Structural characterization of LiCrxMn2-xO4 via a simple reflux technique
Dyah Purwaningsih, Roto Roto, Hari Sutrisno, and Agus PurwantoCitation: AIP Conference Proceedings1823, 020100 (2017); doi: 10.1063/1.4978173
View online: http://dx.doi.org/10.1063/1.4978173
View Table of Contents: http://aip.scitation.org/toc/apc/1823/1
Structural Characterization of LiCr
xMn
2-xO
4via A Simple Reflux Technique
Dyah Purwaningsih
1,2,a, Roto Roto
1,a, Hari Sutrisno
2, Agus Purwanto
31Department of Chemistry, Faculty of Mathematics and Natural Sciences,
Gadjah Mada University, Yogyakarta, Indonesia.
2Department of Chemistry Education, Faculty of Mathematics and Natural Sciences,
Yogyakarta State University, Indonesia.
3Department of Chemical Engineering, Faculty of Engineering
Sebelas Maret University, Indonesia
a)Corresponding author: [email protected] or [email protected]
Abstract. LiCrxMn2-xO4 (x=0; 0.02; 0.04; 0.06; 0.08, 0.10) have been successfully synthesized via a facile and simple
reflux technique. The SEM-EDS data confirm the presence of Cr, Mn and O elements in the products, while the XRD pattern suggests that the materials have well-developed cubic crystals. Direct method was applied to extract structural parameters of LiCrxMn2-xO4 using the Fullprof and Oscail software in WinPlotr package program. Materials were refined
in the crystal system, and space group of structures Fd3m phase were then identified. The lattice parameters decrease with the decrease in Cr content. The highest Li-O bond length was found for LiCr0.10Mn1.90O4. It was observed that there
is no significant change in particle size as Cr content increased.
INTRODUCTION
LiMn2O4 has recently become an attractive material for the cathode of rechargeable lithium-ion batteries because
it has high capacity and is cheap [1]–[3][4]. However, the pure LiMn2O4 with spinel structure experience has a big
capacity fading during cycling especially at high temperature (T>55oC). The capacity fading mechanism of these
cathodes is very complex and depends on many factors including structural transition due to Jahn-Teller distortion, dissolution in the electrolyte, and oxidation of the electrolyte on the electrolyte at the high potential charging [5]. In the past few years, researchers have focused on synthesizing LiMn2O4 doped with various elements to improve its
specific capacity by using such metals as Co [6]–[8], Ni[9]–[12] and Cr [13]
Microstructure characterization of LiMn2O4 from the XRD-powder data is usually conducted on computer
programs which are usually classified into two: the traditional approach (Rietveld method) and direct space approach including simulated annealing, genetic algorithm, Monte Carlo techniques and Direct Method [14] . Both approaches necessitate the minimum required information on experimental diffraction profile, cell parameters, space group and unit cell content. However, the direct space methods also requires the information on the structure molecular geometry.
We report here that the synthesis of LiCrxMn2-xO4 at various mol ratios Cr/Mn mole ratios using reflux technique
followed by solid-state reaction has been successfully conducted. The obtained data from the XRD-powder were then analyzed as single crystal-like data by using the Direct Methods applied on FullProf Pattern Matching and Oscail Program. In addition, we also investigated the physical properties and the microstructures of the material.
EXPERIMENTAL SECTION
Synthesis of MnO
2and LiCr
xMn
2-xO
4.
An analytical grade of Mn(CH3COO)2 and Na2S2O8 (Aldrich) were used to prepare MnO2. The chemicals were
deionized distilled water at room temperature by magnetic stirring to form a clear solution. The solution was transferred into the boiling flask and heated at 120 oC for 12 hours. The solid was separated and dried in the air at
110 oC for 12 h [15].
Synthesis of LiCr
xMn
2-xO
4is as follows.
One mole of LiOH, 0.02 moles of Cr(CH3COO)3 and 1.98 moles of as-synthesized MnO2, were dispersed into
pure ethanol to form a thick slurry and stirred for several hours followed by separation and drying at room temperature. The process was repeated 2-3 times. The LiCr0.02Mn1.98O4 solid was then ignited at 750oC for 10 hours.
The same steps were done for other Cr to Mn mole ratios.
Determination of the LiCr
xMn
2-xO
4microstructure.
