Short and long-term responses of whole-plant gas exchange
to elevated CO
2in four herbaceous species
Catherine Roumet *, Eric Garnier, He´le`ne Suzor, Jean-Louis Salager,
Jacques Roy
Centre d’Ecologie Fonctionnelle et E6oluti6e(C.N.R.S.-U.P.R.9056),1919Route de Mende,34293Montpellier Cedex5,France
Received 14 August 1999; received in revised form 15 October 1999; accepted 15 October 1999
Abstract
Four Mediterranean herbaceous species, the two grasses Bromus madritensis (annual) and B.erectus (perennial), and the two legumesMedicago minima(annual) andM.glomerata(perennial) were grown in glasshouses at two levels of atmospheric CO2(350 and 700mmol mol−1), under non-limiting nutrient conditions. After 6 months of growth,
short and long-term responses of whole plant photosynthesis and stomatal conductance to elevated CO2 were
measured, together with changes in leaf total non-structural carbohydrate concentration, leaf nitrogen concentration and specific leaf area. Short-term exposure to elevated CO2increased whole plant photosynthesis by 30% on average.
However, this stimulation did not persist in the long term, indicating a down-regulation of photosynthesis in plants grown at elevated CO2. By contrast, stomatal conductance was similarly or more decreased after long-term than after
short-term exposure to elevated CO2. As a result, the short-term effect of CO2on instantaneous water use efficiency
was conserved in the long-term and theci/caratio remained nearly constant after both short and long-term exposure
to elevated CO2. Analysis of the main leaf components revealed that when grown at elevated CO2, leaves of the two
grass species showed a large accumulation of total non-structural carbohydrates and a decrease in their nitrogen concentration, while leaf total non-structural carbohydrate and nitrogen concentrations of the two legume species were unaffected by elevated CO2. Species-specific differences in down-regulation of photosynthesis were positively
correlated with the long-term response of stomatal conductance and negatively correlated with changes in total non-structural carbohydrate concentration. This suggests that source – sink relationship may play a role in the control of photosynthetic response to high CO2concentration. © 2000 Elsevier Science B.V. All rights reserved.
Keywords:CO2; Grasses; Legumes; Stomatal conductance; Water use efficiency; Whole-plant photosynthesis
www.elsevier.com/locate/envexpbot
1. Introduction
Interspecific variability in the response of plants to elevated atmospheric carbon dioxide concen-tration may play an important role in determining productivity of ecosystems in a future climate.
* Corresponding author. Tel.:+33-4-67613238; fax: +33-4-67412138.
E-mail address:[email protected] (C. Roumet)
C.Roumet et al./En6ironmental and Experimental Botany43 (2000) 155 – 169
156
Since the primary, short-term effects of elevated CO2 are an increase in the rate of carboxylation
and a decrease in stomatal conductance (Stitt, 1991), it is worthwhile to understand the causes of interspecific differences in the response of photo-synthesis and stomatal conductance to elevated CO2.
Depending upon species and environmental conditions, the photosynthetic enhancement oc-curring after short-term exposure to elevated CO2
either persists, or is partly or fully reversed on the long term (Sage et al., 1989; Gunderson and Wullschleger, 1994; Greer et al., 1995). This vari-ability in the long-term response of photosynthe-sis is often associated with interspecific differences in the response of leaf chemical composition and leaf structure to elevated CO2. In plants grown
under elevated CO2, leaf non-structural
carbohy-drate concentration generally increases (Poorter et al., 1997), as a result of changes in the source – sink balance in the whole plant (Stitt, 1991) and there is a decrease in leaf nitrogen concentration as well as in specific leaf area (the ratio between leaf area and leaf biomass) (Curtis, 1996; Poorter et al., 1997).
By contrast with the extensive research con-ducted on the acclimation of photosynthesis to elevated CO2, considerably less has been reported
on the acclimation of stomatal conductance and its coupling with photosynthetic acclimation to elevated CO2 (S&antru˚cˇek and Sage, 1996). The
response of stomatal conductance to elevated CO2
is reported to be as variable as that of photosyn-thesis. The decrease of stomatal conductance ob-served after short-term exposure to elevated CO2
is either maintained (Radoglou et al., 1992; Morison, 1998), increased (Xu et al., 1994b; S&antru˚cˇek and Sage, 1996), or decreased (Ryle et al., 1992; Read et al., 1997) after long-term expo-sure. While short-term responses reflect a change in stomata aperture, long-term response can result from a change in stomatal density, or from a physiological adjustment to match any photosyn-thetic acclimation, in order to keep the usual tight coupling between stomatal conductance and pho-tosynthesis (Morison, 1998). The ratio of intercel-lular to ambient CO2 concentration (ci/ca), which
reflects such a coupling, is often found to be
unaffected by elevated CO2 (Sage, 1994; Drake et
al., 1997), while both photosynthesis and stomatal conductance responses to elevated CO2 are highly
variable. This suggests that stomata acclimate in parallel to photosynthesis (Morison, 1998), and that interspecific differences in CO2 response of
stomata are strongly linked to differences in pho-tosynthesis response. The magnitude of the long-term response of photosynthesis and stomatal conductance to elevated CO2may have important
consequences on instantaneous water and nitro-gen use efficiencies (defined as the ratio of photo-synthesis to transpiration and of photophoto-synthesis to leaf nitrogen concentration, respectively), which have often been reported to increase under elevated CO2 (Drake et al., 1997).
How these effects, mainly described at the leaf level, translate to the whole plant level is not clear. To understand the disproportional re-sponses of photosynthesis and plant biomass to elevated CO2 (Luo et al., 1997) and to predict
plant and community responses to environmental changes, it is crucial to analyse interspecific differ-ences in the response of photosynthesis and stom-atal conductance at the whole plant level. Since source – sink relationships as well as nitrogen status appear to play an important role in deter-mining photosynthetic response to elevated CO2,
we may expect that much of the variability be-tween species in photosynthetic response to CO2
could be accounted for by interspecific differences in sink capacities and nitrogen economy. In this context, in this study, four species were studied at ambient and elevated CO2: two grasses and two
legumes, with one annual and one congeneric perennial within each family. Compared to peren-nials, annuals have higher relative growth rate and specific leaf area (Garnier, 1992; Garnier et al., 1997), two traits associated with large sink capacity; in addition they are more responsive to elevated CO2 than perennials (Roumet and Roy,
1996). For legumes, the presence of N2-fixing
Hartwig, 1996; Hebeisen et al., 1997; Lu¨scher et al., 1998). In the present study, we tested the hypothesis that legumes and annuals would show little or no change in total non-structural carbo-hydrates and therefore no down regulation of photosynthesis, while the opposite effects are ex-pected in grasses and perennials. The link between the response to photosynthesis and stomatal con-ductance response to elevated CO2 and
conse-quences on water use efficiency will be also analysed. To test these hypotheses we examined whether: (i) short-term effect of elevated CO2 on
whole plant gas exchange and resource use effi-ciencies are maintained over the long-term, and (ii) interspecific differences in the degree of down-regulation of whole plant photosynthesis are cor-related with interspecific differences in the response of total non-structural carbohydrate concentration, leaf-nitrogen concentration and stomatal conductance to elevated CO2.
