Ethylamine in maize callus: isolation, identification and first
approach to the physiological role of its hyper-production
Massimo Zacchini
a, Antonio Graverini
a, Stefano Grego
b, Marina de Agazio
a,*
aIBEV,CNR,Via Salaria Km.29,300,00016Monterotondo Scalo,Rome,Italy bDABAC,Uni6ersita` della Tuscia,01100Viterbo,Italy
Received 10 June 1999; accepted 9 September 1999
Abstract
A primary amine, ethylamine, was isolated, purified and identified by 1H-NMR from perchloric acid-extracts of maize
embryogenic callus, where it occurred in high amount (2mmol/g fresh weight, an amount ten-fold higher than that of other
well-known amines such as polyamines) while only a faint trace was found in maize plant organs. Ethylamine amount was measured in different phases of callus proliferation and differentiation (somatic embryogenesis). Results indicated that ethylamine mostly accumulates in the proliferation phase. It decreased to very low concentration in cells that have acquired a new competence for differentiation or are already differentiated, except for in the developing somatic embryo phase. Undifferentiated callus appears to be an ethylamine hyper-accumulator. © 2000 Elsevier Science Ireland Ltd. All rights reserved.
Keywords:Zea mays; Callus culture; Biosynthesis; Amines; Ethylamine
www.elsevier.com/locate/plantsci
1. Introduction
Multicellular organisms synthesise secondary products from precursors supplied by primary metabolism determining a sort of competition be-tween growth processes and secondary metabolism [1,2]. In in vitro tissue cultures the production of natural products often exceeds that of differenti-ated plants [3]. Bio-transformation of exogenous molecules by plant tissue cultures induces the pro-duction of a number of new phytochemicals be-cause of the high biochemical capacity of cultivated cells to perform specific bio-transforma-tion under in vivo condibio-transforma-tions [4]. This conversion rate might eventually compete economically with chemical synthesis [5]. The identification of new secondary substances present in the proliferation tissues, absent in the differentiated tissues, makes possible to detect unknown metabolic routes
use-ful in the bioproduction and biotransformation of natural products.
In living organisms, amines are essential for growth and cell proliferation and can have benefi-cial or harmful effects. In animal, amines play a variety of physiological roles, such as regulation of body temperature, stomach volume and acid lev-els, and alteration of brain activity [6]. Extracellu-lar sources of polyamines are important for the growth of tumours in mammals. Diet is the most important source of polyamines. It has been stressed the necessity to develop vegetables and cereals with low dietary polyamine content for cancer patients due to the direct relationship be-tween the dietary content of polyamines and tu-mour growth [7].
Amines are widespread in plant kingdom and they have aromatic and alyphatic structures. They are formed during the metabolic processes and show diverse characteristics and biological func-tions. Aromatic amines (dopamine) stimulate GA3
effect in isolated lettuce hypocotyls and ethylene biosynthesis in isolated chloroplast of sugar beet * Corresponding author. Tel.: +39-6-90672534; fax: +
39-6-9064492.
E-mail address:[email protected] (M. de Agazio)
leaves [8]. Alyphatic monoamines are often pro-duced in flowers at anthesis and their smell attract insects [9]. Alyphatic di- and poly- amines are involved in wide array processes, ranging from triggering organogenesis to protecting against stress [10 – 12].
In the present work we performed a comparison of chromatographic patterns of dansylated amines extracted from different organs of maize plantlets and maize embryogenic callus. The goal was to focus our attention on those poorly investigated amines that occurred in higher amount in maize in vitro tissue cultures with respect to maize plants. In this paper we report on the high level of an amine, isolated and identified as ethylamine, found in undifferentiated callus tissue. Analysis of its content during proliferation and differentiation (somatic embryogenesis) steps opens an intriguing question on the role of this substance.
2. Materials and methods
2.1. Plant material
Maize seeds (Zea mays L., hybrid cultivar Samodek, Dekalb, USA) were rinsed in tap water and germinated in the dark at 27°C in a controlled growth chamber in a 0.5 mM CaSO4 solution.
Primary roots and coleoptiles were excised from 3-day-old seedlings, frozen in liquid N2and stored
at −80°C until use; other seedlings were then transplanted to soil and, after 15 days, leaves were also collected, frozen and stored. All the samples were analysed for amine content.
