Summary Flowering and vegetative growth were assessed in 19 half-sib families of Alnus rubra Bong. planted in a replicated field trial near Olympia, Washington, USA. The trial consisted of three square spacings (0.5, 1.0 and 2.0 m), two irrigation regimes (low and high), and two fertilization treatments (0 and 300 kg P ha−1). Male and female flowers were surveyed in all plots for all families at plantation ages 4 and 5 years. Female strobili were surveyed for seven families in the 2-m spaced plots at plantation age 6 years. The percentage of trees flower-ing and the number of flowers per tree were always greatest, and height and diameter growth were always least, in the low-irrigation regime. Phosphorus fertilization had no effect on the percentage of trees flowering or on 5-year height or diameter growth; it had a positive but small effect on the number of female flowers per tree at age 5 years. Wider spacing resulted in larger trees, higher rates of flowering, and higher tree survival. Within each irrigation regime, the percentage of trees flowering increased as tree size increased. There was substantial variation in flowering among families, with positive but low correlations between tree size and flowering attributes. At ages 4 and 5 years, the ratio of number of trees flowering in the low-irrigation regime to number of trees flowering in the high-irrigation regime differed among families. By age 6 years, many more trees flowered than in previous years, and differ-ences between irrigation regimes were reduced. Early growth rates were rapid and resulted in substantial crown recession and mortality in the closer spacings by age 5 years. We conclude that spacings less than 2 m should only be used in seed production areas if roguing can be done by age 2 to 3 years. Keywords: diameter growth, flower production, height growth, phosphorus.
Introduction
Alnus rubra Bong. occurs naturally from central California to southeastern Alaska and is the most abundant hardwood tree species in western Oregon, Washington and British Columbia. Although considered to have no value for many decades, Alnus rubra is now appreciated for its unique ecological attributes (e.g., N2-fixation and immunity to Phellinus root rot) and its contribution to the forest products economy of the region. Several operational plantations of A. rubra have recently been
established, and the biology and management of the species have been summarized (Hibbs et al. 1994). General guidelines are available for collection and treatment of A. rubra seed (Hibbs and Ager 1989), but detailed information on reproduc-tive processes, including variability in flowering within and among Alnus stands and the effects of management practices, is limited (cf. Brown 1986 and Ager et al. 1994). We have investigated the effects of spacing, fertilization and irrigation on vegetative growth and flowering in 19 half-sib families of A. rubra under short-rotation intensive culture regimes.
Materials and methods
The study reported here is one of several trials undertaken to evaluate short-rotation intensive culture regimes for wood, fiber or biomass production. This replicated trial, planted on a site with uniform conditions and good access, provided the opportunity to make repeated observations on reproductive development and vegetative growth of A. rubra in response to various treatments. The trial was installed in spring 1986 on a xeric site near Olympia, Washington (47°00′ N, 122°45′ W). The soil is a somewhat excessively drained, loamy fine sand formed in sandy glacial outwash. Slope is 0--1%; elevation is 50 m. During the study, precipitation and temperature were monitored with an on-site weather station. Based on a long-term weather station located 12.5 km from the study area, mean annual precipitation is 129 cm with only 19 cm falling from May 1 through September 30 (US Dept. of Commerce 1961).
The experimental design was a randomized complete block design on three adjacent blocks. Tested were three square spacings (0.5, 1.0 and 2.0 m), two irrigation regimes (low and high), and two phosphorus fertilization treatments (0 and 300 kg P ha−1 as triple superphosphate). Irrigation regimes were applied to whole plots, fertilization treatments to split plots, and spacings to split-split plots. The low-irrigation regime was intended to provide just enough supplemental water to ensure survival and tree health in this regime; approximately 15 cm of water per year was applied during June, July and August. The high-irrigation regime was intended to increase tree growth and thus accelerate the rate of stand development on this dry site; approximately 55 cm of water per year was applied in this regime. In most years, the high-irrigation treatment began
Effects of irrigation, spacing and fertilization on flowering and growth
in young
Alnus rubra
CONSTANCE A. HARRINGTON and DEAN S. DEBELL
Pacific Northwest Research Station, Forestry Sciences Laboratory, 3625 93rd Ave. S.W., Olympia, WA 98512-9193, USA
Received May 31, 1994
between late May and mid-June; however, in 1991, irrigation was not begun until July 5 because of equipment failures. Phosphorus fertilization was chosen because A. rubra is a nitrogen-fixing species whose growth is closely linked with P status (Radwan and DeBell 1994). The P fertilizer was applied with a spreader and disked into the surface soil. All plots were maintained in a weed-free condition throughout the experi-ment.
