www.elsevier.com/locate/ibmb
Effect of temperature and photoperiod on juvenile hormone
biosynthesis and sexual maturation in the cotton bollworm,
Helicoverpa armigera
: implications for life history traits
Xiaofeng Zhou, Moshe Coll, Shalom W. Applebaum
*Department of Entomology, The Faculty of Agricultural, Food and Environmental Quality Sciences, The Hebrew University of Jerusalem, PO Box 12, Rehovot 76100, Israel
Received 31 October 1999; received in revised form 31 December 1999; accepted 25 January 2000
Abstract
The Israeli population of the cotton bollworm, Helicoverpa armigera (Hu¨bner), undergoes a short-day, low-temperature pupal diapause and is also suspected of being a seasonal migrant in the eastern Mediterranean region.H. armigera were reared in the laboratory under several constant temperature and photoperiodic combinations which simulate average conditions encountered in the spring, summer, early-autumn and late-autumn in Israel. Juvenile hormone (JH) biosynthesis, the onset of calling behavior, sex pheromone production and ovarian development were examined in virgin female moths subsequent to eclosion. Allatal maturation, defined as acquisition of competence to synthesize JH, was significantly delayed in moths reared under simulated spring conditions. This was probably the cause for the observed delay in ovarian development and the onset of calling behavior, and to the reduction in sex pheromone biosynthesis. The delay in female sexual maturation, commonly associated with migratory flight, is consistent with presumptive pre-reproductive migration inH. armigera. 2000 Elsevier Science Ltd. All rights reserved.
Keywords: Helicoverpa armigera; Juvenile hormone; Temperature; Photoperiod; Sex pheromone; Reproductive maturation; Migration
1. Introduction
Diapause and various degrees of regional displace-ment, ranging from short-distance flights to long-dis-tance classical migration, are alternative strategies used by many insects to escape, in space or in time, from adverse environmental conditions and/or habitat deterio-ration. (Southwood, 1977). The cotton bollworm, Hel-icoverpa armigera (Hu¨bner) (Lepidoptera: Noctuidae), seems to exhibit both strategies in different parts of its global distribution: temperate populations undergo facul-tative diapause under short-day conditions, and popu-lations are reported to migrate in Australia (Daly and Gregg, 1985; Fitt and Daly, 1990; Gregg et al., 1993a), India (Pedgley et al., 1987; Riley et al., 1992; Vaisham-payan and Singh, 1996), Sudan (Haggis, 1981), Europe
* Corresponding author. Tel.:+972-8-948-9154; fax:+ 972-8-946-8586.
E-mail address:[email protected] (S.W. Applebaum).
0965-1748/00/$ - see front matter2000 Elsevier Science Ltd. All rights reserved. PII: S 0 9 6 5 - 1 7 4 8 ( 0 0 ) 0 0 0 5 9 - X
(Pedgley, 1985; Palmqvist, 1997) and China (Wu et al., 1998).
Seasonal conditions in the Israeli autumn induce short-day pupal diapause, which terminates after winter. Various considerations led us to question whether migration also occurs in the Israeli population of H. armigera. We exposed larvae to combinations of photo-period and temperature designed to simulate the spring, summer, early-autumn and late-autumn conditions in Israel, and later examined, after adult eclosion, several physiological parameters upstream of reproductive maturity and presumptive migratory flight.
noc-tuid moths,Pseudaletia unipuncta(Cusson and McNeil, 1989) andAgrotis ipsilon (Gadenne, 1993; Picimbon et al., 1995). It has recently been found that JH primes the pheromone glands of pharate adult H. armigerato later respond to Pheromone biosynthesis Activating Neuro-peptide PBAN (Fan et al., 1999). Beside reproduction, JH also plays an important role in flight in many species (Rankin and Riddiford, 1978; Rankin et al., 1986; Ran-kin, 1989; McNeil et al. 1995, 1996; Schneider et al., 1995).
It is generally assumed that if migratory behavior occurs, it does so during the pre-reproductive period. Once a virgin female calls for the first time, this indicates the termination of the pre-reproductive period. McNeil (1986) proposed that the pre-calling period could be used as an indicator of migratory species. In most Lepidop-tera, calling occurs during sex pheromone production, or shortly thereafter.
