---
--- (Received; Sep. 15, 2006: Accepted; Dec. 8, 2006)
*
Corresponding author: tkoike@for.agr.hokudai.ac.jpEffects of Soil and Vegetation Types on Soil Respiration Rate in Larch
Plantations and a Mature Deciduous Broadleaved Forest in Northern Japan
Y
ANAGIHARAYuko
1, S
HIBATAHideaki
2, M
ATSUURAYojiro
3and K
OIKETakayoshi
4*
1
Graduate School of Agriculture, Hokkaido University, Sapporo 060-8589, Japan 2
North Research Station, Field Science Center for Northern Biosphere, Hokkaido University, Nayoro 096-0071, Japan
3 Forestry and Forest Products Research Institute, Tsukuba, 305-8687, Japan 4
Hokkaido University Forests, FSC, Sapporo 060-0809, Japan; present address: Research Faculty of Agriculture, Hokkaido University,
Sapporo 060-8589, Japan
Abstract
Seasonal changes in the soil respiration rate, soil water content, temperature at 5cm depth in soil and C/N in soil were detected in four different forests in Hokkaido, northern Japan for evaluating CO2 efflux from forest ecosystem for two years. Each forest was mature deciduous broadleaved forest (more than 300 years old after eruption of Mt. Tarumae) and declining larch plantation (as of 46 years old at 2000) invaded several deciduous broadleaved saplings in Tomakomai Experimental Forest, a mature larch plantation in National Forest (as of about 49 years old at 2000) and a young larch plantation (as of 27 years old at 2000) with dense coverage of dwarf Sasa bamboo in Teshio Experimental Forest. Soil respiration of each site correlated exponentially with soil temperature but not soil water content. Prior to the measurement of soil respiration, its variation was determined with a 22 x 22m plot for 112 points in June. According to this, we selected the place for soil respiration measurement. Root density was almost constant of 1.0 g 100m-3 for the old larch plantation but increased from 1 to 3 g 100m-3 to for young one, which positively correlated with soil respiration. C/N ratio of a young larch plantation was slightly larger than that of old one. Based on these results, we discussed the factors affecting soil respiration in northern Japan.
Key words: Soil respiration, larch plantation, mature deciduous broadleaved forest, C/N ratio, environmental factors
Abbreviations:
TE: 1arch Larch Forest in Teshio TO-larch: Larch Forest in Tomakomai
TO-d-larch: Declining Larch Forest in Tomakomai TO-broad: Broad-Leaved Forest in Tomakomai Rs: Soil Respiration Rate ( μmol m-2 s-1 ) Ts: Soil Temperature at 10cm depth Cs: Soil Carbon Content (%)
Introduction
Atmospheric carbon dioxide (CO2) concentration is increasing yearly, which inducing global greenhouse conditions, due to use of fossil fuels and the destruction of tropical forests (Strain 1985). Hougton et al. (1990) reported that during the 21st century, atmospheric CO2 concentration would become twice and temperature would increase 3-5℃ at high latitude in Northern
Hemisphere. Moreover, according to the monitoring record of Mauna Loa in Hawaii, seasonal depression of CO2 concentration is coincided with the growth period of terrestrial plants in Northern Hemisphere (Butcher et al. 1998). According to the Kyoto Protocol (1998), forest ecosystems are expected to be a big CO2 sink. Many studies have been conducting CO2 flux of forests developing through Western Europe and U.S.A. to evaluate the CO2 Sink capacity (Valentini et al. 2000,
Valentini 2003). Some previous studies revealed that carbon in soil was as twice or third times higher as carbon in plants (Schimel et al. 1994, Houghton et al.
1996). Therefore, it has become to be appeared that an understanding of carbon efflux is necessary if we would evaluate whether forest soil as a source of the carbon evolution or as a carbon pool.
Carbon cycling and budget in terrestrial ecosystems fluctuate with the balance of photosynthesis and respiration by mainly vegetation and microorganisms (Shibata et al. 2005). The integrated monitoring of carbon exchange between atmosphere and forest has been recently conducted in various biomes (Baldocchi
Carbon dynamics in forest also varied with different vegetation types in each climate zone (Valentini 2003). Larch forests are broadly distributed on the eastern part of Eurasian continent, where permafrost layers are well developed (Koike et al. 2000). Larch forests may act as an important role in carbon balance in the atmosphere. When we would estimate global carbon budgets, we need to know about not only rates of CO2 released from a larch forest floor but also factors affecting CO2 efflux from the larch forest ecosystem.
In Japan, natural larch forests are found at Nagano, central Japan and Miyagi, northern Honshu Island. Many larch plantations have been afforested in all Hokkaido Island, except for high altitude (Koike et al.
2000). These areas are suitable to estimate CO2 flux of larch forest ecosystems because many larch plantations are established on flatland. Among several plantations of larch species in Hokkaido, northern Japan, some of them were made on unsuitable soil conditions for tree growth. Moreover, many larch plantations have been suffering from several stresses, such as low temperature damage, the disease caused by shoot blight, root rot, grazing damage by voles etc. (Koike et al. 2000). Afterwards, many larch plantations have been showing declining symptom, which may allow the invasion of broad-leaved tree seedlings to the open space provided by dead larch trees. It seems that growth and health condition of larch would be strongly affected by soil conditions.
Soil respiration is affected by various factors, such as soil temperature (Anderson 1973, Crill 1991, Gupta et al. 1981, Mathes et al. 1985, Grahammer et al. 1991), soil moisture (Grahammer et al. 1991, Kowalenko et al.
1987) and content of nitrogen (Maier et al. 2000, Yanagihara et al. 2000). Moreover, the amount of organic matter, root biomass, and carbon in soil may also affect soil respiration rates. If the environment condition, such as soil type and vegetation difference would change also these factors and total interactions would be responded to them. Therefore, our overall objective was to know about the seasonal trend of soil respiration of various larch plantations and a mature deciduous forest.