2. Material and methods
2.1. Species and growth conditions
This study was conducted on Mediterranean populations of the two grassesBromus madritensis
L. (annual) andBromus erectusHuds. (perennial), and of the two legumes Medicago minimaGrufb. (annual) and Medicago glomerata Balb. (peren-nial). Seeds were collected in old-fields near Montpellier (France) for the first three species, and near Aix en Provence (France) for the fourth one.
Seeds were germinated in the dark at 22°C. For legumes, seeds were placed on filter paper moist-ened with deionised water, inside plastic booklets maintained vertically. For grasses, the seeds were germinated on moistened perlite. When seedlings reached a total length of 4 – 5 cm (day 0, 15 – 18 October 1993), individual plants were transferred into 15.5 L plastic pots (77 cm high×16 cm diameter) filled with calcinated clay, and placed in four glasshouses on the C.N.R.S. campus in Montpellier (43°38% N, 3°52% E). The glasshouses were divided into two replicated CO2 treatments,
i.e. two ‘ambient’ CO2 glasshouses and two
‘ele-vated’ CO2glasshouses. The environmental
condi-tions (CO2, temperature, relative humidity and
global radiation), which did not differ signifi-cantly between the four glasshouses (PB0.05, data not shown), are reported in Table 1. Plants were watered weekly with deionised water; 8.7 l per pot from October to April (corresponding to 500 mm rainfall). During this period, nutrients were supplied eight times with the nutrient solu-tion described by Koch et al. (1987); each pot received the equivalent of 90 kg N ha−1 year−1,
as KNO3, 73 kg P ha
−1 year−1 and 120 kg K
ha−1 year−1.
Legumes were inoculated with a solution con-tainingRhizobium meliloti, strain CM51 No. 4 for
M. minima and CS41 No. 25 for M. glomerata. These strains were isolated from nodules of these species harvested in the field (INRA, Laboratoire des Symbiotes des Racines, Montpellier, France). After 6 months of growth, nodule biomass repre-sented 4 – 7% of the root system biomass (Table
Table 1
Summary of environmental conditions inside the glasshouses for 4 selected months of the experimental perioda
Global radiation CO2 (mmol mol−1)
Relative humidity (%) Temperature (°C)
(MJ m−2day−1)
Day Night Day Night Ambient Elevated
3.1 70393 37092
11.690.1 88.191
November 8.790.1 79.191.4
11.890.1 9.090.1 75.790.7
January 84.590.7 39594 75191 3.6
March 15.790.1 13.090.1 70.890.4 80.090.5 39194 75194 7.4 37093 69691
April 15.090.1 11.790.1 70.690.5 78.890.4 7.6
aValues referring to air day and night temperatures, relative humidity and global radiation represent the mean (9S.E.) of data
from the four glasshouses. Values concerning atmospheric CO2concentrations represent means (9S.E.) of data recorded in the two
C.Roumet et al./En6ironmental and Experimental Botany43 (2000) 155 – 169
158
Table 2
Summary of dry matter distribution between leaves, sheaths, roots and nodules, and leaf area of the four species grown either at ambient or elevated atmospheric CO2concentrationsa
B.madritensis B.erectus M.minima M.glomerata
Variables CO2
3.5990.77 0.8390.17
Leaf DM (g) Ambient 0.8390.13 0.5490.06
Elevated 4.0690.43 1.3890.27 0.8490.11 0.7090.09
4.3691.14 0.5890.11 1.0790.21
Sheath DM (g) Ambient 0.5290.08
1.7590.75 0.8690.21 1.1590.16
Elevated 0.6490.06
Root DM (g) Ambient 4.2190.61 1.4590.29 1.1390.21 0.9090.12
4.5290.24 2.1090.47 0.8390.13 1.0090.15
Elevated
– –
Nodule DM (g) Ambient 0.05290.008 0.06290.010
Elevated – – 0.04390.007 0.07890.008
Leaf area (cm2) Ambient 7119148 140927 161920 12595
8169104 212935 175925 150913
Elevated
aEach value is the mean (9S.E.) of four to six replicate plants per species and CO
2growth concentration.
2), and the nitrogenase activity, estimated by the acetylene reduction method (Hardy et al., 1968), ranged between 500 and 590mmol. C2H2g−1nod.
h−1, indicating that nitrogen fixation was
effec-tive and not inhibited by NO3 fertilisation.
Photosynthesis (A) and stomatal conductance (gs) were measured in April, on four to six
repli-cates per species and CO2 treatment. Within each
CO2treatment, plants were chosen in either of the
two replicate glasshouses, so as to present the same phenological stage, before flowering for the twoMedicago species and before heading for the two Bromus species. Dry matter partitioning be-tween leaves, sheaths, roots and nodules as well as leaf area are reported in Table 2 for ambient and elevated CO2-grown plants. Total biomass of B.
madritensiswas four to six times larger than that of the other three species; within grasses and legumes, the annual species were larger than the perennials.
2.2. Gas-exchange measurements
The gas exchange system used had two identical shoot chambers (1.1 l) that operated simulta-neously. Two plants grown at the same CO2
concentration were watered and brought to the laboratory. Their shoot and root atmospheres were separated with a ‘Plexiglas’ disk placed at the soil surface, in which a hole was drilled to let
the stem through. To ensure air tightness between shoot and root compartments, a silicone sealant (Rhodorsil, Rhoˆne Poulenc, Lyon, France) was applied to the base of stems or tillers. The whole shoot of each plant was introduced into one of the two measurement chambers, except for B.
madritensis, which was too large to fit the cham-ber; for this species, measurements were con-ducted on one attached tiller per plant. Two metal halide lamps (OSRAM HQIT 1000 W) provided light. The mean (9S.E.) photon flux density of photosynthetically active radiation (PAR) and air temperature at the mid-height of shoots were set at 60394 mmol photons m−2 s−1 and 24.49
0.1°C, respectively. Leaf to air vapour pressure difference was 1.1590.04 kPa. Gas exchange of plants grown at ambient CO2 was first measured
at 700mmol mol−1 (Aamb700 orgs amb700), and then
at 350 mmol mol−1 (Aamb350 or gs amb350); gas
exchange of plants grown at elevated CO2
mea-sured at 700mmol mol−1only (Aelev700orgs elev700).
The measurements were conducted in the open system described by Larigauderie et al. (1986). CO2 concentration of the air entering the
cham-bers was measured with an infrared gas analyser (IRGA) functioning in the absolute mode, and the CO2 exchange in the shoot chamber was
measurements, from Analytical Development, Hoddesdon, United Kingdom). Water vapour concentrations of the air entering and leaving the shoot chambers were measured with dew-point hygrometers (EG & G, models 880 and 911 re-spectively, Waltham, MA, USA). Gas exchange parameters were calculated according to equa-tions from von Caemmerer and Farquhar (1981).