In the present study, sex pheromone production and calling were examined in the adult female H. armigera emerging under different rearing regimens. Ovarian maturation in these adults was also compared, the assumption being that the ovaries of females pro-grammed for pre-reproductive flight would be less developed than those of reproductively active females. We report herein that JH biosynthesis and sexual matu-ration are delayed when larvae are exposed to regimens simulating spring conditions.
2. Materials and methods
2.1. Insect culture and season simulation
H. armigerawas maintained individually under stan-dard laboratory conditions, at a constant temperature of 26°C and 14:10 LD (Rafaeli and Soroker, 1989). Pupae were sexed and males and females were held separately. Moths were fed with 10% sugar water. Three combi-nations of temperature and photoperiod were used to simulate the conditions that the larval stages would be exposed to in Israel during spring (19°C, 13:11 LD), summer (26°C, 14:10 LD), early-autumn (25°C, 12:12 LD) and late-autumn (21°C, 11:13 LD). Larvae were reared under standard conditions until the third instar, and then transferred to the designated conditions.
Virgin female H. armigera moths, reared under the defined seasonal regimens as described, were transferred at emergence to a constant temperature of 26°C and 14:10 LD photoperiod. Clearly, standardized conditions may reduce the effects of the previous rearing con-ditions, and this would lead to an underestimate of dif-ferences amongst the moths emerging from defined sea-sonal regimens. Therefore, the progression of allatal biosynthetic maturation and of acquisition of repro-ductive behavior should not be taken as representative
of normal rates which might have been recorded, had the temperatures and photoperiods of the different treat-ments been maintained after the inductive larval period. The purpose of this transfer was therefore to provide equalized conditions of adult development across all experimental groups during the time of assay.
About half of the population reared under late-autumn conditions entered pupal diapause. The fifth and more-so sixth instars are reportedly the instars sensitive to photoperiodically induced diapause (He et al., 1995; Jiang et al., 1999). The criterion for determining diapause was the persistence of larval eyespots (Wilson et al., 1979), as nondiapausing pupae lose them before day 12 under 21°C. The age of the female moth on the night of its eclosion is designated D0 and subsequent nights are numbered accordingly.
2.2. Total JH biosynthesis
Rates of JH biosynthesis were determined at D0, D1, D3, and D5, using the radiochemical assay of Pratt and Tobe (1974) for JH biosynthesis, as adapted by Fan et al. (1999). Pairs of excised CA were preincubated for 1 h in a Petri dish at 26°C in 50 µl of minimal essential medium (MEM), without methionine, containing 2% Ficoll 400, 50 mM HEPES buffer (pH 7.2) and 5×1027 M of the JH esterase inhibitor 3-octylthio-1,1,1-trifluoro-2-propanone (Hammock et al., 1984). CAs were then incubated for an additional 2 h in fresh medium with 5 µCi of l-[3H-methyl]-methionine (80 Ci/mmol specific
activity, 1.2µM final concentration in the medium). The medium and tissue together were then extracted with 150 µl ice-cold hexane. Hexane-soluble extract was further analyzed by thin-layer chromatography (TLC). JH I, JH II, and JH III were used as standards to identify radioac-tively labeled JHs produced in vitro byH. armigeraCA. Synthetic JH I and II were kindly provided by Dr Zdenek Wimmer, of the Institute of Organic Chemistry and Bio-chemistry, Prague, The Czech Republic. JH III was pur-chased from Sigma. Hexane-soluble extracts (25 µl) of individual incubations were applied to silica gel plates (Polygram SIL G/UV254; Macherey-Nagel, Doren, Germany) and developed in hexane:ethyl acetate (2:1) according to Moshitzky and Applebaum (1995). JH I, II, and III do not separate clearly in this system. They were, therefore, collected together and counted as “total JH” in a beta-counter (1209 RackBeta; LKB). We found total JH biosynthesis to be linear for incubation periods of 1– 3 h, so a 2 h incubation was routinely used throughout this study.