For this objective, physiological and biological environments affecting CO2 efflux from forest floor was detected in situ. First specific objective is to understand how different soil types affect soil CO2 flux. Therefore, we lead first prediction; “soil respiration under unsuitable soil condition is lower than that of suitable condition.” In addition, some declining larch plantations, which were unmanaged under inadequate soil conditions for larch species, may change other types of forests mainly composing potential or original vegetation.
The second objective was to access the reason why metabolic activities were changed in the rhizosphere by the modification of vegetation from the original plant community to a larch plantation. We thought that in these larch forests, soil biological activity, such as metabolism of roots and microorganisms, maybe degraded than that of natural vegetation. Therefore, we lead next hypothesis, “soil respiration rate of declining
larch stand plot is lower than that of the natural vegetation”.
To examine these predictions, we measured seasonal changes in soil respiration, temperature in soil, soil moisture content in two types of larch plantations and a mature deciduous broadleaved forest in northern Japan for two years. Based on the results, we discussed the factors affecting soil respiration.
Materials and Methods Study Sites
We established two study sites of larch plantations. First we made three plots in Tomakomai National Forest (Compartment No. 1198) in Tomakomai city (call this plot as “TO-larch”), southeast of Hokkaido (42 o 40'N, 141 o 36'E) in northern Japan. In Tomakomai, the mean annual temperature is 5.6 oC and the mean annual precipitation is 1161 mm (Hiura et al. 1998). In TO-larch, the soil type was immature soil derived from volcanic ash and there is a thin organic horizon (Eguchi
et al. 1997). Soil profile is shown in Appendix 1. In Tomakomai Experimental Forest of Hokkaido University (TOEF; 42o40'N, 141o36'E), the other two sites were established in Japanese larch plantations along to the different degree of mixing with invaded broad-leaved trees. Namely, there were three plots including the TO-larch.
The second plot was the larch plantation in TOEF, which have been unmanaged and invaded by several broad-leaved trees (called this plot as TO-d-larch). Original vegetation in this area is several kinds of deciduous broad-leaved tree species (Takahashi et al.
1999). The third plot was selected in a mature broad-leaved forest (called this plot as TO-broad) (more than 300 years old after eruption of Mt. Tarumae). Dominant species were Ostrya japonica, Acer mono, Ceridiphyllum japonicum and Quercus mongolica var.
crispula (Hiura et al. 1998). In these three sites in Tomakomai region, soil type was all the same. Moreover, these areas belong to cool temperate region with little snow-fall and the soils are usually frozen from December to late April. We conducted field research from May to November 1999 and from May and to October 2000.
We also determined the other plot in Teshio Experimental Forest in Horonobe town (call this plot as “TE-larch”) which was located m northern most Hokkaido (44o53'N, 142o 03'E). In Teshio, mean annual temperature is 5.7 oC and the mean annual precipitation is 1124 mm. In TE-larch, the soil type was classified as brown-forest soil. Soil profile in TE-larch is shown in Appendix 2.
Sizes of both plots in Tomakomai and Teshio were 50 m in square. Stand age of plantations was 27-year old in TE-larch and of 46-year old in TO-larch as of 2000. Basal area in these both sites (cm2 m-2) was almost the same value (Table 1).
Measurement of Soil Respiration Rate and Environmental Factors
2000. Soil respiration rate was measured with a portable gas analyzer (LI-6400; LiCor, Lincoln, NE, U.S.A). The analyzer had a closed air circulation system with a null balance chamber (71.6cm soil surface area), which enhanced the accuracy of measurements. Soil temperature (0-10cm depth) was concurrently measured with an attached temperature prove.
Soil collars were placed in the soil at least 24h before measurements to avoid influence of soil disturbance and root injury on the measurements (Law et al. 1999). Inside diameter of soil collar was 10cm and height from the ground was 4.4cm. Living vegetation was clipped at the soil surface inside the soil collar at the time of placement. Replication of the measurement of soil respiration rate was 4 to 12 (in 1999, the first year) and 11 to 13 (in 2000, the second year) per each study plot. These points were randomly selected from each plot. These replication numbers were determined by the pre-measurements of soil respiration of 112 different points (the size was 22 x 22m) conducted in mid June, 1999 (Appendix 3).
Two soil samples (20 cm2 x 5cm) were immediately collected using a soil core sampler after the soil respiration rate was measured. The other soil cores were also sampled from the depth of 0-8cm at every measuring point. These collected soils were wrapped with a plastic back, and then brought immediately to the laboratory. Being sieved from the soil by hand to exclude coarse gravels, the soil was used for determining the amount of root samples, and as being measured of fresh weight and dry mass (after oven-dried at 105℃ for 48h). The other soil cores were
used for calculating volumetric water content (g cm-3) and soil bulk density (g cm-3). The latter two parameters were also used as measuring total pool of carbon and nitrogen in soil. The concentration of carbon and nitrogen was determined with use of a NC analyzer (NC- 900, Shimadzu, Kyoto).
Annual Carbon Input and Output
In order to estimate annual carbon input to soil as litter fall, we put 10 circular-shaped traps of litter-fall (diameter = 1m) in each site. Litter-falls were collected in every month, brought immediately to the laboratory, dried up at 80℃ and measured their dry mass. We
assumed the carbon content of dry mass is roughly equal to 50 % (Okubo 1984, Shibamoto 1977), which enabled to estimate annual carbon inputs.
In order to know about daily mean temperature, we buried soil thermometer with data logger (Thermo-recorder Mini, Nagano, Japan), which recorded temperature at 10cm depth for every one hour interval, during May 1999 - December 2000. We developed the empirical relationship between observed soil respiration rate and soil temperature based on the actual measurements. With these data, we estimated annual soil respiration rate.
Statistical analysis
Soil respiration, soil temperature, root density, soil water volume, soil carbon content and root biomass were transformed for parametric testing, respectively. Relationship between soil respiration rate and soil temperature was analyzed by regression analysis. Comparison of regression lines between soil respiration rate and soil temperature was made by analysis of cover analysis (ANCOVA). Bonfferoni test was applied for multiple comparison among sites.