2.3. Resource use efficiencies
Photosynthetic nitrogen use efficiency (see Gar-nier and Aronson, 1998, for a discussion of the terminology) was calculated as the ratio between
Aand leaf-organic nitrogen concentration ([N]lf).
Water use efficiency was assessed in two different ways: (i) intrinsic instantaneous values were com-puted as the ratio between photosynthesis and stomatal conductance (A/gs), as measured by gas
exchange; and (ii) integrated values were esti-mated from the carbon isotope ratio of leaf biomass (13C/12C, see below). Carbon isotope
dis-crimination partly depends on stomatal opening, which strongly influences water use efficiency (see Farquhar et al., 1989).
2.4. Har6est and chemical analysis
As soon as the gas exchange measurements were finished, plants were harvested. Leaf blades and sheaths for grasses or leaflets and stems for legumes were separated. Leaf area was measured with a leaf area meter (Delta-T Devices, model MK2, Cambridge, United Kingdom). Leaves were oven-dried for 48 h at 60°C prior to weighing.
Determination of total carbon, nitrogen con-centrations and isotopic abundance of 13C [i.e.
molar ratio 13C/(12C+13C)] were measured on
ground material using an elemental analyser cou-pled with a mass spectrometer (Tracermass, Eu-ropa Scientific). Results are expressed in terms of carbon isotopic composition (d, ‰) as:
d=Rs−Rb
Rb
where Rs and Rb are the ratio
13C/12C in the
sample and in the Pee Dee Belemnite (PDB), respectively. Since the carbon isotopic
composi-tion of the air in the glasshouses was different between the two CO2 treatments and was not
assessed, the carbon isotopic composition of leaves can be used to compare species, but not CO2 treatments.
Nitrate was extracted from sub-samples of the ground material with 10 ml HCl 0.1 N, and kept at 4°C for 48 h. The nitrate content of each sample was assayed colorimetrically according to Henriksen and Selmer-Olsen (1970). Nitrate was undetectable in any of the species (data not shown); the total nitrogen concentration mea-sured can thus be considered to be the organic nitrogen concentration of the samples.
Total non-structural carbohydrate (TNC) anal-ysis was carried out on ground material following the method of Farrar (1993). Samples were ex-tracted in 90% (v/v) ethanol at 80°C to yield soluble sugars (ethanol-soluble sugars). Starch and fructans (water-soluble sugars) contained in the residue were extracted at 100°C for 1 h in water; starch was then hydrolysed using amy-loglucosidase in acetate: acetic acid buffer at pH 4.5. Total carbohydrate in each fraction was de-termined using the phenol – sulphuric acid method of Dubois et al. (1956). Data were expressed per unit of total dry mass and per unit of structural dry mass, after subtraction of tissue non-struc-tural carbohydrates content from the total dry mass.
2.5. Treatment of data
Data from all four species were first combined to summarise the overall species and CO2 effects
using a two-way ANOVA on the different vari-ables measured. Fisher’s LSD test was used to test for species differences and for differences between CO2 combinations, i.e. amb350(plants grown and
measured at ambient CO2), amb700(plants grown
at ambient CO2 and measured at elevated CO2)
and elev700 (plants grown and measured at
ele-vated CO2). Secondly the effect of CO2
combina-tion on each individual species was assessed with a one-way analysis of variance followed by a Fisher’s LSD test.
C.Roumet et al./En6ironmental and Experimental Botany43 (2000) 155 – 169
160
at ambient CO2when variables are measured both
at ambient and elevated CO2 (amb350vs. amb700:
data collected on the same plants); ’long-term’ effect is used to compare variables measured on plants grown and measured at ambient CO2 to
that of plants grown and measured at elevated CO2(amb350vs. elev700: data collected on different
plants). Down-regulation is said to occur when plants grown at elevated CO2 have lower
photo-synthetic rates than plants grown at ambient CO2,
when both are measured at a common CO2
con-centration (Rey and Jarvis, 1998). The degree of down-regulation was estimated by the ratio
Aelev700/Aamb700, called the ‘assimilation ratio’
(Sage, 1994). The relationships between the assim-ilation ratio and the long-term response of leaf characteristics to CO2 enrichment (i.e. elev700/
amb350 for [TNC]lf, [N]lf, SLA, gs or total leaf
area) were tested using Pearson’s correlation co-efficient. Statistical analyses were conducted with the STATGRAPHICS PLUS software (Manugistics,
USA).
3. Results
3.1. Whole shoot gas exchange
For the four species, the rate of photosynthesis (A) of plants grown at ambient CO2 was
signifi-cantly increased by 30% after a short-term expo-sure to elevated CO2(Aamb350vs.Aamb700), but was
not significantly affected after long-term exposure to elevated CO2(Aamb350vs.Aelev700) (Table 3). The
average assimilation ratio (Aelev700/Aamb700) was
0.8, indicating a down-regulation of photosynthesis.
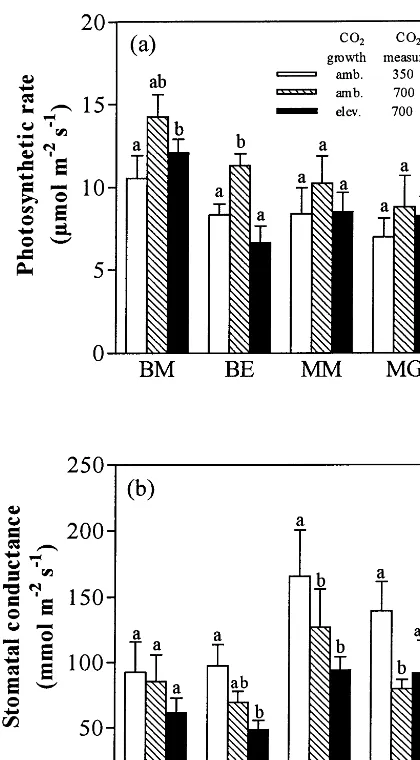
The same trends were observed at the individ-ual species level (Fig. 1a), but the magnitude and the significance of the photosynthetic response to elevated CO2 varied among the four species. In
the twoMedicagospecies, no significant difference was observed between the three CO2
combina-tions (Fig. 1a), while the two Bromus species grown at ambient CO2 exhibited an increase inA
after short-term exposure to elevated CO2(35% in
B. madritensis and 36% in B. erectus) and a down-regulation of photosynthesis. The larger as-similation ratio was observed in B. erectus (0.58 vs. 0.85 inB.madritensis). The results were quali-tatively similar for leaf area-based and mass-based photosynthesis (Table 3).
For the four species, stomatal conductance (gs)
of plants grown at ambient CO2 was significantly
reduced by 27% after a short-term exposure to elevated CO2 (gs amb350 vs. gs amb700) and by 60%
after long-term exposure (gs amb350 vs. gs
elev700)(Table 3). Althoughgs elev700was lower than
gs amb700in three of the four species studied,
differ-ences between these two CO2 treatments were
statistically not different (Table 3). These trends were confirmed at the individual species level for all species except forB.madritensis, which showed no significant difference between the three CO2
combinations (Fig. 1b).