2.3. Ovarian development
Female moths from all experimental conditions were transferred to 26°C, 14:10 LD in the first photophase after eclosion (D0). Moths were weighted on D0 and on subsequent days, after which their ovaries were collected in physiological medium, blotted on filter paper for sev-eral minutes and individually weighed.
2.4. Onset of female calling and sex pheromone production
Female H. armigera pupae, reared under the defined temperature and photoperiodic regimens as described above, were transferred to 19°C, 13:11 LD photoperiod when pupal eyespots blackened (an indication of pharate adult development). Preliminary observations showed that some of the H. armigera virgin females, held con-tinuously at standard conditions of long-day and 26°C, already start calling the night after emergence (D1). To evaluate the effect of the different temperatures and pho-toperiods on the onset of calling, pre-eclosion females were placed at a lower temperature (19°C, 13:11 LD). The purpose of this transfer was to standardize the rate of reproductive maturation following apolysis to the pharate adult across all experimental groups, and meas-ure calling and pheromone production under equal con-ditions. Eclosion of the pharate moths at this lower tem-perature and photoperiod occurred about 10 days after transfer. Thus, the transfer to 19°C could be expected to minimize any treatment effect.
Calling behavior was observed at 15 min intervals under a dim red light during the last 5 h of scotophase (Rafaeli and Soroker, 1989). The time from eclosion to the night in which each female commenced calling was regarded as the pre-calling period. In preliminary experi-ments, we recorded production of sex pheromone during the sixth to tenth hour of scotophase. The highest values were recorded at the ninth hour, but the values obtained were not significantly different during the whole interval. We therefore routinely removed ovipositor tips at the ninth hour of scotophase and extracted them in hexane containing 25 ng of internal standard. The extracts were subsequently analyzed by gas chromatography (GC) for quantification of Z11-16:Ald, the main sex pheromone component of H. armigera, using conditions described previously (Soroker and Rafaeli, 1989).
2.5. Data analysis
Analyses of variance (ANOVA) and Fisher’s pro-tected least significance tests were used for data analyses (Steel and Torrie, 1980; SAS Institute, 1988). Unless noted differently, tests of significance used a probability level of 0.05.
3. Results
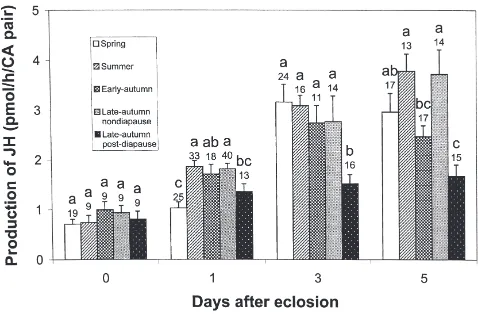
3.1. JH biosynthesis rate
There was a significant interaction in JH production between seasonal conditions and moth age (F12, 319=3.28,
P≪0.001). Virgin D0 females produced very little JH under all season simulations (Fig. 1). For older females, however, the increase in JH biosynthesis rate was sig-nificantly lower in post-diapause moths (F4,122=4.88,
P,0.001; F4,76=5.04, P,0.001; and F4,71=6.80,
P,0.001 on D1, D3, and D5, respectively). Spring moths produced a significantly smaller amount of JH on D1 (F4, 122=4.88, P,0.001; Fig. 1), suggesting a delay in allatal maturation.
3.2. Ovarian development
Virgin female ovarian development was determined by the ratio of ovarian wet weight to body weight (O/B) (Fig. 2). There was a significant interaction in ovary weight between seasonal conditions and female age (F16,144=4.18, P,0.001). The overall ovary weight increased significantly from D0 to D5, and all virgin female moths attained a similar O/B value by D5. On D0 and D1, ovarian development was the most advanced in summer moths (F4,33=10.33, P≪0.001; F4,33=18.62,
P≪0.001, respectively). In contrast, the rate of ovarian maturation in the spring moths was slower; on D2, spring moths had significantly lower O/B values than moths from other seasonal simulations except post-diapause females (F4,21=8.58, P≪0.001).