Root density, soil carbon content, C/N ratio and root biomass were compared among sites and months using two-way analysis of variance (two-way ANOVA). Once significant interaction between site and month was observed, separate ANOVAS was performed for comparison among sites and months. Tukey's HSD tests were applied for multiple comparisons.
To understand the effect of various factors on soil respiration rate, multiple regression statistics for forward stepwise models was performed. Dependent variable was soil respiration rate. Moreover, independent variables that carried out stepwise were soil temperature, volumetric soil water, soil nitrogen content, soil carbon content and root density. Significant level for all statistical analysis was of 0.05.
Results
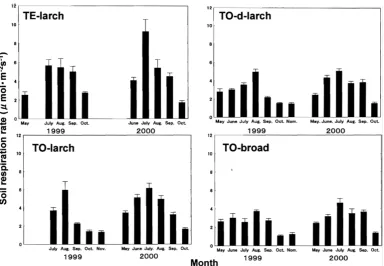
Seasonal change in soil respiration rate
0.7-8.8, 1.0-6.7 and 0.8-9.8 μmol m-2 s-1, respectively. Figure 2 shows seasonal change of volumetric water content in soil core measured of soil respiration rate.
Affecting of different soil types
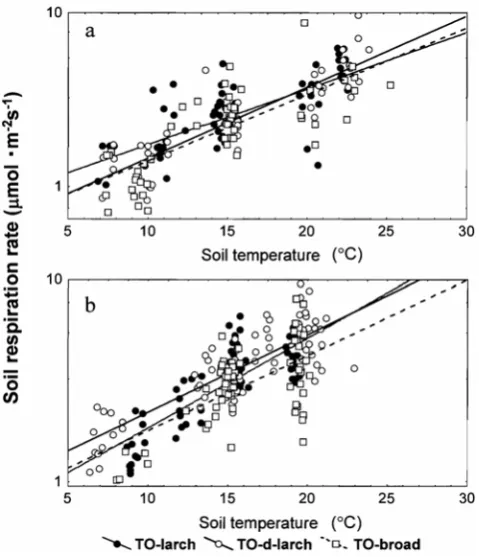
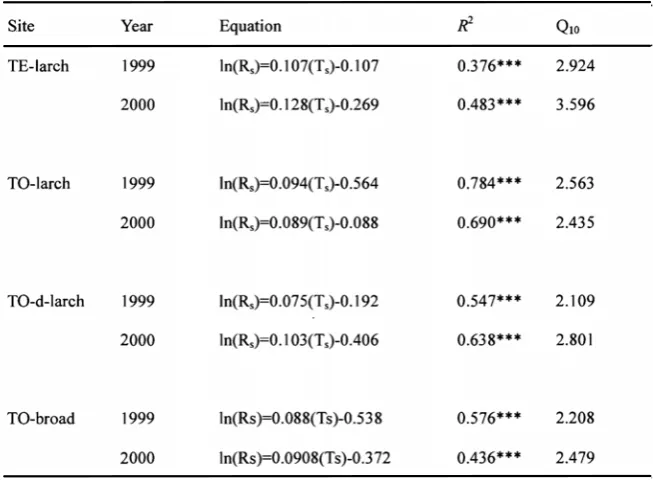
Figure 3 shows the relationship between soil respiration (Rs) and soil temperature at 10cm (Ts) in TE-larch and TO-larch (the equation of the simple regressions was listed in Table 2). ANCOVA revealed that, in 1999, the slopes of Rs on Ts did not differ significantly between TE-larch and TO-larch (P>0.05), but the intercepts were larger for TE-1arch (P<0.001). Therefore, if the soil temperature is the same range, Rs of TE-1arch was significantly higher than that of TO-larch in 1999.
In 2000, the ANCOVA revealed that the slopes of Rs on Ts differed significantly between TE-larch and TO-larch (P<0.05). It is indicated that the response of Rs to Ts was higher in TE-larch than that in TO-larch during 2000.
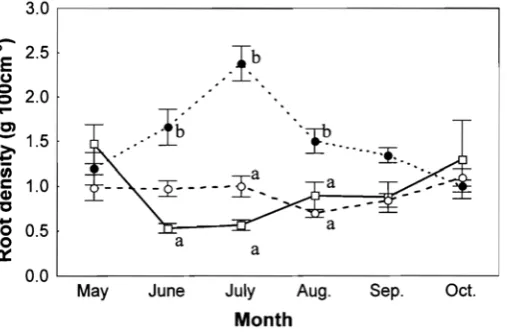
Figure 4 shows seasonal change in root density of the soil core in 2000. Two-way ANOVA showed that there was no difference between the root densities through the months (P>0.05), but there was a significant difference for root density among sites (P<0.01). Moreover, there was not significant interaction between sites and months (P=0.107). Therefore, the root density in TE-1arch was significantly higher than that in TO-larch through the year.
Seasonal course of soil carbon content (SOC) in the soil core is shown in Figure 5. There was a significant difference in SOC between two sites (P=0.01) but not significant difference in SOC among months (P>0.05). Moreover, there was not significant interaction between sites and months (p>0.05). Therefore, throughout the year, SOC in TO-larch was significantly higher than in TE-larch, especially from May to July.
Seasonal change of C/N ratio in the soil cores is given in Figure 6. Two-way ANOVA revealed that there were significant differences in C/N ration both among months (P<0.001), and among sites (P<0.01). In addition, there was no significant interaction between sites and months (p>0.05). According to this result, the C/N ratio of TE-larch was higher than that of TO-larch throughout the year. Moreover, multiple comparison among sites revealed that the C/N ratio of June was significantly lower than that of May (Tukey HSD; P<0.001 ) and August (P<0.0 1).