Each measurement plot contained 100 trees and was sur-rounded by a minimum of three buffer rows. Within each measurement plot, there were five to seven container-grown seedlings from each of 19 half-sib families of A. rubra. Fami-lies were randomly assigned to planting spots. The famiFami-lies were from locations that provided a range in latitude and elevation (Table 1). All trees were measured annually for total height and basal diameter (0.3 m above the ground); a selected subsample of trees in each plot was measured periodically for height growth during the growing seasons of 1986--1988 and for diameter growth during the growing seasons of 1987--1991.
In spring 1990 and 1991, all plots (approximately 3000 trees) were surveyed for the presence of reproductive struc-tures. In both years, each tree was coded as not flowering or as having a low, medium or high number of flowers. For some analyses, the categorical values were transformed to numerical values based on the following conversion: none = 0, low = 5, medium = 35, and high = 70 (the numerical values were midpoints of categories based on ocular estimates of the num-ber of inflorescences per tree). In spring 1993, seven of the 19
families in the 2-m plots were selected for an additional assess-ment of the number of woody ‘‘cone’’ clusters (i.e., female inflorescences that had opened and shed seed during the pre-ceding months) per tree. At least 15% of the trees in each selected family had female flowers in the 2-m spacing + low-irrigation treatment in 1991; in addition, the selected families exhibited a range in apparent sensitivity of flowering to irrigation. Because the 1993 survey provided a measure of the reproductive output for 1992, the information is reported here as 1992 female flowers. The estimates for 1992 are prob-ably conservative because flowers may have aborted or been destroyed between the time of flowering and when they were surveyed.
Total oven-dry, aboveground woody biomass, foliar biomass and leaf area were predicted for each tree from site-specific equations based on cultural regime, diameter and total height (DeBell et al. 1991). Statistical tests of the effects of the irrigation, fertilization and spacing treatments on tree size and flower production were done by ANOVA for a split-split plot experiment (Snedecor and Cochran 1980). Treatment effects were judged significant at P≤ 0.05; however, actual prob-ability levels are provided. In 1990, almost all trees that flow-ered had similar quantities of male and female flowers, so separate analyses were not made for each flower type. Because the frequency of male and female flowering differed in 1991, separate analyses were made for each sex and for both types combined for that year. Basic data on flowering by family were summarized by block and treatment and examined graphically. Correlations among flowering variables and tree size attributes were calculated based on values for individual trees. Use of values from individual trees in the correlation analyses as-sumes each tree was an independent observation and ignores the basic experimental design; thus, results from these analy-ses were only used to describe the general relationships present in the data and not to test for significant associations between variables.
Results
By the end of the 1990 growing season, both the spacing and irrigation treatments had markedly influenced tree size and survival (Table 2). Tree size was significantly greater in the 1.0- and 2.0-m spacing treatments than in the 0.5-m spacing treatment, and it was also significantly greater in the high-irri-gation regime than in the low-irrihigh-irri-gation regime. Tree size was not affected by the fertilization treatments, and there were no significant interactions between the fertilization and other cul-tural treatments (Table 3).
The high-irrigation regime altered the seasonal pattern of growth. For example, in 1987, 1989 and 1990 in the 2-m spacing treatment, diameter growth of the trees in the high-ir-rigation regime peaked later in the growing season and high growth rates occurred over a longer period than in the low-ir-rigation regime (Figure 1). In a study of the same trees, DeBell and Giordano (1994) noted that seasonal height growth contin-ued for a longer period in the 1986--1988 growing seasons in the high-irrigation regime than in the low-irrigation regime. Table 1. Parent location and relative rank based on height of half-sib
families of Alnus rubra.
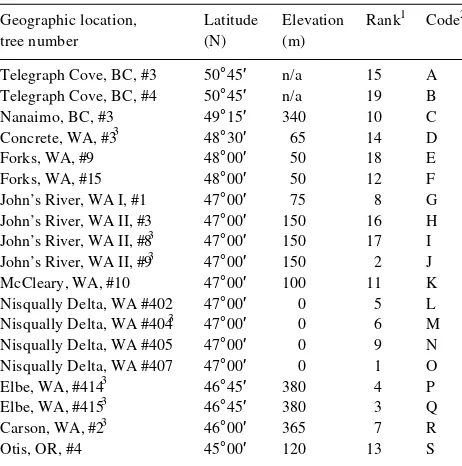
Geographic location, Latitude Elevation Rank1 Code2 tree number (N) (m)
1 Rank based on fall 1992 height in 2-m plots in the high-irrigation
regime (1 = tallest).
