Fertile pea plants regenerate from protoplasts when calluses have
not undergone endoreduplication
Sergio J. Ochatt *, C. Mousset-De´clas, M. Rancillac
INRA,URGAP,B.V. 1540,21034 Dijon,cedex, France
Received 24 December 1999; received in revised form 17 February 2000; accepted 6 March 2000
Abstract
Large numbers of viable protoplasts were isolated and cultured from five pea genotypes. Calluses obtained (percent final plating efficiency (% FPE)=0.65 – 2.82% of initially plated protoplasts) exhibited great differences in proliferation and regeneration competence between and within genotypes. Flow cytometric analyses showed the occurrence of endoreduplication processes correlated with such differences, and could serve as a tool for the early prediction of plant regeneration competence from protoplasts. Fertile plants were produced only from calluses with a normal DNA level. © 2000 Elsevier Science Ireland Ltd. All rights reserved.
Keywords:Endoreduplication; Flow cytometry;Pisum sati6um; Plant regeneration; Protoplast
www.elsevier.com/locate/plantsci
1. Introduction
The pea (Pisum sati6um L.) is well known as
being recalcitrant to biotechnological approaches in general [1] and in particular to those based on protoplasts [2], with the competence for whole fertile plant regeneration as a recurrent bottleneck. Protoplasts have been isolated from a range of tissues [2 – 4] and regeneration has been reported via somatic embryogenesis [5] and caulogenesis [6 – 8]. However, none of these protocols has proven widely applicable, efficient, reproducible and fast, as underlined by Jacobsen [1]. In addi-tion, whenever regeneration occurred via cauloge-nesis authors had to rely on the grafting of protoplast-derived shoots, that remained non-rootable and also showed varying degrees of fertil-ity thereafter.
The enhancement of disease resistance and an improved protein quality, as pursued in our labo-ratory, will require the availability of reliable methods for the recovery of normal plants from protoplasts. This will permit the introduction of traits by direct gene transfer [9] or protoplast fusion, where somatic hybridisation will be needed to overcome barriers for crossing with wild rela-tives carrying those agronomic traits such as the grasspea (Lathyrus sati6us) and Pisum ful6um [10]. Therefore, for a successful exploitation of these approaches, the tissues and plants deriving from protoplasts must be assessed in terms of their ploidy level and true-to-typeness. Only then will it be possible to compare such protoplast-derived plants with those regenerated from complex ex-plants under standard conditions or following
transformation mediated by Agrobacterium tume
-faciens, and to fully assess their utility for
breed-ing, particularly by exploiting the solid,
non-chimaeric nature commonly recognised for plants deriving from protoplasts.
The occurrence of endoreduplication, somatic cells of different nuclear DNA levels, is
wide-Abbre6iations: BAP, 6-benzylaminopurine; FPE, final plating effi-ciency; IPE, initial plating effieffi-ciency; Kin, kinetin; NAA, a
-naph-thaleneacetic acid; TDZ, thidiazuron; 2,4-D, 2,4-dichlorophenoxyacetic acid.
* Corresponding author. Tel.: +33-3-80693161; fax: + 33-3-80693263.
E-mail address:[email protected] (S.J. Ochatt).
spread among angiosperms [11], and could be enhanced in protoplast-derived cultures thereby accounting for the difficulties observed for plant regeneration from such tissues to date [1]. Flow cytometry is an invaluable tool to determine the nuclear DNA content and the modification of patterns of endoreduplication in the resulting tis-sues, whereby its relationship with cell division activity and regeneration competence may be studied.