Different vegetation types affecting soil respiration Figure 7 shows the relationship between Rs and soil temperature (Ts) in TO-larch, TO-d-larch and TO-broad (the equation of the simple regressions were in Table2). ANCOVA revealed that, in 1999, the slopes of Rs on Ts did not differ significantly among them (P>0.05), but there was a trend that the intercepts were significantly different (P=0.055). Moreover, Rs in TO-d-larch was significantly higher than in TO-broad (Bonferroni) (P<0.05), but there were no significant difference among other pairs. In 2000, ANCOVA revealed that the slopes of Rs on Ts did not differ significantly among three sites (P>0.05), but the intercepts were
significantly different (P<0.001). Moreover, there were significant difference in interception among following pairs TO-d-larch and TO-broad (Bonfferoni) (P<0.05) and TO-larch and TO-broad (P<0.01 ).
Figure 8 shows seasonal change in root density at three sites in Tomakomai in 2000. The estimated root biomass of TE-1arch was significantly higher than that of TO-1arch through the year (TWO-way ANOVA; P<0.01). Two-way ANOVA revealed that there was not a significant difference in root density among months (P>0.05), but significant difference among sites (P<0.001). In addition, there were significant interaction between the sites and months (P<0.05), therefore the multiple comparison could not be made. ANOVA revealed that there was a significant difference of root density in three sites in June, July and August (P=0.001), in July (P<0.001) and in August (P<0.05). According to multiple comparisons, the root density in TO-d-1arch was significantly higher than that in TO-broad in June (Tukey HSD; P<0.001). During July and August, root density in TO-d-larch was highest of all study sites (P<0.05). Therefore, root biomass in TO-d-larch was higher among three sites during summer.
Seasonal SOC in three Tomakomai sites is shown in Figure 9. There was a significant difference in SOC among sites (two-way ANOVA; P<0.001). However, there were not significant differences in SOC among months (P>0.05) and the interaction between site and month (P>0.05). Tukey HSD test revealed that there were significant differences in SOC in all pairs of sites (all pairs of sites; P<0.001). Therefore, throughout the year, SOC was higher in the order of TO-d-larch, TO-larch and TO-broad.
Figure 10 shows seasonal fluctuation of C/N ratio in soil at TO-larch, TO-d-larch and TO-broad sites. Two-way ANOVA revealed that there were significant differences in C/N ratio among months (P<0.05), and sites (P<0.01). In addition, there was not significantly interaction between sites and months (P>0.05). Moreover, multiple comparison among sites revealed that the C/N ratio of TO-broad was lower than that of TO-larch (Tukey HSD, P<0.01) and there were not significant difference between other pairs. Multiple comparison among months revealed that the C/N ratio in June was significantly lower than that of May (Tukey HSD, P<0.01) and there was a tendency that the value of June was lower than that of July (P=0.070) for all sites.
Multiple regression analysis
The results of multiple regression analysis showed that soil respiration was significantly correlated with soil temperature in all sites (Table 3). In addition, soil respiration rate was also significantly correlated with the soil nitrogen content in TO-larch, and with the root mass in TO-broad, respectively.
Annual soil respiration rates
Correlation equations between soil respiration rate and soil temperature (Table 2) were used to calculate daily mean soil respiration based on daily mean temperatures (26 October 1999 - 25 October 2000). In this calculation, we hypothesized that daily change of temperature would be including the regression lines,
then daily soil respiration rate was estimated from daily mean soil respiration, which was cumulated to calculate annual soil respiration rate (Table 4).
Annual carbon input was calculated from the dry mass of litter-fall and annual carbon output was estimated by annual soil respiration rate (all unit was gC m-2 yr-1) (Table 5).
Fig. 1. Seasonal change of soil respiration rate in all sites. Vertical bars represent ±SE of the mean.
Fig. 2. Volumetric soil water (%) in TE-larch ( ), TO-larch (○), TO-d-larch (●) and TO-broad ( ).
Fig. 3. Relationship between soil respiration rate (Rs) and soil temperature at 10cm (Ts) in TE-larch and TO-larch.
a; data from 1999, b;data from 2000. Equation of each regression lines are listed in Table 2.
Fig. 4. Root density in TE-larch (●) and TO-larch (○).
Fig. 5. Soil carbon content in TE-larch (●) and TO-larch (○).
Vertical bars represent ±SE of the mean.
Fig. 6. Seasonal change of C/N ratio in TE-larch (●) and TO-larch (○).
Vertical bars represent ± SE of the means.
Fig. 7. Relationship between RS and TS in TO-larch, TO-d-larch and TO-broad.
Fig. 8. Root density in TO-larch (○), TO-d-larch (●) and TO-broad ( ).
Vertical bars represent ±SE of the mean. Different letters indicate the
statistical differences in the combination.
Fig. 9. Seasonal change of soil carbon content in TO-larch (○),
TO-d-larch (●) and TO-broad ( ).
Vertical bars represents ±SE of the mean.
Fig. 10. Seasonal change of ratio of C/N in TO-larch (○), TO-d-larch (●) and TO-broad ( ).
Fig. 11. Mass of the litter fall in TE-larch (●) and TO-larch (○).
Verticallines represent ± SE of the mean.
Fig. 12. Seasonal change of litter mass in TO-larch (○), TO-d-larch (●) and TO-broad ( ).
Vertical bars represent ± SE of the mean.
Table 2. Correlation between soil respiration rate (RS) (μmol- m-2s-1) and soil temperature at 10cm
Table 5. Annual organic carbon input as litter fall; input (Cg-m-2 yr-1) and annual soil respiration rate; output (Cg-m-2 yr-1) in the study sites cited from Table 4.
Table 3. The results of multiple regression statistics for forward stepwise models. Variables that did not enter the model at any step are not shown.
Discussion
Soil respiration rate is composed of respiration by root respiration, soil microorganisms and soil animals. Therefore, the productivity and nutrient of the ecosystems affect soil respiration rate (Lieth and Ouellette 1962, Raich and Schlesinger 1992, Singer and Munns 1999).