2 Code used for plotting family values in Figures 3 and 4.
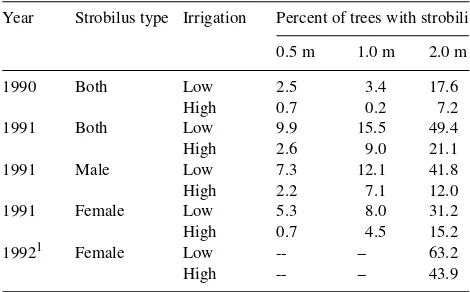
Flowering was enhanced by the 1.0- and 2.0-m spacing treatments. Spacing and the spacing × irrigation interaction were significant in the ANOVA of all measures of flowering in 1990 and 1991 (Table 3). The high-irrigation regime sup-pressed flowering, and the difference between irrigation re-gimes was greatest in the 2-m spacing treatment (Table 4). Fertilization with P did not significantly affect the percentage of trees with inflorescences in any year, but it significantly increased the number of female inflorescences per tree in 1991 (Tables 3 and 5). In 1992, the high-irrigation regime was again
associated with poor flowering, and fertilization had no effect on the percentage of trees flowering or on the mean number of mature female flower clusters per tree (Table 6). The percent-age of trees with flowers was greater in 1991 than in 1990 and, for the families sampled, greater in 1992 than in 1991 (Ta-ble 4). Although different stages and types of flowers were assessed in the various surveys, it is clear that the percentage of trees flowering increased each year.
Within each irrigation regime, as tree size increased, there was an increase in the percentage of trees within a family with Table 2. Effects of spacing and irrigation treatment (Low-I and High-I) on tree size and survival at the end of the 1990 growing season (5 years after planting). Values are means ± 1 SE.
Spacing (m) Height (m) Diameter (cm) Survival (%)
Low-I High-I Low-I High-I Low-I High-I
0.5 4.5 ± 0.5 7.3 ± 0.1 3.3 ± 0.3 5.1 ± 0.1 53 ± 3.1 33 ± 2.1 1.0 5.5 ± 0.3 8.2 ± 0.1 4.7 ± 0.2 7.1 ± 0.1 94 ± 0.4 67 ± 2.2 2.0 6.1 ± 0.2 8.3 ± 0.2 7.5 ± 0.3 9.5 ± 0.1 99 ± 0.3 98 ± 0.7
Table 3. Significance of ANOVA model components on tree size 5 years after planting and on number of inflorescences by year and type (B = both, F = female, M = male).
Source of variation df Probability of > F-value occurring
Fall 1990 Number of inflorescences by year and type
Diameter Height 90 B 91 B 91 F 91 M
Block 2 0.01 < 0.01 0.44 0.05 0.03 0.22
Irrigation1 1 0.01 0.02 0.24 0.08 0.07 0.11
Fertilization1 1 0.62 0.48 0.95 0.59 0.05 0.80
Irrigation × fertilization1 1 0.55 0.83 0.99 0.94 0.13 0.59 Spacing 2 < 0.01 < 0.01 < 0.01 < 0.01 < 0.01 < 0.01 Irrigation × spacing 2 < 0.01 0.44 0.03 < 0.01 < 0.01 < 0.01 Fertilization × spacing 2 0.81 0.32 0.81 0.92 0.74 0.75 Irrigation × fertilization × spacing 2 0.12 0.15 0.86 0.81 0.45 0.67
1 Irrigation tested using block × irrigation as the error term (df = 2); fertilization and irrigation × fertilization tested using block × fertilization
(irrigation) as the error term (df = 4); the split-split error term had 16 df.
Figure 1. Periodic diameter increment in 2-m plots of Alnus rubra by irrigation regime and year.
Table 4. Effects of irrigation and spacing on the percentage of trees flowering.
Year Strobilus type Irrigation Percent of trees with strobili
0.5 m 1.0 m 2.0 m
1990 Both Low 2.5 3.4 17.6
High 0.7 0.2 7.2
1991 Both Low 9.9 15.5 49.4
High 2.6 9.0 21.1
1991 Male Low 7.3 12.1 41.8
High 2.2 7.1 12.0
1991 Female Low 5.3 8.0 31.2
High 0.7 4.5 15.2
19921 Female Low -- -- 63.2
High -- -- 43.9
1 Only one spacing treatment and seven families surveyed (see text
male strobili (Figure 2). For each family, the relationship between mean tree diameter (averaged across trees within one irrigation, spacing and fertilization combination) and percent flowering was positive. Within all tree sizes but the smallest (which did not flower), there was a substantial range in flow-ering as a result of differences among families in propensity to flower.