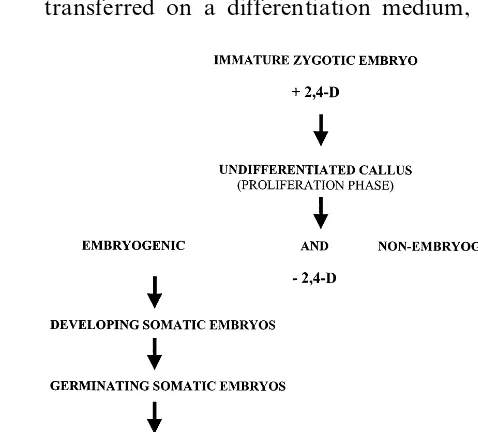
Callus cultures were obtained from immature embryos of the same self-cross maize hybrid culti-var plated on N6 agar-solidified (0.8%) medium [13] supplemented with 3% sucrose and 2 mg/l 2,4-dichlorophenoxyacetic acid (2,4-D). They were subcultured every 3 weeks and maintained in dark-ness in a growth chamber at 28°C. To induce somatic embryogenesis, calli were transferred to an induction medium supplemented with 6% su-crose and deprived of 2,4-D [14]. Plates were maintained in darkness for 1 week and succes-sively in light (18W/33 fluorescent tubes, light intensity 50 mmol/m−2 s−1, photoperiod 16 h
light/8 h dark) for 2 weeks, after which developed germinating embryos were transferred in plastic vessel [14]. Once leaves and roots were well
estab-lished, they were transplanted in pots in a green-house. At the end of each subculture period, samples were harvested and analysed for amine content.
2.2. Analysis of amine content
Samples were homogenised with 5 vols. of a 5% (w/v) cold HClO4 solution in a prechilled mortar.
The homogenates were kept for 1 h at 4°C and then centrifuged at 15000×g for 10 min. Free amines contained in the supernatant fraction were dansylated and separated by silica gel TLC ac-cording to Goren et al. [15]. TLC plates were developed with CHCl3– triethylamine (4:1) and
scanned in a Shimadzu CS9000 flying-spot scanner with fluorimetry attachment. Bi-dimensional TLC plates were developed in CHCl3– triethylamine
(4:1) and then in cyclohexane – EtOAc (5:4). Data reported in figures refer to a single typical experiment with five replicates per thesis. Data obtained were subjected to analysis of variance (ANOVA); means were compared using Tukey’s test. At least three series of independent experi-ments were carried out giving reproducible results.
2.3. Purification, identification and quantification of ethylamine in maize extracts
Dansylamines were separated by preparative TLC plates (silica gel 60, layer thickness 2 mm, 20 g sample loaded) developed with CHCl3–
triethy-lamine (4:1). Bands were revealed by an UV lamp system; spot corresponding to Rf=0.7 was
scraped off, eluted in EtOAc, and loaded on a Lichroprep RP-8 (40 – 63 mm) reverse phase
column (MeOH – H2O, 6:4).
HPLC was performed on a system consisting of two solvent metering pumps programmed with a microprocessor controller. Samples were injected into a fixed 100-ml loop and separated by a reverse
phase C8 pre-packed column (Lichroprep RP-8,
silica gel 10mm, 5×250 mm). Samples were eluted
from the column with a programmed MeOH – H2O solvent gradient changing from 70 to 40%
H2O in 45 min at a flow rate of 2 ml/min. The
column was washed with MeOH 100% for 5 min and re-equilibrated at MeOH – H2O (3:7) for 15
emission wavelength 510 nm); peaks with relative areas and retention time were recorded by an attached integrator.
2.4. 1H-NMR analysis
The NMR spectra were recorded on a Bruker-AMX Spectrometer operating at a 600.13 MHz field. The sample was solved in deutered chloro-form; tetramethylsilane was used as internal standard.
3. Results and discussion
Amines were extracted either from root tips of 3-day-old maize seedlings or from immature em-bryo derived calli, in the proliferation phase. Amine content and their distribution were analysed by silica gel TLC after dansylation. A stronger fluorescence intensity of a spot migrating between spermine and spermidine (Rf=0.7) was
found in extracts of callus tissues with respect to root tips. That was not due to a substance present in the medium utilised for callus growth, since it was missing when the medium was dansylated and chromatographed. Three-day-old seedlings grow-ing for 48 h in the callus growth medium also did not show this spot. This indicates that the fluores-cent spot was not the result of metabolic transfor-mation of one of the medium components. Two questions arose: (i) which molecule corresponds to this unknown fluorescent substance and (ii) what is the significance of its strong increase in the callus tissue.