Then, what kinds of factors do affect soil respiration rate in forest ecosystems? This is an essential question for considering the role of CO2 storage capacity of forest ecosystems. Based on the previous study in Siberian larch forest (Yanagihara et al. 2000), we listed the candidate factors as follows; 1) soil type and 2) vegetation type. In this sense, the 1) and 2) would be classified as abiotic factor and biotic factor, respectively.
In all sites, soil respiration rate peaked towards in summer when the soil temperature was highest. When we measured soil respiration rate in July at TE-larch, there was long-term precipitation of five days in the Teshio district. The results of volumetric water content, which was sampled after measuring soil respiration rate, showed that soil water volume content in July at TE-larch was highest as shown in Figure 2. Sato et al.
(2000) reported that microbe respiration increased with increasing soil moisture content up to 0-50 %, but was constant when soil moisture was over 50 %. In July at TE-1arch, soil moisture was 67.30 (SE 2.95) %. Therefore, in this time, it seemed that microbe respiration rate was not increased by an increase of soil moisture content. However, it is difficult to separate the factors because the fluctuation of soil temperature affecting soil respiration was enough to large. It is considered that CO2, which was dissolved in water during rain, was evolved at one time after the rain was stopped (Jiang et al. 2005).
Different Soil Types Affecting Soil Respiration Soil respiration rate in the larch forest, which is established on the well-developed soil, i.e. TE-1arch was significantly higher than that in immature soil derived from volcanic pumice of TO-1arch. Why were these differences existed?
As mentioned above, component of soil respiration is considered to be respiration by root, soil microorganisms and soil animals. Eguchi et al. (1997) estimated that the ratio of soil animal's respiration (mainly earthworm) to the soil respiration was 3 % of total soil respiration. Paustain et al. (1990) estimated its ratio was 7 %. These studies suggested that soil respiration by soil animals was negligible small to total soil respiration. We, therefore, mainly discussed the respiration from root and microorganisms.
Root density of A horizon in TE-1arch was significantly higher than that in TO-larch (Fig. 4). Eguchi et al. (1997) reported plant root was mainly existed within 12 cm depth, corresponding to A and H/A horizon (that is the integration from H horizon to A horizon). Sato et al. (1982) also reported that the rooted depth was 15cm in A horizon in Teshio Experimental Forest. Based on these studies, we estimated the root biomass in both sites according to the relationship
between the root density and the depth of A horizon (Appendix 4).
With higher value of the estimated root biomass of TE-larch (Fig. 4), it is considered that large root biomass may affect higher soil respiration rate in TE-larch. This is one of the clear evidence to explain the difference in soil respiration of both sites. Of course, turnover rate of fine roots correlates positively root respiration. High turnover rate of fine roots is found at the surface of the forest floor in cool temperate forest (Satomura et al. 2006). Therefore, high respiration of soil may be an indicator of activities of rhizosphere, mainly related to fine roots.
Soil carbon content in TO-larch was significantly higher than in TE-larch through the year (Fig. 5). Generally speaking, biomass of soil microorganisms increases proportionally with soil carbon content (Anderson 1980, Sawamoto 2000). Therefore, it is considered that biomass of microorganism was larger in TO-larch than in TE-larch.
C/N ratio of soil in TO-larch was lower than in TE-larch through the year (Fig. 6). Generally speaking, C/N ratio was used as an index of decomposition of organic matters (Takeda 1994, 1997). Therefore, it is considered that decomposition of microorganisms was much active in TO-1arch than in TE-1arch. Nakadai (=Noguchi, T.) et al. (unpublished data) found that soil microorganisms concentrated in the surface of the soil in TO-larch. Therefore, these results indicate that in the surface of the soil, microorganism's activity was higher in TO-larch than that in TE-larch. Considering about the depth and structure of horizon of soil in both sites, however, this result may not apply in both sites because both A and Ao horizon at TO-larch was only about 15cm. In contrast, Nakadai (1996) reported that CO2 emission from plow layer of farmland was 93 % of soil CO2 efflux. Therefore, it be is indicated that respiration of microorganisms may strongly related to the structure of soil horizon. In both sites, the C/N ratio was lower in June (Fig. 6). Just after snowmelt, increasing temperature may firstly accelerate the activity of microorganisms. Carbon in soil may be exhausted by microorganisms, which induced low C/N ratio.
Q10 value is an useful index for comparing the sensitivity of soil respiration with soil temperature. During two years, Q10 value of TE-1arch was tended to be slightly higher than that of TO-larch. TE-1arch is located in cooler region than TO-1arch. In TE-larch, there may be acclimatized to lower temperature. Under cold climate, Q10 of plant is usually higher (Townsend
et al., 1992, Kirschbaum, 1995) which may be advantageous for plants to produce energy with a small increase in temperature (Körner 1999). Moreover, Raich et al. (1992) reported that soil respiration rate in cooler region expected to be strongly increased by global warming. Therefore, soil respiration at cool and cold regions will be a critical factor for CO2 evolution from soils.
Effect of vegetation types on soil respiration
Rs increment rate of TO-1arch was significantly higher than of TO-broad in 2000. But there was no significant difference between Rs of TO-1arch and of TO-d-larch during two years. These results, however, are contradicted with our prediction. What is the reason why soil respiration rate in TO-broad is lower than that in TO-d-1arch and TO-larch?
Root density in TO-d-larch was significantly higher than the other sites in summer (Fig. 8). A horizon seems to be the same depth at three sites in Tomakomai, because of these three sites on the same soil type originated from immature volcanic ash. Therefore, it is considered that root biomass reflected directly the root density. Consequently, it is considered that TO-d-larch has much root biomass than the other sites. It is indicated that the root invading broad-1eaved trees and understory vegetation, topographies may affect this large root biomass.
The SOC was significantly higher in the order of TO-d-larch, TO-1arch and TO-broad though the year (Fig. 9), suggesting that there was much biomass of microorganisms. It is considered that higher respiration of microorganisms was found in the order of TO-d-larch, TO-larch and TO-broad.