Table 3
Mean photosynthetic ratea
ci/ca A/[N]lf(mmol g−1s−1)
A gs(mmol m−2s−1) A/gs(mmol mol−1)
(mmol m−2s−1) (nmol g−1s−1)
4.68a
amb350 8.56a 171a 124a 0.080a 0.74a
amb700 11.15b 222b 91b 0.137b 0.76ab 6.14b
8.87a 175a 74b
elev700 0.139b 0.79b 5.53ab
a(A) expressed per unit leaf area and unit leaf mass, stomatal conductance (g
s), ratio of intercellular to ambient CO2
concentration (ci/ca), intrinsic instantaneous water use efficiency (A/gs) andA/[N]lf, for: (i) amb350:plants grown and measured at
350mmol mol−1CO2; (ii) amb700: plants grown at ambient CO2and measured at 700mmol mol−1and (iii) elev700: plants grown
at elevated CO2 and measured at 700 mmol mol−1 CO2. Each value is the mean of the four species (n=17–18, i.e. four
Fig. 1. (a) Photosynthetic rate and (b) stomatal conductance (whole shoots) ofB. madritensis(BM), B. erectus (BE), M. minima(MM) andM.glomerata (MG) when plants were (i) grown at ambient CO2 (amb) and measured at 350 mmol
mol−1 CO
2 (open bars); (ii) grown at ambient CO2 and
measured at 700 mmol mol−1 CO2 (hatched bars) or (iii)
grown at elevated CO2 (elev.) and measured at 700 mmol
mol−1 CO
2 (solid bars). Bars represent the means 9S.E.
(n=4 – 6). Bars with different letters are significantly different within a species (LSD, *PB0.05).
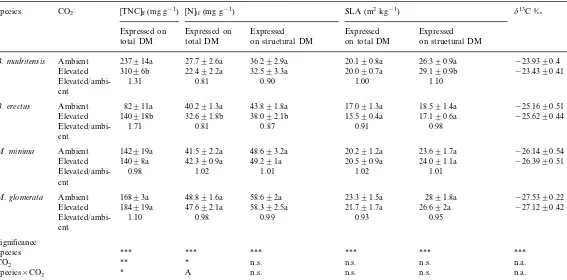
[TNC]lf of B. madritensis being almost twice as
high as that of the other species. Doubling the atmospheric CO2 concentration led to a
signifi-cant CO2×species interaction (Table 4): [TNC]lf
of the two Bromus species was largely increased, 31 and 71% for B. madritensis and B. erectus, respectively, while that of the two Medicago was not significantly affected by elevated CO2.
Leaf nitrogen concentration ([N]lf) expressed on
a dry mass basis differed between species (Table 4) with [N]lf of the two Bromus species being
lower than that of the two Medicago species (31 vs. 45 mg g−1
respectively, averaged over the two CO2 treatments). A nearly significant species×
CO2 interaction (P=0.06,n=40) was found for
[N]lf: leaves of elevated grown legumes were
unaf-fected while those of the two grasses contained on average 19% less nitrogen per unit of dry mass. After correction for non-structural carbohydrates, no significant effect of CO2 on [N]lf could be
detected (Table 4), although [N]lf of the two
grasses was still slightly decreased (10% in B.
madritensis and 13% in B. erectus).
Specific leaf area (SLA) expressed on a total dry mass basis ranged from 16 (B.erectus) to 23 m2
kg−1
(M.glomerata). Whether expressed on a total or structural dry mass basis, SLA was unaf-fected by elevated CO2, except for B.madritensis,
in which the SLA expressed on a structural dry mass basis was slightly increased at elevated CO2
(Table 4).
Leaf carbon isotopic composition differed largely among the four species. Leaf d13C of the
two Bromus species was on average less negative than those of the twoMedicagospecies (Table 4).
3.3. Resource use efficiencies
Intrinsic instantaneous water use efficiency (A/ gs) of the twoMedicagospecies was low compared
to that of the twoBromus(Fig. 2a). As shown on Fig. 3, A/gs was positively correlated with leaf
carbon isotopic composition, indicating that the lower water use efficiency of the twoMedicagoas calculated from gas exchange measurements, was probably maintained over a longer time scale. All species combined (Table 3), as well as at the individual species level (Fig. 2a), short-term
expo-3.2. Characteristics of lea6es
Total non-structural carbohydrate concentra-tion ([TNC]lf) differed widely between species
(Table 4), ranging from 82 to 310 mg g−1
C
.
Roumet
et
al
.
/
En
6
ironmental
and
Experimental
Botany
43
(2000)
155
–
169
162
Table 4
Summary of leaf total non-structural carbohydrate concentration [TNC]lf, leaf nitrogen concentration [N]lf, specific leaf area (SLA), and leaf carbon isotopic
composition (d13C) of the four species used for the gas exchange measurements and grown either at ambient or elevated atmospheric CO
2concentrationsa
SLA (m2kg−1) d13C ‰
[TNC]lf(mg g−1)
Species CO2 [N]lf(mg g−1)
Expressed on Expressed
Expressed on Expressed Expressed
total DM total DM on structural DM on total DM on structural DM
B.madritensis Ambient 237914a 27.792.6a 36.292.9a 20.190.8a 26.390.9a −23.9390.4
32.593.3a 20.090.7a 29.190.9b −23.4390.41
31096b
Elevated 22.492.2a
1.00 1.10
0.90 1.31
Elevated/ambi- 0.81
ent
Ambient 82911a 40.291.3a 17.091.3a 18.591.4a −25.1690.51
B.erectus 43.891.8a
15.590.4a
Elevated 140918b 32.691.8b 38.092.1b 17.190.6a −25.6290.44
0.91 0.98
0.87 Elevated/ambi- 1.71 0.81
ent
48.693.2a 20.291.2a 23.691.7a −26.1490.54
M.minima Ambient 142919a 41.592.2a
24.091.1a −26.3990.51 20.590.9a
49.291a 14098a 42.390.9a
Elevated
1.01
Elevated/ambi- 0.98 1.02 1.02 1.01
ent
2891.8a −27.5390.22
23.391.5a 58.692a
M.glomerata Ambient 16893a 48.891.6a
58.392.5a 21.791.7a 26.692a −27.1290.42
Elevated 184919a 47.692.1a
0.93 0.95
0.99 Elevated/ambi- 1.10 0.98
ent
Significance
*** ***
*** ***
***
Species ***
n.s. n.s. n.a.
** * n.s.
CO2
n.s. n.s. n.s. n.a.
*
Species×CO2 A
a[TNC]
lf, [N]lf, and SLA results are expressed both on a total dry mass (DM) and a structural dry mass basis (stDM). Each value is the mean (9S.E.) of four to
six replicate plants per species and CO2treatment. Levels of statistical significance of the species×CO2ANOVA are: n.s., not significant; a, 0.05BPB0.1; *PB0.05;
sure to elevated CO2 resulted in a large and
significant increase inA/gs(71% on average). This
short-term effect of CO2 was maintained on the
term, despite the lack of a significant long-term response of photosynthesis to CO2. The
in-Fig. 3. Correlation between intrinsic instantaneous water use efficiency (A/gs) and carbon isotopic composition of four
species grown at ambient (open symbols) or elevated (closed symbols) CO2. Each point represents the means 9S.E. (n=
4 – 6). (,B.madritensis; ,B.erectus;,M.minima;
2", M. glomerata). The r values are Pearson’s correlation
coefficients; significance levels are: **PB0.01; ***PB0.001.