2. Materials and methods
2.1. Protoplast isolation culture and plant regeneration
The protoplast source tissues were the epicotyls from embryo axes (and shoot cultures therefrom) of the pea cultivars Frisson, Solara, Terese, Bon-naire and P64, a hypernodulating mutant of Fris-son [12]. For protoplast isolation, several enzyme mixtures dissolved in various media were tested (Table 1), mostly modified from those described in [2,8]. Protoplasts were cultured at various densities
(0.25, 0.5, 0.75, 1.0 or 2.0×105 ml−1), and on a range of media as 1 ml liquid, alginate (1.0%, w/v) or agarose-containing (0.6%, w/v) layers or 50 ml
droplets, dispensed in 12-welled repliplates
(Fal-con® 3043). Media were based on KM [13] or
LMJ [2] formulae that were supplemented with
2,4-dichlorophenoxyacetic acid (2,4-D), a
-naph-thaleneacetic acid (NAA), picloram, kinetin (Kin), thidiazuron (TDZ) and/or zeatin.
Protoplast proliferation, following 4 weekly re-ductions of culture osmolarity with
osmoticum-free medium (1:4, v/v), was assessed as a
percentage of dividing protoplasts (initial plating efficiency (IPE)) and of microcallus production (final plating efficiency (FPE)) (Table 1). Microcal-luses were identified and cloned separately on me-dia KP [13], LP30 [2] or MS [14] with 0.1 mg l−1
picloram and 0.5 mg l−1 6-benzylaminopurine
(BAP), as appropriate. Until the proliferation of microcallus and for callus growth, cultures were kept in the dark at 2492°C. For regeneration via somatic embryogenesis, the sequence of media as reported by Lehminger-Mertens and Jacobsen [5] was employed, while for organogenesis a modified MS salts medium (with fourfold the micronutrient
Table 1
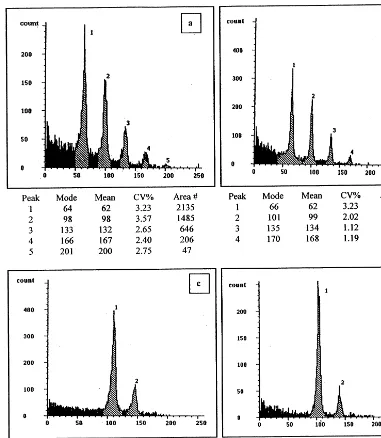
Protoplast isolation and proliferation efficiencies (mean data9S.D. from a minimum of ten replicated experiments) and regeneration competence (mean data from six independent experiments) of five pea genotypesa
Terese Bonnaire
Solara
Parameters Frisson P64
2501 3402YC
Enzyme mixturesb 3402RS 3402RS 3401YC
Yield (106g−1f.wt) 4.7392.44 a 5.6091.62 a 6.1490.62 a 4.9091.16 a 2.9090.77 b
89.596.1 ab 82.893.8 bc
92.395.9 ab
Viability (%) 96.992.1 a 85.691.8 b
Kpic KPic
Mediac KP KP LP
34.4197.28 c 67.1992.39 a
Percent IPEd 59.4299.65 b 71.1496.28 a 49.6695.22 b
2.8290.21 a 1.1790.11 c 2.0090.36 b 0.6590.14 d 0.00 e Percent FPEd
Regeneration pathway Organogenesis Embryogenesis Organogenesis Organogenesis No callus obtained mainly
mainly mainly
only
5B/0.01 N×4 LMJe\o\m\g 5B/0.05 N×4 None
Regeneration media 3B/0.1 N×4
(mg l−1)e
0.052 a 0.034 b
Percent plant regeneration 0.016 c 0.002 d 0.00 e
Percent fertile plants \90 \90 \90 Not recorded 0.00
aFigures within a row followed by different letters were significantly different (a=0.05).
b2501, 2% Macerozyme R-10, 0.1% Pectolyase Y-23, 5% cellulase Fluka; 3402 RS, 3% Macerozyme R-10, 0.2% Pectolyase
Y-23, 4% cellulase Onozuka RS; 3402YC, as 3402RS but with Onozuka YC; 3401YC, as 3402YC but with 0.1% Pectolyase. All enzymes (%, w/v) dissolved in medium LP [2] with 72 g l−1myo-inositol and 5 mM MES (pH 5.6).