However, activities of soil microorganisms may be strongly regulated by the litter quality, namely the C/N ratio. Nitrogen in litters is an essential element for protein synthesis of microorganisms. But during decomposing litters, microorganisms may need energy derived from carbon in litter. Therefore, the C/N ratio of litter is critical factor to regulate the rate of decomposition of litters (Takeda 1994, 1997).
C/N ratio in TO-broad was significantly lower than in TO-1arch (Fig. 10). Moreover, C/N ratio in TO-d-1arch was the medium value of TO-broad and TO-1arch. Generally speaking, soil of broad-leaved forest has lower C/N value, which is found for TO-broad and TO-larch. This result shows that decomposition rate by microorganisms is higher in TO-broad. However, soil respiration of TO-broad was the lowest among three sites examined. If the activity of soil microorganisms would be high by way of using low C/N litter as their food, soil respiration should be higher. In fact, in the Tomakomai sites, there was no direct relationship between the C/N ratio in leaf litter and soil respiration. Raich (1995) compared directly the rate of CO2 emission from the moist temperate forest dominated by deciduous broad-1eaved trees with those from coniferous forests, which had different nutrient-cycling characteristics. However, there was no apparent difference between these forest types with respect to their observed soil CO2 emission. Considering the present results, when we compared for the C/N ratio with the broad-leaved forest and the larch forests which nutrient-cycling characteristics were different, moreover, it produces the conflict about microorganism's activities.
One of the ideas that explain this conflict is the existence of dormant microorganisms. Based on this study and previous researches (e.g. Toda 2000), soil respiration rate is not directly correlated with the soil C/N ratio, even though soil microorganisms have high
nitrogen content in their bodies. We may conclude that we cannot infer the activities of soil microorganisms by way of soil C/N ratio because not all microorganisms are active but some of them are in dormant condition (Takeda 1994, Toda 2000).
But identifying a relationship between substrate quality and CO2 emission is of importance if one exists, as increases in atmospheric CO2 concentrations are predicted to increase the C/N ratio in litter production (Norby et al. 1986, Crill1991).
Getting with seasonal change of C/N ratio, C/N ratio in June was lower (Fig. 10). This result was coincided with the trend of TE-larch (Fig. 6). The same as premise, it is suggested that snowmelt following an increase of soil temperature induce the microorganism's activities, and respiration by microbes was higher in June. Law et al. (1999) reported that soil respiration rate was higher in the younger stands than the older one. Based on the data obtained in this study, high Rs of TO-d-larch may be attributed to the composition of tree species and with younger tree seedlings invaded into gaps and forest floor.
Multiple regression analysis revealed that most of factor affecting soil respiration rate was soil temperature in all sites (Table 3). Moreover, the second factor was the root density in TO-d-larch and was nitrogen content in TO-larch. In TO-d-larch, root biomass was larger than other sites (Fig. 8), which was affecting soil respiration rate. Therefore, these results indicate that higher soil respiration rate of TO-d-larch involved in larger root density. However, the reason why TO-larch has higher soil respiration rate than TO-broad, was still under discussion. Actually, forest stand structure of TO-broad is classified as a typical well matured forest (Takahashi et al. 1999). It is considered that activity of underground of TO-broad is low because of aged composer of tree species.
Annual Soil Respiration Rate
Annual soil respiration rates of the study sites ranged between2936 and 3699 (gCO2 m-2yr-1) (Table 4). This value was higher than the other reports in the similar latitude (Koizumi unpublished data). Mixed broadleaf and conifer forest in U.S.A. have the value of 2000 gCO2 m-2yr-1 (Crill 1991) and that type of forest in Germany have the value of 2313 gCO2 m-2yr-1 (Dorr et al. 1987). This value was lower than temperate forest in central Japan, i.e. 4026-4602 gCO2 m-2yr-1 (Koizumi unpublished data), and higher than in boreal forests in, where 1750 gCO2 m-2yr-1 (Toland and Zak 1994).
rate in TO-broad derived from respiration of microorganisms.
It is well known that litter decomposition rate of broad-leaved forests is higher than that of coniferous forests, such as pine and cypress forests which are considered to be related to the C/N ratio in needle litter (Takeda 1994). With lower C/N litters of broad-leaved tree spices, litters were easily attacked by microorganisms as rich nitrogen resources. In contrast, conifers usually have higher ratio of C/N in leaf litters, which is hardly decomposed by microorganisms because many phenolic compounds, especially high content of lignin prevents to be digested. However, the range of C/N ratio seems to be most indicative parameter, from the viewpoint of nitrogen mineralization limited by carbon availability (Takeda 1994).
In TO-broad, lower root respiration rate may affect lower soil respiration. However, root density in TO-broad was similar to that in TO-larch. Therefore, root respiration per its biomass in broad-1eaved trees may be lower than in larch-trees.
During two years, there was a trend that Q10 was similar value in every site. In these sites, the range of Q10 was from 2.11 to 2.80. Raich et al. (1992) reported medium value of Q10 calculated from value of various forests, was 2.4. Our results showed the similar value.
Conclusion
Soil respiration rate in a larch plantation was changeable by soil types and mixing degree of broad-leaved trees. Therefore, estimation of soil respiration rate would be strongly influenced by several factors. For example, it is reported that soil respiration rate was also affected by gap formation (Matsuura et al.
2001) and the species richness (Appendix 5). It is essential for estimation of soil CO2 efflux to be taken into account for these factors. Further studies will be needed in analysis of the role of microorganisms and the activities of trees for estimating in soil respiration of forests. Moreover, to understand the reason why higher soil respiration in a larch forest, we need to know respectable respiration by root and microorganisms, relationship between C/N ratio and soil respiration rate.
Acknowledgements
We thank Dr. Tsutom Hiura for providing field facilities for the experiments in the Tomakomai Experimental Forest (TOEF). We also thank for the staff of University Forests and students of the Boreal Forest Conservation Studies of Hokkaido University for their assistance and cooperation in field. Especially, Y.Y. appreciates their kindness and friendship. This study was supported in part by Global Environment Research Fund (FY1997-2000) B-2.2 Analysis and modeling of carbon sequestration of forest stands and JSPS Basic Research A to T.K.