Fig. 2. (a) Intrinsic instantaneous water use efficiency (A/gs)
and (b) A/[N]lf of B.madritensis (BM),B. erectus (BE),M.
minima(MM) andM.glomerata (MG) when plants were (i) grown at ambient CO2 (amb) and measured at 350 mmol
mol−1 CO
2 (open bars); (ii) grown at ambient CO2 and
measured at 700 mmol mol−1 CO2 (hatched bars) or (iii)
grown at elevated CO2 (elev) and measured at 700 mmol
mol−1 CO
2 (solid bars). Bars represent the means 9S.E.
(n=4 – 6). Bars with different letters are significantly different within a species (LSD, *PB0.05).
crease in A/gs after prolonged exposure to CO2
was then totally due to the decrease in stomatal conductance (gs). As expected from their highergs
and their slightly lower photosynthetic rate (Fig. 1), the two Medicago operated at a higher ci/ca
ratio than the twoBromusspecies (0.8490.02 vs. 0.6990.03, respectively). Overall, the ci/ca ratio
was not significantly affected after short-term re-sponse to elevated CO2, but it was slightly
in-creased (7%) in plants grown and measured at elevated CO2 (Table 3). These effects were
how-ever not significant for each species taken individually.
The A/[N]lf ratios of three of the four species
were similar (Fig. 2b). B. madritensis showed a higher value. After short-term exposure to ele-vated CO2, as a consequence of the increase in the
photosynthetic rate, A/[N]lf was increased in all
species; however these effects were not statistically significant within each species (Fig. 2b; Table 3). TheA/[N]lfratio of plants grown and measured at
elevated CO2 was not significantly different from
those of plants grown and measured at ambient CO2, except for B. madritensis, which showed a
C.Roumet et al./En6ironmental and Experimental Botany43 (2000) 155 – 169
164
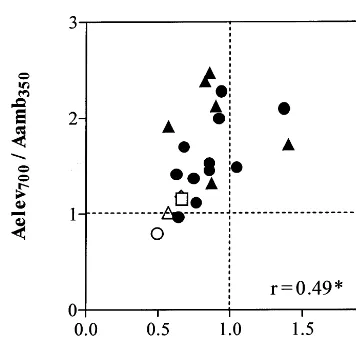
Fig. 4. Relationship between the assimilation ratio (Aelev700/ Aamb700) and the long-term effect of elevated CO2on leaf total non-structural carbohydrates ([TNC]lf 700/[TNC]lf 350) for four
species:,B.madritensis; ,B.erectus;,M.minima;", M. glomerata. The assimilation ratio is defined as the ratio between the photosynthetic rate (expressed per unit of leaf area) of plants grown at elevated and measured at 700mmol
mol−1 CO
2 (Aelev700) and the photosynthetic rate of plants
grown at ambient CO2 and measured a 700 mmol mol−1
(Aamb700). Thervalue is the Pearson’s correlation coefficient.
[TNC]lf to CO2 enrichment ([TNC]lf 700/[TNC]lf
350)(Fig. 4), positively correlated with the
long-term response ofgs(gs elev700/gs amb350), but not with
changes in [N]lf, SLA or total plant leaf area
(Table 5).
4. Discussion
For many of the variables measured, annuals did not differ from perennials but there were marked differences between the two grasses and the two legumes in their response to elevated CO2.
M. minima and M. glomerata had higher leaf nitrogen concentration (Table 4), higher stomatal conductance (gs) and lower A/gs (Figs. 1 and 2)
than B.madritensis andB. erectus; the latter was confirmed over the long term by leaf carbon isotopic composition data. When grown at ele-vated CO2,B.madritensisandB.erectusshowed a
large increase in [TNC]lf and large decrease in
[N]lf, while the [TNC]lfand [N]lfofM.minimaand
M. glomeratawere unaffected. Do these interspe-cific differences translate into differences in pho-tosynthetic response to elevated CO2?
For data pooled over the four species, whole plant photosynthetic rate (A) increased by 30% after short-term exposure to elevated CO2, but 3.4. Whole shoot photosynthesis 6s. characteristics
of lea6es
The assimilation ratio of photosynthesis (Aelev700/Aamb700) for the four species was
nega-tively correlated with the long-term response of
Table 5
Pearson’s correlation coefficients (r) between the assimilation ratio of photosynthesis (Aelev700/Aamb700) and the long-term effect of
CO2 (elev700/amb350) on stomatal conductance (gs), leaf total non-structural carbohydrate concentration ([TNC]lf), leaf nitrogen
concentration ([N]lf), specific leaf area (SLA) and whole plant leaf area of four species (Bromus madritensis,B.erectus,Medicago
minimaandM.glomerata) grown at ambient or elevated CO2a
Long-term CO2response(elev700/amb350) Assimilation ratio (Aelev700/Aamb700)
Aper unit leaf area Aper unit leaf DM
r p
r p
gs 0.89 0.054a 0.87 0.067a
0.069a
−0.86 −0.9
[TNC]lf 0.049*
0.213 ns
0.57 0.57 0.213 ns
[N]lf
0.71 0.144 ns
[N]lfexpressed per structural DM 0.72 0.143 ns
0.41 0.296 ns
SLA 0.58 0.211 ns
−0.69
Leaf area 0.153 ns −0.8 0.102 ns
aThe assimilation ratio is defined as the ratio between the photosynthetic rate of plants grown at elevated CO
2and measured at
700mmol mol−1 CO2 (Aelev700) and that of plants grown at ambient CO2 and measured a 700 mmol mol−1 (Aamb700). The
this effect was not maintained on the long term. After 6 months of growth, photosynthetic rate of CO2 elevated grown-plants (Aelev700) was not
sig-nificantly different from that of plants grown at ambient CO2 (Aamb350) (Table 3). Comparison of
photosynthetic rates measured at a common CO2
concentration (Aelev700 vs. Aamb700), indicated that
the magnitude of the down-regulation varied be-tween species; photosynthesis was significantly down-regulated in the two Bromus species, while it was not in the twoMedicago species (Fig. 1a). The most frequent hypothesis to explain down-regulation is a feedback inhibition of photosyn-thesis by accumulated carbohydrates (Farrar and Williams, 1991; Stitt, 1991; Reining, 1994; Xu et al., 1994a). The experimental evidence supporting a direct linkage between down-regulation and sugar accumulation is not clear however. Some reports have shown that CO2enrichment increases
[TNC]lfwithout decreasing the photosynthetic
en-hancement due to CO2 enrichment (Wullschleger
et al., 1992; Will and Ceulemans, 1997). At the opposite, in Malus domestica (Pan et al., 1998),
Betula pendula(Rey and Jarvis, 1998) and for five boreal tree species (Tjoelker et al., 1998), signifi-cant starch accumulation coincides with a de-crease in photosynthetic rate. Consistent with these later results, in our study, a negative rela-tionship was found between the assimilation ratio (Aelev700/Aamb700) and the proportional change in
[TNC]lf of the four species (Fig. 4, Table 5). B.