Fig. 2. Ratio of ovarian to body weight (w/w mean±SE) as a function of age in virgin Helicoverpa armigerafemales, reared during their developmental stages under different seasonal regimens, and trans-ferred after eclosion to 26°C, 14:10 LD. Within days, bars labeled with different letters are significantly different (Fisher’s protected LSD,
P,0.05). Sample sizes are given above each bar.
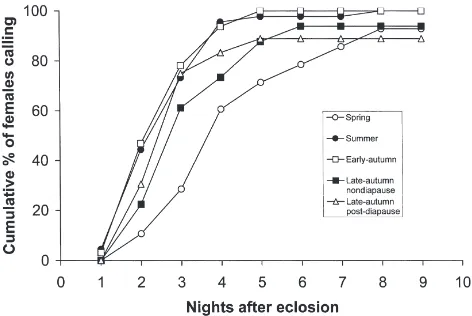
3.3. Onset of female calling and sex pheromone production
Under the standardized conditions, spring-reared H. armigera had a significantly longer (F4,176=8.90;
P≪0.001) pre-calling period than that of moths under the other rearing conditions (Fig. 3). By the third night, less than 30% of the spring moths initiated calling, com-pared to about 75% of the summer and post-diapause moths. By that time, 60% of the nondiapause late-aut-umn moths had called for the first time. A small number of moths under spring and late-autumn conditions (both post-diapause and nondiapause moths) did not call at all throughout the observed period.
Overall, significantly more (F2,250=19.64, P≪0.001) pheromone was produced on D3 and D4 than on D2 (16.68±1.36; 19.92±1.33; and 7.56±1.5, respectively;
Fig. 3. Cumulative proportion of virgin Helicoverpa armigera
females calling for the first time after being reared under different seasonal conditions, and later transferred as pharate adults to 19°C, 13:11 LD. (n$35 for each treatment).
Fig. 4). Moths reared under spring conditions produced significantly less sex pheromone throughout the experi-ment (F4,250=9.02, P≪0.001; Fig. 4). There was no sig-nificant interaction in pheromone production between treatment and moth age (F8,250=1.53, P.0.15).
4. Discussion
Migration is usually, but not always, associated with a delay in reproductive maturation, presumably due to the dichotomy of limited resource allocation (see review by Gatehouse, 1997). The trade-off between migration and reproduction, termed the “oogenesis–flight syn-drome”, was first proposed by Johnson (1969). It has been observed previously in H. armigera (Colvin and Gatehouse, 1993). We found that various aspects of female reproductive development in an Israeli popu-lation ofH. armigeraare delayed or reduced when larvae are reared at low temperature and relatively long day, conditions simulating the spring in Israel. Post-diapause females, which eclose after overwintering, also exhibit a relatively lower rate of JH synthesis and of delayed ovarian development. This reduced reproductive devel-opment observed in the Israeli population is consistent with possible migratory flight deduced from recent field studies using pheromone traps (unpublished data). It was observed that a peak of male moths caught in the traps in early spring preceded the peak of moth eclosion in local diapausing overwintering population in the spring by almost 2 months. Furthermore, these early season moths had significantly fewer wing scales, indicating long distance flight. We hypothesis that these earlier moths are migrants from warmer, more southern areas which experienced meteorological conditions similar to those encountered later in the Israeli spring season. To
Fig. 4. Sex pheromone production by virgin Helicoverpa armigera
females on the second, third and fourth nights after eclosion. Larvae were reared under different seasonal conditions, and then transferred as pharate adults to 19°C, 13:11 LD. Within days, bars labeled with different letters are significantly different (Fisher’s protected LSD,
some extent, moths completing their development under Israeli spring conditions may be inclined to disperse to suitable more northern habitats. Spring migration of H. armigera has been documented in Australia (Fitt and Daly, 1990; Gregg et al., 1990; Gregg et al., 1993b) and India (Vaishampayan and Singh, 1996).