References
Anderson, J. M. (1973) Carbon dioxide evolution from two temperatures, deciduous woodland soils. J
Appl. Ecol., 10: 361-373
Anderson, J. P. E. and Domsch, K. H. (1980) Quantities of plant nutrients in the microbial biomass of selected soils. Soil Science 130: 211-216
Baldocchi, D., Falge, E., Gu, L., Olson, R., Hollinger, D., Running Sthoni, P., Bernhofer, C., Davis, K., Evans, R., Fuentes, J., Goldstein, A., Katul, G., Law, B., Lee, X., Malhi, Y., Meyers, T., Munger, W., Oechel, W., Paw, K.T., Pilegaard, K., Schmid, H.P., Valentini, R., Verma, S., Vesala, T., Wilson, K. and Wofsy, S. (2001) FLUXNET: A New tool to study the temporal and spatial variability of ecosystem-scale carbon dioxide, water vapor, and energy flux densities. Bull Amer. Meteorol. Soc., 82: 2415-2434
Butcher, S. S., Charlson, R. J., Orains, G. H. and Wolfe, G. V. (1992) Global Biogeochemical Cycles. Academic Press, London, p.379.
Bazzaz, F. A. and Williams, E. W. (1991) Atmospheric CO2 concentrations within a mixed forest: implications for seedling growth. Ecology 72:12-1 6.
Crill, P. M. (1991) Seasonal patterns of methane uptake and carbon dioxide release by a temperate woodland soil. Global Biogeochem. Cycles., 5: 319-334.
Ddrr, H. and Munnich, K. O. (1987) Annual variation in soil respiration in selected areas of the temperate zone. Tellus 39B: 114-121
Eguchi, S., Sakata, T., Hatano, R. and Sakuma, T. (1997) Daily changes of CO2 efflux from the soil of a deciduous broad-leaved forest and its significance as a CO2 source for vegetation. Soil Sci. Plant Nutr., 68:138-147 (in Japanese with English summary).
Grahammer, K., Jawson, M.D. and Skoop, J. (1991) Day and night soil respiration from a grassland. Soil Biol. Biochem., 23: 77-81.
Gupta, S. R. and Singh J. S. (1981) Soil respiration in a tropical grassland. Soil Biol. Biochem., 13: 1681-1690.
Hiura, T., Fujito, E., Ishii, T., Naniwa, A., Sugata, S., Ishida, K., Murakami, M., Kato, E., Maeno, H., Fukushima, Y. and Sakai, T., (1998) Stand structure of a deciduous broad-leaved forest in Tomakomai Experimental Forest, based on large-plot data. Res. Bull. Hokkaido Univ. For., 55: 1-10 (in Japanese with English summary). Houghton, R. A. and Woodwell, G. M. (1989) Global
climate change. Scientific America 260: 18-26. Houghton et al. (1990) Climate Change. The IPCC
scientific assessment. Cambridge University Press. Cambridge.
Hoghton, J.T., Meiro Filho, L.G., Callamder, B.A., Harris, N., Kattenberg, A. and Maskell, K. (1996) Climate change 1995 -Science of climate change-. Cambridge Univ. Press. U.K., p.572.
Koike, T., Yaszaki, K., Funada, R., Maruyama, Y., Mori, S., and Sasa, K. (2000) Forest health and vitality in northern Japan- A history of larch plantation. Res. Notes, Fac. Forestry, Univ. of Joensuu, 92: 49-60.
Koizumi, H. (unpublished) Biomass and respiration of various ecosystems. (in Japanese)
(http://www.green.gifu-u.ac.jp/~koizumi/)
Kowalenko C. G., Ivarson K. C. and Cameron D. R. (1987) Effect of moisture content, temperature and nitrogen fertilization on carbon dioxide evolution from field soils. Soil Biol. Biochem., 10: 417-423. Körner, Ch. (1999) Uptake and loss of carbon. In:
Körner, Ch., Alpine Plant Life. Springer-Verlag Berlin, Heidelberg, New York. p.l90- 196.
Lieth, H. and Ouellette, R. (1962) Studies on the vegetation of the Graspe Peninsula. Can. J. Bot. 40: 127-140.
Law, B. E., Ryan, M. G. and Anthoni, P. M. (1999) Seasonal and annual respiration of a ponderosa pine ecosystems. Global Change Biol., 5: 169-182. Maier, C. A. and Kress, L.W. (2000) Soil CO2 evolution
and root respiration in 11-year-old loblolly pine (Pinus taeda) plantations as affected by nutrient availability. Can. J. For. Res., 30: 347-359. Matsuura, Y., Takahashi, M. and Sanada, E. (2001)
What is happen in flow of carbon after the gap formation? Northern Forestry (Hoppo Ringyo) 53 : 16-19 (in Japanese).
Mathes, K. and Schriefer, Th. (1985) Soil respiration during secondary succession. -Influence of temperature and moisture-. Soil Biol. Biochem., 17: 205-211.
Nakadai, T. (=Noguchi, T.) (1996) Studies on evaluation of carbon dioxide evolution from crop field. Ph.D. Thesis. Tokyo University of Agriculture and Technology, p.123 (in Japanese). Okubo, T. (1984) Fertilization. In: Nishimura, S. et al.
ed. Crop Science, Buneido, Tokyo, p. 109 (in Japanese).
Oikawa, T., (1999) Increase of atmospheric CO2 concentration and biosphere. J. Agr. Met., 47: 191-194 (in Japanese).
Paoustain, K., Andren, O., Clarholm, M., Hanson, A. C., Johonson, G., Lagerlof, J., Lindberg, T., Petterson, R. amd Sohlenius, B. (1990) Carbon and nitrogen budgets of four agro-ecosystems with annual and perennial crops with and without N fertilization. J. Applied Ecol., 27: 60-84.