erectusshowed the larger down-regulation and the larger [TNC]lfaccumulation whileM.minimaand
M. glomeratashowed no significant down-regula-tion of photosynthesis and no [TNC]lf
accumula-tion. Further experiments with more species are however needed to confirm this relationship. Ac-cumulation of [TNC]lf is a common response of
many species to elevated CO2(Ko¨rner et al., 1995;
Roumet et al., 1996; Poorter et al., 1997; Wang et al., 1999), and is often attributed to an imbalance between the production of assimilate by sources and its utilisation by sinks. The lack of a CO2
effect on [TNC]lffor the two legumes was
consis-tent with the hypothesis that the process of N2
fixation could represent an additional sink, allow-ing them to use the excess carbohydrates pro-duced at elevated CO2.
Since Rubisco represents a major pool of nitro-gen within the leaf (Evans, 1983), and since pho-tosynthesis is often found to be correlated with [N]lf (Evans, 1989), a reduction in [N]lf of plants
grown at elevated CO2may have induced a
reduc-tion in photosynthetic capacity when plants are grown at elevated CO2. While B.madritensis and
B. erectus differed in the magnitude of down regulation of photosynthesis, their [N]lf, even
when expressed on a structural dry mass basis, decreased to a similar extent. [N]lf response to
elevated CO2 cannot therefore be responsible for
the difference in down-regulation observed be-tween these two species. More generally, no sig-nificant relationship was found between the assimilation ratio of the four species and their proportional changes in [N]lf due to CO2
enrich-ment, even when [N]lf was expressed per unit of
structural dry mass (Table 5). The lack of a significant CO2 effect on [N]lf in the two legumes
studied has already been observed by Lu¨scher et al. (1996) for Trifolium repens and could be re-lated to their N2 fixation capacities.
Due to the strong relationship between photo-synthesis andgs(Morison, 1985), a reduction ofgs
at elevated CO2 could result in down-regulation
of photosynthesis. A significant positive relation-ship was found between the long-term response of
gsand the assimilation ratio of photosynthesis for
the four species studied (Table 5). A re-analysis of the data of Beerling and Woodward (1995) con-cerning the long-term response of photosynthesis and stomatal conductance of 17 C3yielded similar
trends; the long-term CO2 response of gs was
positively correlated with the long-term response of A (Fig. 5). Whether changes ingsare the cause
or the consequence of changes in photosynthesis is not clear. In the present study, gs was more
decreased after long-term than after short-term exposure to CO2. The same tendency was
ob-served in Trifolium repens (Ryle et al., 1992),
Triticum aesti6um (Tuba et al., 1994) and Pas
-copyrum smithii (Read et al., 1997). On the con-trary, in Glycine maxand inChenopodium album, the decrease in gs was larger after short-term
rather than long-term exposure to elevated CO2
long-C.Roumet et al./En6ironmental and Experimental Botany43 (2000) 155 – 169
166
Fig. 5. Correlation between the long-term effect of elevated CO2 on whole plant photosynthesis (Aelev700/Aamb350) and the
long-term effect of elevated CO2on stomatal conductance (gs
elev700/gs amb350). Data are from Beerling and Woodward
(1995): for 11 grasses andfor six herbs) and from the present study (B.madritensis,B.erectus,M.minima,
2 M. glomerata; n=4 – 6). The r value is the Pearson’s correlation coefficient; significance level is: *PB0.05.
thetic responses.
In agreement with other studies, A/gs of the
four species studied was increased after short-term exposure to elevated CO2. This short-term effect
was maintained in the long-term despite down-regulation of photosynthesis, mainly because the decrease ingswas maintained or increased on the
long-term. Contrary to what was observed in many other studies (see Drake et al., 1997), here theA/[N]lfratio was not increased after long-term
exposure to elevated CO2, except forB.madriten
-sis. This was due to the small differences in both photosynthesis and [N]lf between the two CO2
treatments, especially in the two legumes.
To understand more precisely the whole plant photosynthetic response to elevated CO2,
vari-ables, other variables than [TNC]lf, [N]lf and gs
should be considered. Indeed whole plant photo-synthetic responses may be affected by: (i) leaf age, with young leaves showing less down regula-tion than older ones, as demonstrated for tomato (Yelle et al., 1989; Besford, 1993), Pinus radiata
(Turnbull et al., 1998) and soybean (Xu et al., 1994a); (ii) increased plant leaf area for plants grown at elevated CO2. This could induce more
self shading and limit photosynthetic response (Poorter et al., 1988). The negative but non-sig-nificant relationship found between the assimila-tion ratio and the proporassimila-tional change in leaf area, suggests that the latter may indeed occur (P=0.15, Table 5).
In conclusion, species-specific differences in down-regulation could be attributed to interspe-cific differences in the responses of [TNC]lf and
stomatal conductance. As expected, down-regula-tion was less pronounced in legumes than in grasses, and the [N]lf and [TNC]lf concentrations
of the former were not affected by elevated CO2.
However, no clear differences appeared between annual and perennial species. Evidence of impor-tant differences between the response of the two
Bromus species and that of the two Medicago
species, is in line with the hypothesis that grasses and legumes belong to different groups of re-sponse to elevated CO2 (Poorter, 1993). These
differences in chemical composition and in photo-synthetic responses to elevated CO2 are likely to
translate into growth differences and might influ-ence plant – herbivore interaction.
term responses of gs were observed in Phaseolus
6ulgaris (Radoglou et al., 1992), Helianthus an -nuus, Hordeum 6ulgare, Brassica napus and
Triticum aesti6um (Morison, 1998). Despite this
large variability of response ings, in most studies
the ci/ca ratio was found to be maintained after
short-term exposure to CO2and slightly increased
after long-term exposure (Sage, 1994; Drake et al., 1997; Bryant et al., 1998). This was confirmed in our study (Table 3). Therefore, although they have a lower gs, leaves from acclimated plants
always have a similar or higher ci than that of
leaves from ambient grown plants measured at ambient or elevated CO2. Thus stomatal
conduc-tance appeared to interact with CO2 uptake to
maintain ci as a constant proportion of ca (Rey
and Jarvis, 1998). This does not support the hy-pothesis that decreased gs is a major cause of
photosynthetic down-regulation. In addition, the stomatal limitation to CO2 uptake, calculated
from A/ci curves, has often been found to
de-crease at elevated CO2 (Long et al., 1996; Stirling
photosyn-Acknowledgements
This work was funded by the European Union through the project ‘Plant processes and biodiver-sity in ecosystem response to global climatic changes’ (EV5V-CT94-0428). The authors thank Ge´rard Laurent and Nathalie Bernadou for grow-ing the plant, assistance durgrow-ing photosynthetic measurements and harvests, Franc¸oise Lafont for her precious advice on the use of the elemental analyser, Pascal Tillard (I.N.R.A., Physiologie et Biochimie Ve´ge´tales, Montpellier) for the carbon isotopic composition determination, Jean-Claude Cleyet-Marel and He´le`ne Bertrand (I.N.R.A., Symbiotes des racines, Montpellier) for isolating and providing the two strains of Rhizobium meliloti, Jean-Marie Prosperi (I.N.R.A., Station de Ge´ne´tique et d’Ame´lioration des Plantes, Montpellier) for providing seeds of Medicago glomerata, Jacques Fabreguettes and Franc¸ois Jardon for improving and maintaining the gas exchange system and the staff of the C.E.F.E. experimental garden for the management of glasshouses. Meg Crookshanks is also warmly thanked for her comments.