The morphologies were obtained on JEOL JSM-6510LA SEM. The content of the doping metal in LiMn2O4 was
studied using EDS, along with the content of Mn and O in the material. The MnO2 and LiCrxMn2-xO4 powders
were examined Rigaku Miniflex 600-Benchtop XRD instrument, using Cu Kα radiation (O = 1.5406 Å) at ambient temperature. The XRD was operated at accelerating voltage of 40 kV and current of 15 mA, the scan rate of 0.02o/s,
2ϴ interval 20o to 90o. Direct Method analysis was carried out by using the Fullprof software developed by Roisnel
and Rodriguez-Carvajal in WinPLOTR package program, Oscail and Diamond. The parameters for refining are a unit cell, scale factor, and full width at half-maximum (FWHM). The BET surface area was obtained by gas sorption.
RESULTS AND DISCUSSION
(a)
(b)
(d)
(e)
(f)
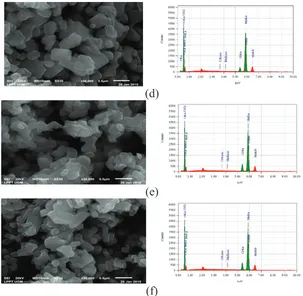
FIGURE 1. SEM photographs and EDS of (a) LiMn2O4, (b) LiCr0.02Mn1.98O4, (c) LiCr0.04Mn1.96O4,(d) LiCr0.06Mn1.94O4, €
LiCr0.08Mn1.92O4, and (f) LiCr0.10Mn1.90O4.
Fig. 1 shows the SEM images of LiCrxMn2-xO4 particles at various mole fractions. The pure LiMn2O4 has cubic
shape with the size smaller than that of ones doped with Cr, LiCrxMn2-xO4. The size of the particles is in the range of
150-500 nm. The LiCr0.10Mn1.90O4 has uniform particle size. The doped powder consisted of aggregates of
well-developed crystals. The EDS data of the materials show that the Cr content is expected as according to the predetermined Cr to Mn molar ratio. It suggests that the Cr content in the LiMn2O4 corresponds to the amount of Mn
to be replaced in the structure. Also, as expected, the peak intensity of Cr increases with an increase in Cr content.
(c) (d)
(e) (f)
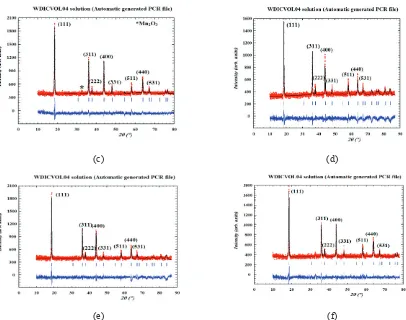
FIGURE 2. XRD patterns of (a) LiMn2O4, (b) LiCr0.02Mn1.98O4, (c) LiCr0.04Mn1.96O4, (d) LiCr0.06Mn1.94O4, € LiCr0.08Mn1.92O4,
and (f) LiCr0.10Mn1.90O4.
Fig. 2 shows the XRD patterns of LiCrxMn2-xO4. The microstructure analysis using winPLOTR package
program and Diamond indicates that the products can be identified as a single-phase cubic with a space group of Fd3m where lithium ions occupy the tetrahedral (8a) sites. Further, Mn3+ or Mn4+ or Cr3+ ions positioned at the centers of the octahedron (16d), whereas O2- ions placed at the edge of the octahedron (32e). The LiCr
xMn2-xO4
structure is in accord with that of the pure LiMn2O4. The difference in XRD patterns of among LiCrxMn2-xO4 with
different doping compositions was not obvious.