Raich, J. W. and Schlesinger, W. H. (1992) The global dioxide flux in soil respiration and its relationship to vegetation and climate. Tellus 44: 81-99. Sato, F., Imai, H. and Okajima, H. (1982) Ionic
composition of soil solutions separated from forest soils and stability of clay minerals in terms of chemical equilibrium. Soil Sci. Plant Nutr., 53: 219-226 (in Japanese).
Sato, A., Tsuyuzaki, T. and Seto, M. (2000) Effect of soil agitation, temperature or moisture on microbial biomass carbon of a Forest and an arable soil. Microbes and Environments 15: 23-30 (in Japanese).
Sato, A. and Seto, M. (2000) Rate of microbial respiration per unit biomasss and carbon balance in a volcanic soil -comparison among three land uses-. Soil Microorganisms 54: 13-21.
Satomura, T., Hoshimoto, Y., Koizumi, H., Nakane, K. and Horikoshi, T. (2006) Seasonal patterns of fine root demography in a cool-temperate deciduous forest in central Japan. Ecol. Res., 21: 741-753. Shibamoto, T. (1977) Forest soil and fertilization.
Nourin-syuppan Tokyo, p.23 (in Japanese). Shibata, H., Hiura, T., Tanaka, Y., Takagi, K. and Koike,
T. (2005) Carbon cycling and budget in a forested basin of southwestern Hokkaido, northern Japan. Ecol. Res., 20: 325-331.
Strain, B.R. (1985) Direct effects of increasing atmospheric CO2 on plants and ecosystems. Trends Ecol. Evol., 2: 18-21.
Singer, M. J. and Munns, D. N. (1999) Soils: an introduction. Fourth ed. Prentice-Hall, Inc. Simon & Schster / A Viacom Company. Printed in the United States of America, 163-226.
Schimel, D.. Enting, I., Heimann, M., Wigley, T., Raynaud, D., Alves, D. and Siegenthaler, U. (1994) CO2 and the carbon cycles. In: Houghton, J.T., Meira Filho, L.G., Bruce, J., Hoesung Lee, Callander, B.A., Harris, N. and Maskell, K. eds., Climate Change. Cambridge Univ. Press, Cambridge, UK. p.35-71.
Tadaki, Y. (1977) Forest biomass. In: Shidei, T. and Kira, T. (eds.) Primary Productivity of Japanese Forests, JIBP Synthesis, Univ. Tokyo Press, 39-44. Takahashi, K., Yoshida, K., Suzuki, M., Seino, T., Tani,
T., Tashiro, N., Ishii, T., Sugata, S., Fujito, E., Naniwa, A., Kudo, G., Hiura, T. and Kohyama, T. (1999) Stand biomass, net production and canopy structure in a secondary deciduous broad-leaved forest, northern Japan. Res. Bull. Hokkaido Univ. For., 56: 70-85.
Takeda, H. (1994) Interaction between plant and decomposer populations in forest ecosystems -a mechanism of biodiversity maintenance-. Jpn. J. Ecol. 44: 211-222 (in Japanese).
Takeda, H. (1997) Carbon and nutrient cycling in forest ecosystems. Chemistry and Biology :35 26- 31 (in Japanese).
Toda, H. (2000) Characteristics of nitrogen mineralization in forest soil. Forest Resources Environ. 38: 1-95 (in Japanese with English summary).
Toland, D. E. and Zak, D. R. (1994) Seasonal patterns of soil respiration in intact and clear-cut northern hardwood forests. Can. J. For. Res., 24: 1711-1716.
Valentini, R. (2003) Fluxes of carbon, water and energy of European forests. Ecological Studies 163, Springer Verlag, Heidelberg, p.270.
Guomundsson, J., Thorgerrsson, H., Ibrom, A., Morgenstern, K., Cleuremans, R., Moncneff, J., Montagnani, L., Minerbi, S. and Jarvis, P. G. (2000) Respiration as the main determinant of carbon balance in European forests. Nature 404: 861-865.
Vose, J. M., Elliott, K. J., Johonson, D. W., Tingey, D. T. and Johonson, M. G. (1997) Soil respiration response to three elevated CO2 and N fertilization
in Ponderosa pine. Plant and Soil 190: 19-28. Yanagihara, Y., Koike, T. Matuura, Y., Mori, S., Sibata,
H., Satoh, F., Masuyagina, O. V., Zyryanova, O. A., Prokusnkin, A. S., Prokushnkin, S. G.. and Abaimov, A. P. (2000) Soil respiration rate on the contrasting north- and south-facing slopes of a larch forest in central Siberia. Eurasian J. For. Res., 1: 1 9-29.
Appendix 4. The mean value of total root biomass (g) under the measuring point taking the depth of A horizon into consideration (± SE in parenthesis).
Appendix 3. Spatial variation of soil respiration rate in a larch plantation.
To understand dispersion of soil respiration rate , We measured soil respiration rate for 100 point s in one day. This measurement performed in TO-larch in 10th September 1999. It is not avoid that diurnal change of soil respiration, but soil respiration rate have large dispersion among every points.
We measured the soil respiration rate at area the where the variation was small. m
m
Soi
l respiration rate (
μ
mol
m
-2
s
-1)
Point NO.
mm
m
m
m
Soi
l respiration rate (
μ
mol
m
-2
s
-1)
Point NO.
mHow to calculate this value was from the following equation; total root biomass-Rd × Ad/5 where Rd (g・100cm
-3
Appendix 5. Relationship between soil respiration rate and the umber of plant species.
We measured soil respiration rate in nine plots where there were different number of plant species (in TOEF). This study was performed in 15-16th August 2000 when whether condition was almost the same in two days. Replication was 10 points at random in every site. Soil respiration was measured with LI-6400. At the same time, We measured soil temperature and volumetric water content. Volumetric soil water content was measured by Thermal Domain Reflectmetry (TRIME-FM, IMKO).
To understand what the factors that affecting on soil respiration rate, multiple regression statistics forward stepwise models were performed. In this case, dependent variables were soil respiration rate, and independent variables were soil temperature, the number of plant spices and volumetric water content.