(i) Free amines extracted from callus tissue were dansylated and separated by silica gel preparative TLC. The compounds corresponding to the spot with Rf=0.7, were scraped off, eluted in EtOAc,
and loaded on a Lichroprep RP-8 reverse phase column. Eluted fractions containing the most fluorescent compound were collected and tested for purity using bi-dimensional TLC and HPLC. The identification of this substance was performed by 1H-NMR. The analysis confirmed the purity of
the sample and the substance was identified as a dansylethylamine, being the 1H-NMR (600.13
MHz, CDCl3) chemical shifts: d 1.034 (3H, t,
J=7.2 Hz, H-2), 2.927 (6H, s, CH3– N), 2.980
(2H, m, H-1), 4.510 (1H, bt, NH), 7.231 (1H, d, H-4%), 7.540 (1H, t, H-7%), 7.578 (1H, t, H-3%),
8.256 (1H, d, H-2%), 8.32 (1H, bd, H-6%), 8.59 (1H,
b, H-8%). Hydrolysis of callus and root extracts revealed no presence of bound or conjugated ethy-lamine forms (data not shown).
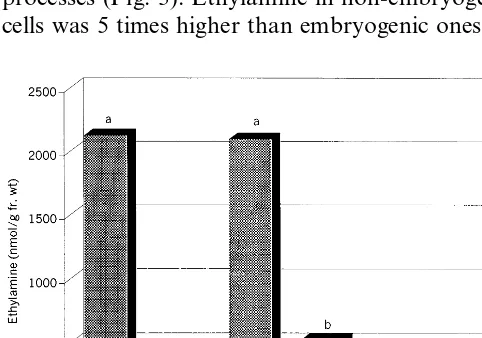
(ii) Ethylamine has been described in a few plants including maize [9] and it is synthesised by decarboxylation of alanine or transamination of acetaldehyde [16,17]. Its role in higher plants is not sufficiently understood. It has been reported that ethylamine enters the formation of theanine by its condensation with glutammic acid [18]. Sponholz [19] reports the presence of ethylamine during must fermentation as the result of anaerobic trans-formation. Maize callus tissue that hyper-produces this substance (2 mmol/g fresh weight, an
amount ten-fold higher than that of other well-known amines such as polyamines) can be an useful material to investigate on its physiological role. For this purpose the changes of ethylamine content during different phases of maize callus differentiation and propagation were analysed by following the experimental protocol reported in Fig. 1. In young undifferentiated callus (prolifera-tion phase) grown in maintenance medium con-taining 2,4-D, where cells are forced to re-program its ontogenetic pathway into an unorganised par-enchymous tissue, ethylamine concentration was
20-fold higher than in in vivo plant organs (Fig. 2). Callus well established in culture was then transferred on a differentiation medium, without
Fig. 2. Ethylamine contents in maize: (1) undifferentiated callus; (2) root; (3) coleoptile; (4) leaf. Letters indicate statisti-cally different values according to Tukey’sP=0.05 test.
all successive developmental steps ethylamine con-centration remained at low level except in the developing somatic embryo phase where ethy-lamine increased to values similar to the non-em-bryogenic tissue. These data indicate that ethylamine accumulates mostly in cells that un-dergo to a unorganised proliferation developmen-tal pathway and decreases significantly at very low concentration in cells that have acquired a new competence for differentiation (embryogenic callus cells, Fig. 3 bar 2) or already started the differenti-ation (germinating somatic embryo, Fig. 3 bar 4). Finally, in regenerated plantlets, where a high degree of differentiation has been reached, ethy-lamine content is further reduced (Fig. 3 bars 5 and 6). The ethylamine peak observed in the devel-oping somatic embryo phase (Fig. 3 bar 3), that interrupts the decreasing trend of ethylamine con-tent during differentiation processes, could be ex-plained with the requirement and accumulation of amino compounds taking place in this particular phase of somatic or zygotic embryogenesis [21]. To obtain more insight on the mechanism by which ethylamine interacts with proliferation pro-cess, callus lines with low proliferation rate must be selected and experiments must be planned in-volving also the feeding of exogenous ethylamine to the culture.
Acknowledgements
Authors wish to thank Roberto Buffone for technical assistance and NMR service, Area della Ricerca di Montelibretti (Rome), Italy.
References
[1] L.H.W. van der Plas, C. Eijkelboom, M.J.M. Hagen-doorn, Relation between primary and secondary metabolism in plant cell suspension, Plant Cell Tissue Organ Cult. 43 (1995) 111 – 116.
[2] M.W. Fowler, Interactions and interelationship between primary and secondary metabolism, in: P. Morris, A.H. Scragg, A. Stafford, M.W. Fowler (Eds.), Secondary Metabolism in Plant Cell Cultures, Cambridge Univer-sity Press, Cambridge, 1986, pp. 103 – 107.