cKPic, KM medium [13] with (mg l−1) picloram (0.1), zeatin (0.2) and NAA (1.0); KP, as KPic but with 2,4-D instead of picloram; LP, as reported [2]. All data for 105protoplasts per ml liquid medium.
dIPE, initial plating efficiency (% of cultured protoplasts dividing once); FPE, final plating efficiency (% of cultured protoplasts
giving microcalluses).
content) plus BAP and NAA [15], and with cal-luses transferred over four successive monthly pas-sages on the same medium was adopted. Dishes were kept at 22/24°C, under a 16/8 h light pho-toperiod of 100 mmol m−2 s−1 from warm white
fluorescent tubes. For all genotypes, both regener-ation pathways and all media were systematically tested. Rooting of regenerated shoots with elon-gated internodes occurred in half-strength MS
medium [14] with 1.0 mg l−1 NAA (5.4
mM) and
0.8% agar (pH 5.7), as described in [15]. Rooted plants of the different genotypes were acclimatised in the greenhouse within 10 days, whereafter they were cultured in a 1:1 mixture of peat and soil until flowering and seed set.
Data presented are (except when stated
other-wise) the mean9S.D. of results from a minimum
of ten replicated experiments with at least five replications per medium and culture condition tested, and were statistically analysed by ANOVA (a=0.05).
2.2. Endoreduplication analysis by flow cytometry
The DNA content of calluses at different devel-opmental stages (of various ages, with or without differentiation) was examined by flow cytometry and compared to a diploid pea (cv. Frisson) leaf control. Nuclei were mechanically isolated from
3 mm3 of calluses by chopping them with a
sharp razor blade in about 2 ml of nucleus isola-tion buffer [16,17]. The suspension was filtered through a 40 mm nylon mesh, and 4%
,6-diamidino-2-phenylindole (DAPI), AT binding specific
fluorochrome was added to the filtrate to a final concentration of 1mg ml−1. The DNA contents of
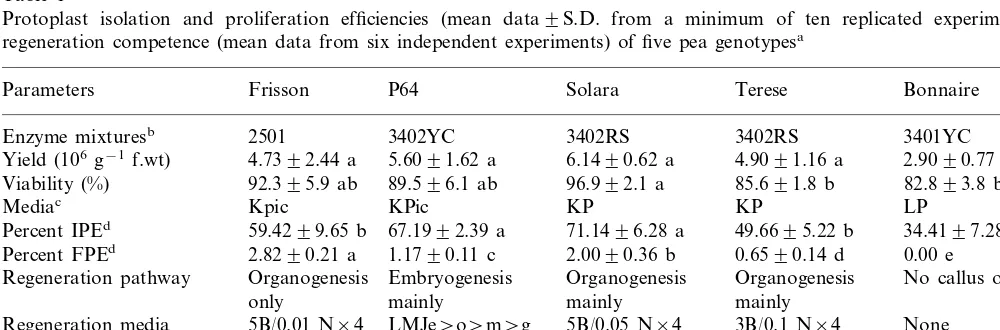
the isolated nuclei suspensions were analysed using a Partec PAS-II flow cytometer equipped with an HBO-100 W mercury lamp and a dichroic mirror (TK420). The data were plotted on a semi-loga-rithmic scale (Fig. 1). In this way, the histogram peaks from 2C to 64C are evenly distributed along the abscissa. Integrals of each peak in the his-tograms were obtained using the built-in software Partec DPAC V2.0.
For one callus portion at one developmental stage, generally two independent DNA content analyses were made, each including a minimum of 2000 nuclei (Table 2). A portion of the tissues analysed was kept in culture for the subsequent
assessment of growth and differentiation
competence.
3. Results
Table 1 gives data on the best protoplast isola-tion efficiency, IPE, FPE and plant regeneraisola-tion results for the various genotypes studied. The use of any enzyme mixture other than the best for each genotype gave protoplast populations that entered division only occasionally and never pro-duced significant numbers of protoplast-derived microcalluses.