TABLE 1. Lattice constants, unit cell volume and agreement factor of LiCrxMn2-xO4 produced
x in LiCrxMn2-xO4
Lattice
Parameter (A) Volume (A)3 Agreement Factor from Direct Methods (Oscail Programs)
a=b=c R1 wR2 GooF
0 8.2452 560.5360951 0.0917 0.1640 3.518
0.02 8.233768 558.2077703 0.0780 0.2125 1.880
0.04 8.239864 559.4485222 0.0698 0.2005 1.602
0.06 8.233999 558.2547535 0.0846 0.1709 2.393
0.08 8.237355 558.9376295 0.0583 0.1203 0.443
0.10 8.233329 558.1184891 0.0821 0.2526 2.292
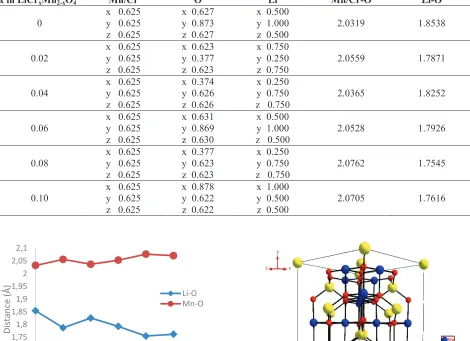
Table 1 shows the lattice parameters and volumes of the materials after being refined using FullProf and Oscail. We used FullProf Pattern Matching for extract the intensity data from the powder diffractions and followed by Oscail program to refine as single crystal-like using Direct Method [14]. It shows that both lattice parameters and cell volumes decrease with increase in Cr to Mn mole fraction. It may be due to the partial substitution of Mn3+
Mn4+ content are detected in all LiCr
xMn2-xO4 solids. We expect the Jahn-Teller distortion is minimum during
cycling with this modification [16]. The reason may be suggested as (1) ionic radius of Mn3+ (0.645 Å) is larger than
that of Mn4+ ions (0.53 Å) and (2) the Cr/Mn-O interatomic distance increases with increase in Cr content (see Fig.
3.). It is also believed that the lattice contraction of LiCrxMn2-xO4 causes the decrease in the volume of the unit cell.
The increase in Cr composition is accompanied by drop of lattice parameters. Table 2 shows the atomic position of Mn/Cr, O and Li in LiCrxMn2-xO4
the highest in LiCr0.10Mn1.90O4. As a result, Li-O bond distance of LiCr0.10Mn1.90O4 is easily separated from that of
(a)
(b)
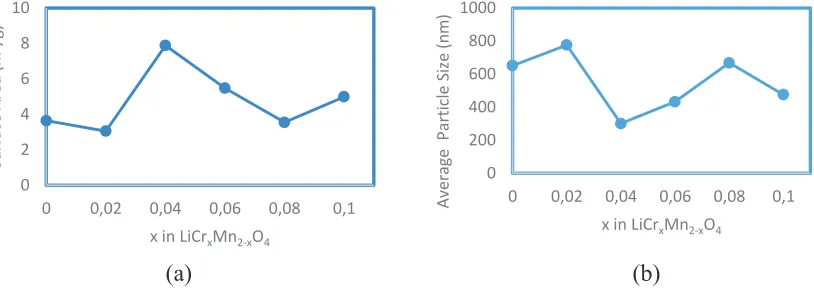
FIGURE 4. (a) BET surface area of LiCrxMn2-xO4 (b) Average Particle Size of LiCrxMn2-xO4.
Fig. 4(a) shows the BET surface area of LiCrxMn2-xO4 with different Cr to Mn mole ratio. The calculated BET
surface area suggests that doping with Cr at various Cr to Mn mole ratios do not affect changes in the structure of LiCrxMn2-xO4. Fig. 4(b) also shows that the substitution of Mn by Cr in the structure causes the particle size of the
LiCrxMn2-xO4 to increase. However, the variation in BET surfaces area is not clear cut to be related to the addition of
Cr.
CONCLUSION
The LiCrxMn2-xO4 materials have been synthesized via reflux technique. The SEM-EDS analysis suggests the
presence of Cr, Mn and O elements in the products. Direct methods using FullProf Pattern Matching and Oscail Program has been successfully applied to refine the crystal system and space group. The XRD result reveals that the materials exhibit well-developed cubic Fd3m. The lattice parameters decrease with the decrease in x values. The highest Li-O bond length is found in the LiCr0.10Mn1.90O4. There was no significant change in the particle size that
accompanied by mole fraction escalation.
6. D. Purwaningsih, R. Roto, Narsito and H. Sutrisno, Adv. Mater. Res.1123, 100–103 (2015). 7. A. D. Robertson, A. R. Armstrong and P. G. Bruce, J. Power Sources98, 332–335 (2001). 14. A. Altomare, R. Caliandro, C. Cuocci, C. Giacovazzo, A. G. G. Moliterni, R. Rizzi and C. Platteau, J. Appl.