According to current theory, neither migration nor reproduction occurs below a certain critical level of very low JH hemolymph titers. Migratory flight is presumably initiated and sustained by low or intermediate titers of JH in newly emerged adults, whereas higher JH titers presumably terminate flight and initiate reproduction. Our results are in agreement with this scenario. Moths reared under spring conditions synthesize JH at a reduced rate, which may be insufficient for reproduction, but might permit migratory flight. Limited migration could even be completed during the short period of the first two nights after eclosion (D0 and D1). In the field, this period of allatal immaturity could be extended even further. In adult moths migrating from the south in the early spring, the extended flight might depress allatal activity or accelerate the degradation of JH in vivo, ther-eby maintaining a relatively low level of JH on sub-sequent days. These possibilities are yet to be explored. The fastest rate of ovarian development in virgin females reared under summer conditions may explain the observations that moths are more sedentary in summer (Gregg et al., 1993a). On D2, the ovaries of spring moths still remain undeveloped, whilst in other season simula-tions, ovarian weight increases significantly. The ovarian development rate in D2 females presumably depends on the D2 rate of JH biosynthesis, which is lower in spring moths. Increases in JH levels are known to stimulate vit-ellogenesis and egg production.
The onset of calling generally occurs during or shortly after maximal sex pheromone production (see review by Delisle and Royer, 1994). By limiting release of sex pheromone during calling, female spring moths of an Indian population ofH. armigera reduce the probability of mating and increase the probability of sustained flight (Armes and Cooter, 1991). The pre-calling period is delayed in the Israeli population ofH. armigerafemales reared under simulated spring conditions, during which presumptive migration might occur. The absolute values of both the onset of calling and pheromone titer may have been affected by the transfer of the pupae to the standardized lower temperature regimen in the experi-mental protocol. Nevertheless, the recorded differences strongly suggest that pre-calling development is delayed, and that the synthesis rate of pheromone is reduced in the moths emerging after larval rearing, which simulates spring conditions in this region. Documented examples of an extended pre-calling period in migratory moths are found in the sunflower moth, Homoeosoma electellum, (McNeil and Delisle, 1989), and the oriental armyworm, Mythimna separata (Han and Gatehouse, 1991). In the
true armyworm moth, Pseudaletia unipuncta, JH is essential for the initiation of both calling behavior and sex pheromone production (Cusson and McNeil, 1989). In conclusion, the temperature and photoperiod con-ditions during larval and pupal development significantly affect adult reproductive physiology. The data presented herein suggest that spring conditions may induce migration of H. armigera.
Acknowledgements
This research was supported in part by US–Israel CDR grant (# TA-MOU-C14-126) to M.C. and S.W.A. It comprises part of the doctoral thesis of X.Z.
References
Armes, N.J., Cooter, R.J., 1991. Effects of age and mated status on flight potential ofHelicoverpa armigera(Lepidoptera: Noctuidae). Physiological Entomology 16, 131–144.
Colvin, J., Gatehouse, A.G., 1993. The reproduction–flight syndrome and the inheritance of tethered-flight activity in the cotton-boll-worm moth, Heliothis armigera. Physiological Entomology 18, 16–22.
Cusson, M., McNeil, J.N., 1989. Involvement of juvenile hormone in the regulation of pheromone release activities in a moth. Science 243, 210–212.
Daly, J.C., Gregg, P.C., 1985. Genetic variation inHeliothisin Aus-tralia: species identification and gene flow in the two pest species
H. armigera(Hu¨bner) andH. punctigeraWallengren (Lepidoptera: Noctuidae). Bulletin of Entomological Research 75, 169–184. Delisle, J., Royer, L., 1994. Changes in pheromone titer of
oblique-banded leafroller, Christoneura rosaceana, virgin females as a function of time of day, age and temperature. Journal of Chemical Ecology 20, 45–69.
Fan, Y., Rafaeli, A., Gileadi, C., Applebaum, S.W., 1999. Juvenile hormone induction of pheromone gland PBAN-responsiveness in
Helicoverpa armigerafemales. Insect Biochemistry and Molecular Biology 29, 635–641.
