hormone in depression.Acta Psychiatr Scand79:597– 602. Mason PTK, Plotsky P, Archer D (1998): Growth failure caused
by early maternal separation and chronic isolation.Neurosci Abstr1997:1435.
Meyer WJ III, Richards GE, Cavallo A, Holt KG, Hejazi MS, Wigg C, Rose RM. (1991): Depression and growth hormone.
J Am Acad Child Adolesc Psychiatry30:335.
Nutt DJ (1989): Altered central alpha 2-adrenoceptor sensitivity in panic disorder.Arch Gen Psychiatry46:165–169. Orvaschel H, Puig-Antich J (1987): Schedule for Affective
Pine DS, Cohen P, Brook J (1996): Emotional problems during youth as predictors of stature during early adulthood: Results from a prospective epidemiologic study.Pediatrics97:856 – 863.
Puig-Antich J, Goetz R, Davies M, Fein M, Hanlon C, Chambers WJ, et al (1984a): Growth hormone secretion in prepubertal children with major depression. II. Sleep-related plasma concentrations during a depressive episode.Arch Gen Psy-chiatry41:463– 466.
Puig-Antich J, Goetz R, Davies M, Tabrizi MA, Novacenko H, Hanlon C, et al (1984b): Growth hormone secretion in prepubertal children with major depression. IV. Sleep-related plasma concentrations in a drug-free, fully recovered clinical state.Arch Gen Psychiatry41:479 – 483.
Puig-Antich J, Kaufman J, Ryan ND, Williamson DE, Nelson B, Stull S, et al (1993): The psychosocial functioning and family environment of depressed adolescents. J Am Acad Child Adolesc Psychiatry32:244 –253.
Puig-Antich J, Novacenko H, Davies M, Chambers WJ, Tabrizi MA, Karwiec V, et al (1984c): Growth hormone secretion in prepubertal children with major depression. I. Final report on response to insulin-induced hypoglycemia during a depres-sive episode.Arch Gen Psychiatry41:455– 460.
Puig-Antich J, Novacenko H, Davies M, Tabrizi MA, Ambrosini P, Goetz R, et al (1984d): Growth hormone secretion in prepubertal children with major depression. III. Response to insulin-induced hypoglycemia after recovery from a depres-sive episode and in a drug-free state.Arch Gen Psychiatry
41:471– 475.
Rao U, Ryan ND, Birmaher B, Dahl RE, Williamson DE, Kaufman J, et al (1995): Unipolar depression in adolescents: Clinical outcome in adulthood. J Am Acad Child Adolesc Psychiatry34:566 –578.
Rapaport MH, Risch SC, Gillin JC, Golshan S, Janowsky DS (1989): Blunted growth hormone response to peripheral infusion of human growth hormone-releasing factor in pa-tients with panic disorder.Am J Psychiatry146:92–95.
Rosenblum LA, Coplan JD, Friedman S, Bassoff T, Gorman JM, Andrews MW (1994): Adverse early experiences affect nor-adrenergic and serotonergic functioning in adult primates.
Biol Psychiatry35:221–227.
Ryan ND, Dahl RE, Birmaher B, Williamson DE, Iyengar S, Nelson B, et al (1994): Stimulatory tests of growth hormone secretion in prepubertal major depression: Depressed versus normal children.J Am Acad Child Adolesc Psychiatry 33: 824 – 833.
Ryan ND, Puig-Antich J, Ambrosini P, Rabinovich H, Robinson D, Nelson B, et al (1987): The clinical picture of major depression in children and adolescents.Arch Gen Psychiatry
44:854 – 861.
Ryan ND, Puig-Antich J, Rabinovich H, Ambrosini P, Robinson D, Nelson B, Novacenko H (1988): Growth hormone re-sponse to desmethylimipramine in depressed and suicidal adolescents.J Affect Disord15:323–337.
Sallee FR, Richman H, Sethuraman G, Dougherty D, Sine L, Altman-Hamamdzic S (1998): Clonidine challenge in child-hood anxiety disorder.J Am Acad Child Adolesc Psychiatry
37:655– 662.
Siever LJ, Trestman RL, Coccaro EF, Bernstein D, Gabriel SM, Owen K, et al (1992): The growth hormone response to clonidine in acute and remitted depressed male patients.
Neuropsychopharmacology6:165–177.
Skare SS, Dysken MW, Billington CJ (1994): A review of GHRH stimulation test in psychiatry. Biol Psychiatry 36: 249 –265.
Tancer ME, Stein MB, Black B, Uhde TW (1993): Blunted growth hormone responses to growth hormone-releasing factor and to clonidine in panic disorder.Am J Psychiatry
150:336 –337.