Frisson, P64 and Solara proliferated best (FPE above 1% of initially cultured protoplasts), while Terese was intermediate and Bonnaire protoplasts entered mitosis but were incapable of sustained division (Table 1). In this context, LMJ-based media permitted initial division of Frisson and P64 protoplasts but failed to do so for the other geno-types and, in any case, never supported sustained proliferation for either of the genotypes studied, with growth arrest consistently occurring at around the ten-celled colony stage. This was in fact the result observed for Bonnaire protoplasts on all media and conditions assessed.
Differences were apparent in terms of plating density requirements, with Frisson and P64 proto-plasts dividing best at 1.0×105, but at 2.0×105 ml−1 for Terese and 0.5×105 ml−1 for Solara. Any deviation from these optimal densities re-sulted in protoplast aggregation, browning and ultimate death.
Maximum plant regeneration from Frisson, So-lara and Terese calluses was obtained via
organo-genesis of shoot buds (Table 1), with
embryogenesis never observed for Frisson and ex-tremely rare for Solara and Terese. Conversely, for P64, optimum plant regeneration was mainly via somatic embryogenesis while regeneration via caulogenesis was seldom observed. For all four genotypes, a requisite for production of whole protoplast-derived plants was the transfer of buds
and/or embryos formed to a hormone-free MS
medium over four passages for growth, then to a
half-strength MS medium with 1 mg l−1 NAA
(medium NAA) for root induction and, finally, to a half-strength hormone-free MS medium (R) right before transfer to the glasshouse of regener-ated rooted plants.
Fig. 1. Typical flow cytometry profiles of pea protoplast-derived tissues. Peak numbers correspond to a DNA level of 2C for peak 1, 4C for peak 2, 8C for peak 3, 16C for peak 4 and 32C for peak 5. Abscissas give the fluorescence intensity in arbitrary units of nuclei, with the peak index position for each 2C peak (channel number) varying dependent on the photo-multiplier level (gain) of analyses. The 2C value is related to the position of the 2C peak of a leaf control. (a) Non-regenerating calluses of Frisson; (b) P64 calluses regenerating embryos but no plants; (c) P64 calluses regenerating plants through embryogenesis; (d) Frisson calluses regenerating plants through organogenesis.
(organogenesis and/or embryogenesis).
Accord-ingly, the best regeneration responses for these genotypes differed for a same medium (Table 1). Thus, large numbers of shoot buds were regener-ated from cultures of Frisson on a medium with
5.0 mg l−1 BAP and 0.01 mg l−1 NAA, while
regeneration from protoplast-derived tissues of P64 occurred mostly via somatic embryogenesis, and on the sequence of media reported in [2]. Solara and Terese protoplast-derived cultures were
of culture, and without any reduction in the
num-ber of shoots/embryos produced or of normal
plants recovered. Conversely, non-regenerating calluses consistently failed to produce any shoots or embryos irrespective of the number of succes-sive passages (up to 12) on regeneration medium. Depending on the genotype, a minimum of 12 months and up to 18 months in average elapsed between protoplast isolation and the harvest of seeds from the protoplast-derived plants, which were produced at a range of as little as 1 (Terese) to more than 50 (Frisson) per ml of initially cultured protoplasts.
Also of interest were the large differences in regeneration competence observed between callus protoclones within a given genotype. The flow cytometry analysis of the different callus cate-gories analysed, pooled for Frisson and P64, per-mitted to correlate such differences in regeneration competence with their respective nuclear DNA contents (Table 2). Thus, whenever an endomitotic DNA replication process (endoreduplication) had occurred during the proliferation phase preceding transfer to the regeneration media, the resulting tissues were incapable of differentiation but tended to proliferate faster than true-to-type tissues. Like-wise, calluses that were true-to-type but failed to respond, once on the regeneration media, consis-tently exhibited a higher DNA content than the
regenerating ones. As above, this was not affected by the time in culture up to a maximum of 2 years from the first transfer on regeneration media. Typ-ical flow cytometric profiles of regenerating and non-regenerating cultures of Frisson and P64 are shown in Fig. 1. In this context, the cytometric analysis of non-regenerating protoplast-derived calluses of Solara and Terese (of a reduced regen-eration competence) revealed that these, too, had undergone a process of endoreduplication (profiles not shown).