[3] Y. Fujita, M. Tabata, Secondary metabolites from plant cells-pharmaceutical applications and progress in com-mercial production, in: C.E. Green, D.A. Somers, W.P. Hackett, D.D. Biesboer (Eds.), Plant Tissue and Cell Culture, Liss, New York, 1987, pp. 169 – 185.
2,4-D, to restore the differentiation program, lead-ing to formation of somatic embryos developlead-ing from the pre-determinated embryogenic cells. Em-bryogenic cells were discriminated from non-em-bryogenic ones by staining for 5 min with Lugol solution because of their strong starch accumula-tion according to Vasil and Vasil [20]. Then so-matic embryos were induced to germinate and to develop in plantlets and, after an acclimatization step, transplanted in a greenhouse (ex vitro plantlets). Ethylamine levels were measured at each step of the differentiation and propagation processes (Fig. 3). Ethylamine in non-embryogenic cells was 5 times higher than embryogenic ones. In
[4] K. Ushiyama, Food and food additives, in: A. Koma-nine, M. Misawa, F. DiCosmo (Eds.), Plant Cell Culture in Japan, CMC, Japan, 1991, pp. 92 – 113.
[5] J. Stockigt, P. Obitz, H. Falkenhagen, R. Lutterbach, S. Endress, Natural product and enzymes from plant cell cultures, Plant Cell Tissue Organ Cult. 43 (1995) 97 – 109. [6] S. Bardocz, G. Grant, D.S. Brown, A. Ralph, A. Pusztai, Polyamines in food-implications for growth and health, J. Biochem. Nutr. 4 (1993) 66 – 71.
[7] S. Bardocz, The role of dietary polyamines, Eur. J. Clin. Nutr. 47 (1993) 683 – 690.
[8] C.M. Protacio, Y. Dai, E.F. Lewis, H.E. Flores, Growth stimulation by catecholamines in plant tissue/organ cul-tures, Plant Physiol. 98 (1992) 89 – 96.
[9] T.A. Smith, Plant amines, in: A. Pirson, M.H. Zimmer-man (Eds.), Encyclopedia of Plant Physiology, Sec-ondary Plant Product, vol. 8, Springer-Verlag, Berlin, 1980, pp. 433 – 460.
[10] M. de Agazio, R. Federico, S. Grego, Involvement of polyamines in the inhibiting effect of injury caused by cutting on K+ uptake through the plasma membrane,
Planta 177 (1989) 388 – 392.
[11] M. Zacchini, A. Marotta, M. de Agazio, Tolerance to salt stress in maize callus lines with different polyamine content, Plant Cell Rep. 17 (1997) 119 – 122.
[12] A.W. Galston, R. Kaur-Sawhney, T. Altabella, A.F. Tiburcio, Plant polyamines in reproductive activity and response to abiotic stress, Bot. Acta 110 (1997) 197 – 207.
[13] C.C. Chu, C.C. Wang, C.S. Sun, C. Hsu, K.C. Yin, C.Y. Chu, F.Y. Bi, Establishment of an efficient medium for anther culture of rice through comparative experiments on the nitrogen sources, Sci. Sin.(Peking) 18 (1975) 659 – 668.
[14] J.C. Sellmer, S.W. Ritchie, I.S. Kim, T.K. Hodges, Ini-tiation, maintenance, and plant regeneration of type II callus and suspension cells, in: M. Freeling, W. Walbot (Eds.), The Maize Handbook, Springer-Verlag, New York, 1994, pp. 671 – 677.
[15] R. Goren, N. Palavan, H. Flores, A.W. Galston, Changes in polyamine titer in etiolated pea-seedlings following red-light treatment, Plant Cell Physiol. 23 (1982) 19 – 26.
[16] T. Takeo, L-alanine decarboxilase in Camellia sinensis, Phytochemistry 17 (1978) 313 – 314.
[17] M. Luckner, Secondary Metabolism in Plant and Ani-mals, Chapman and Hall, London, 1972, pp. 184 – 201. [18] T. Suzuki, Metabolism of methylamine in the tea plant
(Thea sinensisL.), Biochem. J. 132 (1973) 753 – 763. [19] W.R. Sponholz, Nitrogen compounds in grapes, must
and wine, in: J.M. Rantz (Ed.), International Symposium on Nitrogen in Grapes and Wine, Seattle, WA, ASEV, Davis 1991, pp. 67 – 77.
[20] V. Vasil, I.K. Vasil, Plant regeneration from friable embryogenic callus and cell suspension cultures of Zea maysL., J. Plant Physiol. 124 (1986) 399 – 408.
[21] C.L. Armstrong, C.E. Green, Establishment and mainte-nance of friable, embryogenic maize callus and the in-volvement ofL-proline, Planta 164 (1985) 207 – 214.