Correlations between number of female strobili in 1991 and woody biomass, leaf biomass, leaf area and basal diameter were positive but low (r≈ 0.20). When the trees were separated by irrigation regime, the correlations increased to 0.33--0.36 for trees in the low-irrigation regime but were unchanged for trees in the high-irrigation regime (0.22--0.24). Correlations between height and number of female strobili in 1991 were
lower than those for biomass, leaf area or diameter (r = 0.05 for both regimes, r = 0.20 for trees in the low-irrigation regime, r = 0.13 for trees in the high-irrigation regime). In the low-ir-rigation regime, the highest correlations were between total number of flowers produced in 1990 + 1991 and leaf area (r = 0.40) or leaf biomass (r = 0.40). A similar correlation value was observed between flowering and stem diameter, because both leaf area and leaf biomass were highly correlated with basal diameter (r = 0.96--0.97). Correlations between numbers of clusters per tree and height and diameter growth the year the flowers were initiated (1991) were low, but were indicative of a stronger relationship between flower production and diame-ter growth (r = 0.18) than between flower production and height growth (r =−0.06).
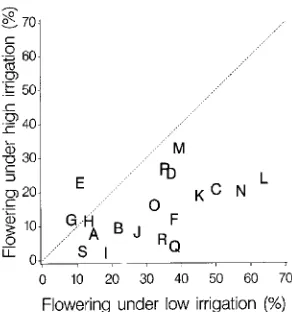
Although some families were more likely to flower than others, individual trees did not exhibit a tendency to produce a consistently high or low number of flowers (or to alternate high and low flower production). There were also differences among families in their flowering response to irrigation re-gime. In 1991 at the 2-m spacing, the percentage of trees with female flowers ranged from 2.8% for John’s River II, #8 in the high-irrigation regime to 63.6% for Nisqually Delta, #402 in the low-irrigation regime (Figure 3). The ratio of the percent-age of trees flowering in the low-irrigation treatment to the percentage flowering in the high-irrigation treatment ranged by family from 0.5 to 8.5, with an overall mean of 2.05. Differences between irrigation regimes in percentage of trees with female strobili was less in 1992 than in 1991 (range for Table 5. Effects of irrigation, fertilization and spacing on number of female inflorescences per tree in spring 1991.
Spacing (m) Low irrigation High irrigation
0 kg P ha−1 300 kg P ha−1 0 kg P ha−1 300 kg P ha−1
0.5 0.1 0.9 0.0 0.1
1.0 0.8 0.7 0.2 0.6
2.0 4.4 5.9 1.4 1.4
Table 6. Significance of ANOVA model components on percentage of trees with 1992 female strobili and mean number of inflorescences (only 2-m spacing sampled).
Source of variation df Probability of > F-value
% With strobili No. of clusters
Block 2 0.98 0.09
Irrigation1 1 0.01 0.06
Fertilization 1 0.28 0.26
Irrigation × fertilization 1 0.83 0.78
1 Irrigation tested using block × irrigation as the error term (df = 2);
the split-plot error term had 4 df.
Figure 2. Percentage of trees per family with 1991 male strobili versus basal diameter at the end of the 1990 growing season. Each point is based on approximately 15 trees and represents the mean value for a family by irrigation, spacing, and fertilization regime.
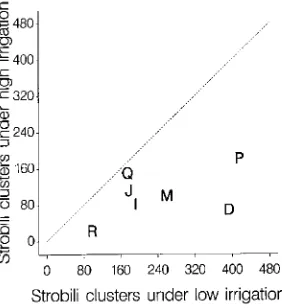
1992 of 1.22 to 2.00, range for same families in 1991 of 1.3 to 6.5) (Figure 4); however, the 1992 ratio of the mean number of strobili clusters per tree between irrigation regimes varied from 1.0 to 4.7 among families, indicating that family differences in flowering response to irrigation were still being expressed.