Fitt, G.P., Daly, J.C., 1990. Abundance of overwintering pupae and the spring generation ofHelicoverpaspp. (Lepidoptera: Noctuidae) in northern New South Wales, Australia: implications for pest man-agement. Journal of Economic Entomology 83, 1827–1836. Gadenne, C., 1993. Effects of fenoxycarb, juvenile hormone mimetic,
on female sexual behaviour of the black cutworm,Agrotis ipsilon
(Lepidoptera: Noctuidae). Journal of Insect Physiology 39, 25–29. Gatehouse, A.G., 1997. Behavior and ecological genetics of wind-borne migration by insects. Annual Review of Entomology 42, 475–502.
Gregg, P.C., Fitt, G.P., Zalucki, M.P., Twine, P., 1990. Evidence for spring migration ofHeliothisspp. from inland Australia to cotton areas. In: Proceedings of the fifth Australian Cotton Conference, Broadbeach, Queensland. Australian Cotton Growers Research Association, Brisbane, pp. 327–335.
W.M. (Eds.), Pest Control and Sustainable Agriculture. CSIRO, Australia, pp. 460–463.
Haggis, M.J., 1981. Spatial and temporal changes in the distribution of eggs byHeliothis armigera(Hu¨bner) (Lepidoptera, Noctuidae) on cotton in the Sudan Gezira. Bulletin of Entomological Research 71, 183–193.
Hammock, B.D., Abdel-Aal, Y.A.I., Mullin, C.A., Hanzlik, T.N., Row, R.M., 1984. Substituted thiofluoropropanones as potent selective inhibitors of juvenile hormone esterase. Pesticide Biochemistry and Physiology 22, 209–223.
Han, E., Gatehouse, A.G., 1991. Effect of temperature and photoperiod on the calling behaviour of a migratory insect, the oriental army-wormMythimna separata. Physiological Entomology 16, 419–427. He, Z., Xi, R., Zhang, L., Zhang, C., Zhou, F., 1995. The sensitive larval stadium of diapause in cotton bollworm, Helicoverpa armigera. Sinozoologia 12 (Suppl), 40–43.
Jiang, M., Xie, L., Zhang, X., 1999. Characteristics of diapause induc-tion of cotton bollworm. Chinese Journal of Applied Ecology 10, 60–62.
Johnson, C.G., 1969. Migration and Dispersal of Insects by Flight. Methuen, London.
Koeppe, J.K., Fuchs, M., Chen, T.T., Hunt, L.M., Kovalick, G.E., Bri-ers, T., 1985. The role of juvenile hormone in reproduction. In: Kerkut, G.A., Gilbert, L.I. (Eds.), Comprehensive Insect Physi-ology, Biochemistry and PharmacPhysi-ology, vol. 8. Pergamon Press, Oxford, pp. 166–203.
McNeil, J.N., 1986. Calling behaviour: can it be used to identify migratory species of moths? Florida Entomologist 69, 78–84. McNeil, J.N., Delisle, J., 1989. Host plant pollen influences calling
behaviour and ovarian development of the sunflower moth, Homo-eosoma electellum. Oecologia 80, 201–205.
McNeil, J.N., Cusson, M., Delisle, J., Orchard, I., Tobe, S.S., 1995. Physiological integration of migration in Lepidoptera. In: Drake, V.A., Gatehouse, A.G. (Eds.), Insect Migration: Tracking Resources in Space and Time. Cambridge University Press, Cam-bridge, pp. 279–302.
McNeil, J.N., Laforge, M., Be´dard, C., Cusson, M., 1996. Juvenile hormone production and sexual maturation in true armyworm,
Pseudaletia unipuncta(HAW.) (Lepidoptera: Noctuidae): a com-parison of migratory and non-migratory populations. Archives of Insect Biochemistry and Physiology 32, 575–584.
Moshitzky, P., Applebaum, S.W., 1995. Pathway and regulation of JHIII-bisepoxide biosynthesis in adult Drosophila melanogaster
corpus allatum. Archives of Insect Biochemistry and Physiology 30, 225–237.