Generally, the medium on which the calluses
had been obtained and/or maintained played a
significant role on the occurrence of endo-reduplication, but such medium as well as the one for regeneration from the
protoplast-derived tissues was strongly
genotype-de-pendent. The use of picloram was particularly prone to provoking an increase in the ploidy level of the protoplast-derived tissues, and the same occurred when TDZ was used to induce shoot bud regeneration (data not shown). This was particu-larly clear when comparing flow cytometric profi-les of the source tissues used for protoplast isolation or the young microcalluses derived from them with those of calluses transferred to various proliferation media, and subsequently with those showing (or not) a competence for plant regenera-tion.
Table 2
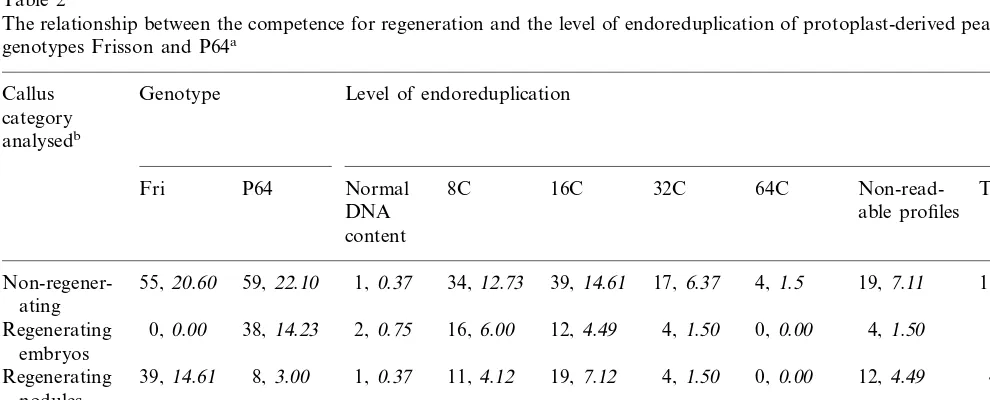
The relationship between the competence for regeneration and the level of endoreduplication of protoplast-derived pea calluses of genotypes Frisson and P64a
Callus Genotype Level of endoreduplication category
Non-regener- 34, 12.73 39,14.61 17, 6.37 4, 1.5 19,7.11
ating
12,4.49 4, 1.50 0, 0.00 4, 1.50 38,14.23 Regenerating 0, 0.00 38, 14.23 2, 0.75 16, 6.00
embryos
Regenerating 37,13.86 31, 11.61 67,25.09 1, 0.37 0, 0.00 0, 0.00 68,25.47
plants
71,26.58 62, 23.22 70,26.22 25, 9.36 4, 1.5 35,13.10 267,100.0 Total
aData are the total number and, in italics, the percentage of calluses for each category analysed.
4. Discussion
The results for IPE and FPE fit well with those reported by other authors, but optimum prolifera-tion for each genotype occurred on media different from those published in the past [2,6,8]. In turn, the development of novel strategies for protoplast isolation and culture was needed for success. The closely related genotypes Frisson and P64 [12] responded similarly and clearly detached from the rest, in line with previous reports including other related genotypes [2].