References
Beerling, D.J., Woodward, F.I., 1995. Leaf stable carbon isotope composition records increased water-use efficiency of C3 plants in response to atmospheric CO2 enrichment.
Funct. Ecol. 9, 394 – 401.
Besford, T., 1993. Photosynthetic acclimation in tomato plants grown in high CO2. Vegetatio 104/105, 441 – 448.
Bryant, J., Taylor, G., Frehner, M., 1998. Photosynthetic acclimation to elevated CO2 is modified by source: sink
balance in three component species of chalk grassland swards grown in a free air carbon dioxide enrichment (FACE) experiment. Plant Cell Environ. 21, 159 – 168. Curtis, P.S., 1996. A meta-analysis of leaf gas exchange and
nitrogen in trees grown under elevated carbon dioxide. Plant Cell Environ. 19, 127 – 137.
Drake, B.G., Gonza`lez-Meler, M.A., Long, S.P., 1997. More efficient plants: a consequence of rising atmospheric CO2?
Annu. Rev. Plant Physiol. Plant Mol. Biol. 48, 607 – 637. Dubois, M., Gilles, K.A., Hamilton, J.K., Rebers, P.A.,
Smith, F., 1956. Colorimetric method for determination of sugars and related substances. Anal. Chem. 28, 350 – 356. Evans, J.R., 1983. Nitrogen and photosynthesis in a flag leaf
of wheat (Triticum aestiumL.). Plant Physiol. 14, 59 – 68.
Evans, J.R., 1989. Photosynthesis and nitrogen relationships in leaves of C3 plants. Oecologia 78, 9 – 19.
Farquhar, G.D., Ehleringer, J.R., Hubick, K.T., 1989. Carbon isotope discrimination and photosynthesis. Annu. Rev. Plant Physiol. Plant Mol. Biol. 40, 503 – 537.
Farrar, J.F., Williams, M.L., 1991. The effects of increased atmospheric carbon dioxide and temperature on carbon partitioning, source – sink relations and respiration. Plant Cell Environ. 14, 819 – 830.
Farrar, J.F., 1993. Carbon partitioning. In: Hall, D.O., Scur-lock, J.M.O., Bolhar-Nordenkampf, H.R., Leegood, S.C., Long, S.P. (Eds.), Photosynthesis and Production in a Changing Environment. A Field and Laboratory Manual. Chapman and Hall, London, pp. 232 – 246.
Garnier, E., Aronson, J., 1998. Nitrogen-use efficiency from leaf to stand level: clarifying the concept. In: Lambers, H., Poorter, H., van Vuuren, M.M.I. (Eds.), Inherent Varia-tion in Plant Growth. Physiological Mechanisms and Eco-logical Consequences. Backhuys, Leiden, pp. 515 – 538. Garnier, E., Cordonnier, P., Guillerm, J.L., Sonie´, L., 1997.
Specific leaf area and leaf nitrogen concentration in annual and perennial grass species growing in Mediterranean old-fields. Oecologia 111, 490 – 498.
Garnier, E., 1992. Growth analysis of congeneric annual and perennial grass species. J. Ecol. 80, 665 – 675.
Greer, D.H., Laing, W.A., Campbell, B.D., 1995. Photosyn-thetic responses of thirteen pasture species to elevated CO2
and temperature. Aust. J. Plant Physiol. 22, 713 – 722. Gunderson, C.A., Wullschleger, S.D., 1994. Photosynthetic
acclimation in trees to rising atmospheric CO2: a broader
perspective. Photosyn. Res. 38, 369 – 388.
Hardy, R.W.F., Holsten, R.D., Jackson, E.K., Burns, R.C., 1968. The acetylene – ethylene assay for N2fixation:
Labo-ratory and field evaluation. Plant Physiol. 43, 1185 – 1207. Hebeisen, T., Lu¨scher, A., Zanetti, S., Fischer, B.U., Hartwig, U.A., Frehner, M., Hendrey, G.R., Blum, H., No¨sberger, J., 1997. Growth response of Trifolium repens L. and Lolium perenneL. as monocultures and bi-species mixture to free air CO2 enrichment and management. Global
Change Biol. 3, 149 – 160.
Henriksen, A., Selmer-Olsen, A.R., 1970. Automatic methods for determining nitrate and nitrite in water and soil ex-tracts. Analyst 95, 514 – 518.
Koch, G.W., Winner, W.E., Nardone, A., Mooney, H.A., 1987. A system for controlling the root and shoot environ-ment for plant growth studies. Environ. Exp. Bot. 27, 365 – 377.
Ko¨rner, C.h., Pelaez-Riedl, S., Van Bel, A.J.E., 1995. CO2
responsiveness of plants: a possible link to phloem loading. Plant Cell Environ 18, 595 – 600.
Larigauderie, A., Roy, J., Berger, A., 1986. Long-term effects of high CO2 concentration on photosynthesis of water
hyacinth [Eichhornia crassipes(Mart.) Solms]. J. Exp. Bot. 37, 1303 – 1312.
C.Roumet et al./En6ironmental and Experimental Botany43 (2000) 155 – 169
168
J.M., Agren, G.I. (Eds.), Global Change: Effects on Conif-erous Forests and Grasslands. Scope 56. Wiley, Chichester, pp. 121 – 159.
Luo, Y., Chen, J.L., Reynolds, J.F., Field, C.B., Mooney, H.A., 1997. Disproportional increases in photosynthesis and plant biomass in a Californian grassland exposed to elevated CO2: a simulation analysis. Funct. Ecol. 11, 696 –
704.
Lu¨scher, A., Hebeisen, T., Zanetti, S., Hartwig, U.A., Blum, H., Hendrey, G.R., No¨sberger, J., 1996. Differences be-tween legumes and nonlegumes of permanent grassland in their responses to free-air carbon dioxide enrichment: its effect on competition in a multispecies mixture. In: Ko¨rner, Ch., Bazzaz, F. (Eds.), Carbon dioxide, Populations, and Communities. Academic Press, San Diego, CA, pp. 287 – 300.