Palmqvist, G., 1997. Remarkable records of Macrolepidoptera in Sweden. Entomologisk Tidskrift 118, 11–27.
Pedgley, D.E., 1985. Windborne migration of Heliothis armigera
(Hu¨bner) (Lepidoptera Noctuidae) to the British Isles. Entomol-ogist’s Gazette 36, 15–20.
Pedgley, D.E., Tucker, M.R., Pawar, C.S., 1987. Windborne migration ofHelicoverpa armigera(Hu¨bner) (Lepidoptera Noctuidae). Eco-logical Entomology 11, 467–470.
Picimbon, J.F., Becard, J.M., Sreng, L., Clement, J.L., Gadenne, C., 1995. Juvenile hormone stimulates pheromonotropic brain factor release in the female black cutworm Agrotis ipsilon. Journal of Insect Physiology 41, 377–382.
Pratt, G.E., Tobe, S.S., 1974. Juvenile hormone radiobiosynthesized by corpora allata of adult female locusts in vitro. Life Sciences 14, 575–586.
Rafaeli, A., Soroker, V., 1989. Influence of diel-rhythm and brain hor-mone on pherohor-mone production in two Lepidopteran species. Jour-nal of Chemical Ecology 15, 447–455.
Ramaswamy, S.B., Shu, S., Park, Y.I., Zeng, F., 1997. Dynamics of juvenile hormone-mediated gonadotropism in the Lepidoptera. Archives of Insect Biochemistry and Physiology 35, 539–558. Rankin, M.A., 1989. Hormonal control of flight. In: Goldsworthy, G.J.,
Wheeler, C.H. (Eds.), Insect Flight. CRC Press, Boca Raton, Flor-ida, pp. 139–163.
Rankin, M.A., Riddiford, L.M., 1978. Significance of haemolymph juvenile hormone titer changes in timing of migration and repro-duction in adultOncopeltus fasciatus. Journal of Insect Physiology 24, 31–38.
Rankin, M.A., McAnelly, M.L., Bodenhamer, J.E., 1986. The oogen-esis–flight syndrome revisited. In: Danthanarayana, W. (Ed.), Insect Flight, Dispersal and Migration. Springer-Verlag, Berlin, pp. 27– 48.
Riley, J.R., Armes, N.J., Reynolds, D.R., Smith, A.D., 1992. Nocturnal observations on the emergence and flight behaviour ofHelicoverpa armigera(Lepidoptera: Noctuidae) in the post-rainy season in cen-tral India. Bulletin of Entomological Research 82, 243–256. SAS Institute, 1988. SAS/STAT User’s Guide, Release 6.03 edn. SAS
Institute, Cary, NC.
Schneider, M., Wiesel, G., Dorn, A., 1995. Effects of JH III and JH analogues on phase-related growth, egg maturation and lipid metab-olism in Schistocerca gregariafemales. Journal of Insect Physi-ology 41, 23–31.
Soroker, V., Rafaeli, A., 1989. In vitro hormonal stimulation of [14
C]-acetate incorporation by Heliothis armigera pheromone glands. Insect Biochemistry 19, 1–5.
Southwood, T.R.E., 1977. Habitat, the templet for ecological stra-tegies? Journal of Animal Ecology 46, 337–365.
Steel, R.G.D., Torrie, J.H., 1980. Principles and Procedures of stat-istics: A Biometrical Approach, 2nd edn. McGraw-Hill, New York. Vaishampayan, S. Jr., Singh, H.N., 1996. Evidences on the migratory nature ofHeliothis armigera(Hu¨bner) adults collected on light trap at Varanasi. Indian Journal of Entomology 57, 224–232. Wilson, A.G., Lewis, T., Cunningham, R.B., 1979. Overwintering and
spring emergence of Heliothis armigera (Hu¨bner) (Lepidoptera, Noctuidae) in the Namoi Valley, New South Wales. Bulletin of Entomological Research 69, 97–109.
Wu, K., Xu, G., Guo, Y., 1998. Observations on migratory activity of cotton bollworm moths across the Bohai Gulf in China. Acta Phytophylacica Sinica 25, 337–340.