The heterogeneity of a freshly-isolated proto-plast population is immediately apparent, and is most obviously linked with the source tissue that was originally digested [18,19]. We have verified that the epicotyls used here as source tissues for protoplast isolation were constituted by cells with a 2C level of DNA. In this context, the replication of DNA and mitosis have been shown to be basically independent processes [20,21], with mito-sis restricted normally to rapidly-dividing (e.g. meristematic and reproductive [17]) tissues only. Endoreduplication in this work may have resulted from using Picloram for initial protoplast culture [22]. Indeed, as a part of the developmental pro-gramme of the plant, the differentiating tissues can undergo nuclear processes other than DNA repli-cation and mitosis alone including endoreduplica-tion, as already discussed [17,23,24]. It could also be argued that, besides endoreduplication, sponta-neous protoplast fusions might have also con-tributed to the different ploidy levels observed. However, this would have led to a mixoploid flow cytometric pattern, enriched in the two peaks cor-responding to the 4C and 8C DNA levels and coupled with a significantly reduced 2C peak. Conversely, the scheme of multiple decreasing peaks as observed is the typical imprint of an endoreduplication phenomenon.
The exploitation of flow cytometry to assess the DNA content and to confirm or infirm the even-tual true-to-typeness of protoplast-derived tissues is of relevance to provide a further insight on their subsequent ability to differentiate, and could be used in the future as an early predicting method of regeneration competence. This might help to in-crease the low percentages of protoplast-derived calluses that regenerate plants in pea, as only those calluses exhibiting a normal DNA content would be transferred onto the regeneration media,
thereby fostering a more efficient use of proto-plast-based approaches for breeding.
Within the context of protein legumes, such knowledge will be precious for the somatic hy-bridisation of pea and some of its wild relatives (e.g. Lathyrus sati6us L.) for disease resistance
breeding. This rapid and efficient methodology would also be useful for direct gene transfer into pea protoplasts, aimed at an improvement of seed quality and an increased disease resistance. More generally, it may also shed some new light on the long term interactions existing between the re-quirements for in vitro proliferation and the subse-quent regeneration of viable and fertile whole plants from protoplasts not only of legumes but also for other species. Indeed, results in this study strongly suggest a clear effect of the growth regu-lators used during in vitro stages on the DNA
level of the subsequently regenerated tissues/
plants. They also suggest that, in pea, both the recalcitrance for plant regeneration from proto-plasts and the reduced fertility observed after ge-netic transformation [1] might result from an altered DNA content.
Acknowledgements
The authors acknowledge financial support by the Conseil Re´gional de Bourgogne (grant no. DAF98511286).
References
[1] H.-J. Jacobsen, Biotechnology in grain legumes: current state and critical remarks, Grain Legumes 2 (1993) 12 – 13.
[2] R. Lehminger-Mertens, H.-J. Jacobsen, Regeneration of plants from protoplasts of pea (Pisum sati6um L.), in:
Y.P.S. Bajaj (Ed.), Biotechnology in Agriculture and Forestry, vol. 22, Springer, Berlin, 1993, pp. 97 – 104. [3] T. Gram, O. Mattsson, M. Joersbo, Division frequency
of pea protoplasts in relation to starch accumulation, Plant Cell Tissue Organ Culture 45 (1996) 179 – 183. [4] T. Hashimoto, T. Yamada, A. Tada, S. Kawamata, Y.
Tanaka, P. Sriprasertsak, Y. Ichinose, H. Kato, S. Izutsu, T. Shiraishi, H. Oku, Y. Ohtsuki, Transient expression in electroporated protoplasts: elicitor respon-siveness of a phenylalanine ammonia-lyase promoter, Plant Cell Rep. 11 (1992) 183 – 187.
[6] P. Bo¨hmer, B. Meyer, H.-J. Jacobsen, Thidiazuron-in-duced high frequency of shoot induction and plant regeneration in protoplast derived pea callus, Plant Cell Rep. 15 (1995) 26 – 29.
[7] R. Lehminger-Mertens, H.-J. Jacobsen, Protoplast regen-eration and organogenesis from pea protoplasts, In Vitro Cell. Dev. Biol. 25 (1989b) 571 – 574.