Lu¨scher, A., Hendrey, G.R., No¨sberger, J., 1998. Long-term responsiveness to free air CO2 enrichment of functional
types, species and genotypes of plants from fertile perma-nent grassland. Oecologia 113, 37 – 45.
Morison, J.I.L., 1985. Sensitivity of stomata and water use efficiency to high CO2. Plant Cell Environ. 8, 467 – 474.
Morison, J.I.L., 1998. Stomatal response to increased CO2
concentration. J. Exp. Bot. 49, 443 – 452.
Pan, Q., Wang, W., Quebedeaux, B., 1998. Responses of the apple plant to CO2enrichment: changes in photosynthesis,
sorbitol, other soluble sugars, and starch. Aust. J. Plant Physiol. 25, 293 – 297.
Poorter, H., Pot, S., Lambers, H., 1988. The effect of an elevated atmospheric CO2concentration on growth,
photo-synthesis and respiration ofPlantago major. Physiol. Plant. 73, 553 – 559.
Poorter, H., van Berkel, Y., Den Hertog, J., Dijkstra, P., Gifford, R.M., Griffin, K.L., Roumet, C., Roy, J., Wong, S.C., 1997. The effect of elevated CO2 on the chemical
composition and construction costs of leaves of 27 C3
species. Plant Cell Environ. 20, 472 – 482.
Poorter, H., 1993. Interspecific variation in the growth re-sponse of plants to an elevated ambient CO2
concentra-tion. Vegetatio 104/105, 77 – 97.
Radoglou, K.M., Aphalo, P., Jarvis, P.G., 1992. Response of photosynthesis, stomatal conductance and water use effi-ciency to elevated CO2and nutrient supply in acclimated
seedlings ofPhaseolus6ulgarisL. Ann. Bot. 70, 257 – 264. Read, J.J., Morgan, J.A., Chatterton, N.J., Harrison, P.A., 1997. Gas exchange and carbohydrate and nitrogen con-centrations in leaves of Pascopyrum smithii (C3) and
Bouteloua gracilis(C4) at different carbon dioxide
concen-trations and temperatures. Ann. Bot. 79, 197 – 206. Reining, E., 1994. Acclimation of C3 photosynthesis to
ele-vated CO2: hypotheses and experimental evidence.
Photo-synthetica 30, 519 – 525.
Rey, A., Jarvis, P.G., 1998. Long-term photosynthetic accli-mation to increased atmospheric CO2 concentration in
young birch (Betula pendula) trees. Tree Physiol. 18, 441 – 450.
Roumet, C., Roy, J., 1996. Prediction of the growth response to elevated CO2: a search for physiological criteria in
closely related grass species. New Phytol. 134, 615 – 621. Roumet, C., Bel, M.P., Sonie´, L., Jardon, F., Roy, J., 1996.
Growth response of grasses to elevated CO2: a
physiologi-cal plurispecific analysis. New Phytol. 133, 595 – 603. Ryle, G.J.A., Woledge, J., Tewson, V., Powell, C.E., 1992.
Influence of elevated CO2and temperature on the
photo-synthesis and respiration of white clover dependant on N2
fixation. Ann. Bot. 70, 213 – 220.
Sage, R.F., Sharkey, T.D., Seeman, J.R., 1989. Acclimation of photosynthesis to elevated CO2 in five C3 species. Plant
Physiol. 89, 590 – 596.
Sage, R.F., 1994. Acclimation of photosynthesis to increasing atmospheric CO2: the gas exchange perspective. Photosyn.
Res. 39, 351 – 368.
S&antru˚cˇek, J., Sage, R.F., 1996. Acclimation of stomatal con-ductance to a CO2-enriched atmosphere and elevated
tem-perature inChenopodium album. Aust. J. Plant Physiol. 23, 467 – 478.
Soussana, J.F., Hartwig, U.A., 1996. The effects of elevated CO2 on symbiotic N2fixation: a link between the carbon
and nitrogen cycles in grassland ecosystems. Plant Soil 187, 321 – 332.
Stirling, C.M., Davey, P.A., Williams, T.G., Long, S.P., 1997. Acclimation of photosynthesis to elevated CO2 and
tem-perature in five British native species of contrasting func-tional types. Global Change Biol. 3, 237 – 246.
Stitt, M., 1991. Rising CO2 levels and their potential
signifi-cance for carbon flow in photosynthetic cells: commis-sioned review. Plant Cell Environ. 14, 741 – 762.
Tjoelker, M.G., Oleksyn, J., Reich, P.B., 1998. Seedlings of five boreal tree species differ in acclimation of net photo-synthesis to elevated CO2and temperature. Tree Physiol.
18, 715 – 726.
Tuba, Z., Szente, K., Koch, J., 1994. Response of photosyn-thesis, stomatal conductance, water use efficiency and pro-duction to long-term elevated CO2 in winter wheat. J.
Plant Physiol. 144, 661 – 668.
Turnbull, M.H., Tissue, D.T., Griffin, K.L., Rogers, G.N.D., Whitehead, D., 1998. Photosynthetic acclimation to long-term exposure to elevated CO2 concentration in Pinus
radiataD. Don. is related to age of needles. Plant Cell Environ. 21, 1019 – 1028.
von Caemmerer, S., Farquhar, G.D., 1981. Some relationships between the biochemistry of photosynthesis and the gas exchange of leaves. Planta 153, 376 – 387.
Wang, Z., Pan, Q., Quebedeaux, B., 1999. Carbon partitioning into sorbitol, sucrose, and starch in source and sink apple leaves as affected by elevated CO2. Environ. Exp. Bot. 41,
39 – 46.
Will, R.E., Ceulemans, R., 1997. Effects of elevated CO2 on
photosynthesis, respiration and carbohydrate status of coppicePopulushybrids. Physiol. Plant. 100, 933 – 939. Wullschleger, S.D., Norby, R.J., Hendrix, D.L., 1992. Carbon
status of two forest tree species exposed to carbon dioxide enrichment. Tree Physiol. 10, 21 – 31.
Xu, D.Q., Gifford, R.M., Chow, W.S., 1994a. Photosynthetic acclimation in pea and soybean to high atmospheric CO2
partial pressure. Plant Physiol. 106, 661 – 671.
Xu, D.Q., Terashima, K., Crang, R.F.E., Chen, X.M.,
Hes-keth, J.D., 1994b. Stomatal and nonstomatal acclimation to a CO2-enriched atmosphere. Biotronics 23, 1 – 9.
Yelle, S., Beeson, R.C., Trudel, M.J., Gosselin, A., 1989. Acclimation of two tomato species to high atmospheric CO2. I. Sugar and starch concentrations. Plant Physiol. 90,
1465 – 1472.
![Fig. 2. (a) Intrinsic instantaneous water use efficiency (Amolmolgrown at elevated COminima(within a species (LSD, *measured at 700and (b)grown at ambient CO/gs) A/[N]lf of B](https://thumb-ap.123doks.com/thumbv2/123dok/3128014.1380630/9.612.52.251.131.510/intrinsic-instantaneous-efciency-amolmolgrown-elevated-cominima-measured-ambient.webp)