[8] J. Puonti-Kaerlas, T. Eriksson, Improved protoplast cul-ture and regeneration of shoots in pea (Pisum sati6um
L.), Plant Cell Rep. 7 (1988) 242 – 245.
[9] A. De Kathen, T. Wegelin, H. Kiesecker, B. Meyer, H.-J. Jacobsen, Transgenic grain legumes from protoplasts?, in: Third European Conference on Grain Legumes, Val-ladolid, Spain, 1998, pp. 370 – 371.
[10] S.A. Tarawalli, In: G.P. Chapman, S.A. Tarawalli (Eds.), Systems for Cytogenetics Analysis in Vicia faba L., Martinus-Nijhoff/Dr W Junk Publ, 1984, pp. 129 – 137. [11] F. D’Amato, Cytogenetics of plant cell and tissue
cul-tures and their regenerates, CRC Crit. Rev. Plant Sci. 3 (1985) 73 – 112.
[12] G. Duc, A. Messager, Mutagenesis of pea (Pisum sati6um
L.) and the isolation of mutants for nodulation and nitrogen fixation, Plant Sci. 60 (1989) 207 – 213. [13] K.N. Kao, M.R. Michayluk, Nutritional requirements
for growth ofVicia hajastana cells and protoplasts at a very low population density in liquid media, Planta 126 (1975) 105 – 110.
[14] T. Murashige, F. Skoog, A revised medium for rapid growth and bioassays with tobacco tissue cultures, Phys-iol. Plant. 15 (1962) 472 – 497.
[15] S.J. Ochatt, C. Ponte´caille, M. Rancillac, The growth regulators used for bud regeneration and shoot rooting affect the competence for flowering and seed set in regenerated plants of protein peas, In Vitro Cell. Dev. Biol. Plant 36 (2000), in press.
[16] L.J.W. Gilissen, M.J. van Staveren, J. Creemers-Mole-naar, H.A. Verhoeven, Development of polysomaty in seedlings and plants ofCucumis sati6usL, Plant Sci. 91
(1993) 171 – 179.
[17] C. Lemontey, C. Mousset-De´clas, N. Munier-Jolain, J.P. Boutin, Maternal genotype influences pea seed size by controlling both mitotic activity during early embryoge-nesis and final endoreduplication level/cotyledon cell size in mature seed, J. Exp. Bot. 51 (2000), in press. [18] C. Bergounioux, S. Brown, P.X. Petit, Flow cytometry
and protoplast cell biology, Physiol. Plant. 85 (1992) 374 – 386.
[19] Z. Chen, K.-C. Hsiao, C.H. Bornman, Ploidy and divi-sion efficiency of petiolar protoplasts of Brassica napus, Hereditas 120 (1994) 41 – 46.
[20] L.J.W. Gilissen, M.J. van Staveren, J.C. Hakkert, J.M. Smulders, H.A. Verhoenen, J. Creemers-Molenaar, The competence of cells for cell division and regeneration in tobacco explants depends on cellular location, cell cycle phase and ploidy level, Plant Sci. 103 (1994) 81 – 91.
[21] E.L. Rech, S.J. Ochatt, P.K. Chand, B.I. Mulligan, M.R. Davey, J.B. Power, Electroporation increases DNA syn-thesis in cultured plant protoplasts, (Nat.) Biotechnol. 6 (1988) 1091 – 1093.
[22] K. Van den Berg, D. De Craene, R. Van Parijs, Cytoge-netic effects of Picloram on callus induction in Pisum sati6umL. cultivar Finale, Med. Fac. Landbouww. Rijk-suniv. Gent 56 (1991) 1469 – 1481.
[23] W. Nagl, Endopolyploidy and Polyteny in Differentia-tion and EvoluDifferentia-tion, North-Holland, Amsterdam, 1978. [24] D.W. Galbraith, K.R. Harkins, S. Knapp, Systemic
en-dopolyploidy in Arabidopsis thaliana, Plant Physiol. 96 (1991) 985 – 989.