Discussion
Flower initiation occurs during the summer (Furlow 1979), flowering takes place the following February or March, and seeds are ripe in the fall. Mature female strobili are woody and cone-like in appearance, and remain intact and attached to the plant during seed dispersal and for a time after dispersal is completed. Previous-year female strobili were observed on some trees in spring 1990, indicating that female flower initia-tion occurred in 1988 when the trees were 3 years old from seed (2 years since planting). At the widest spacing, approxi-mately half of the trees in seven families produced female strobili in 1992, indicating female flower initiation in 1991 (i.e., 6 years from seed). These results are in agreement with Stettler’s (1978) observation that A. rubra begins to flower at age 3 to 4 years for individual trees and age 6 to 8 years for most dominant trees in a stand. Several species of Betula that naturally flower at young ages have been induced to flower sooner when grown continuously, suggesting that Betula must attain a minimum size before flowering (Longman 1984). Thus, it may be possible to accelerate flowering in A. rubra with growth-promoting treatments similar to those used with Betula.
Crown size or volume is an important factor affecting flow-ering and seed production. Treatments such as wide initial spacing or thinning are known to increase flowering or seed production because they increase crown size and promote tree vigor (Matthews 1963). We found positive correlations be-tween flowering and variables that quantify crown size (e.g., branch biomass and leaf area). We conclude that variables that quantify crown size are better predictors of flowering than stem diameter if trees are growing in stands with considerable variation in age, stocking or site quality.
Although N fertilization promotes flowering (Ross and
Pharis 1985), we did not include an N fertilization treatment in this study because root nodules on A. rubra fix atmospheric N. The P fertilization treatment was chosen because of its poten-tial to increase tree growth rather than to enhance flowering; however, the P fertilization did not increase 5-year height or diameter growth. The lack of response of most flowering variables to P fertilization is consistent with suggestions by Matthews (1963) and Sedgley and Griffin (1989) that the primary purpose of applying nutrients other than N to enhance flowering or seed production should be to correct nutrient deficiencies. Flowering of A. rubra may be responsive to P fertilization on P-deficient sites because growth of the species is strongly linked to P status (Radwan and DeBell 1994).
Although long-term seed production is enhanced by main-taining tree vigor, it has been suggested that flower initiation is promoted by conditions that restrict shoot growth or cause stress during the period when flower initiation can occur (Ross and Pharis 1985, Owens 1991). Vegetative growth in Alnus is primarily monopodial (the apical bud is a persistent leader, and new branches arise laterally below the apex; Swartz 1971); however, shoots that produce flowers exhibit sympodial growth (the terminal bud withers, floral inflorescences are in terminal and subterminal positions, and the main axis is made up of a series of lateral branches). Additional primary shoot extension does not occur after a reproductive structure has been determined. Flower initiation probably occurs in late June or early July for both A. rubra and A. glutinosa (L.) Gaertn. (McVean 1955, Brown 1986, Ager et al. 1994); how-ever, anatomical studies have not been reported. Species such as Alnus with prolonged periods of shoot growth may have greater leeway in the timing of floral initiation than species with short periods of shoot extension (Longman 1985). How-ever, if floral initiation in Alnus only occurs under certain environmental conditions, it is possible that the extended pe-riod of shoot growth in the high-irrigation regime reduced or even precluded floral initiation in some trees. The importance of differences in genetic control of timing of flowering is indicated by the study of O’Reilly and Owens (1988) showing that initiation of seed-cone buds occurs on different dates in different provenances of Pinus contorta Dougl. ex Loud.
We postulate that the increase in the percentage of trees flowering each year was due to increases in the number of trees attaining a minimum size that permits flowering (cf. Longman 1984). It is possible that differences in weather from year to year also influenced flowering; however, in most tree species, abundant seed crops do not occur in consecutive years even if environmental conditions are favorable. Owens (1991) sug-gested that heavy seed crops alter endogenous conditions and thereby inhibit reproductive bud development. We found no indication that occurrence or relative number of flowers in one year affected the occurrence or relative number of flowers in the succeeding year. This finding contrasts with the report by LaBastide and Vredenburch (1970) that seed crops for mature A. glutinosa follow an annually alternating pattern. The num-ber of flowers produced by the young trees in our study was substantially lower than that observed for older trees in the area; thus, it is possible that alternating years of high and low Figure 4. Mean number of 1992 female strobili clusters per tree in 2-m
flower production could occur when these trees begin produc-ing large seed crops. No long-term records of flowerproduc-ing or seed production in A. rubra are available (see Harrington et al. 1994 for summary of short-term records). McVean (1955) con-cluded that the size of seed crops of A. glutinosa could vary substantially from year to year, but that ‘‘boom-and-bust’’ pat-terns of seed production were not typical.
Genetic differences in flowering or seed production have been observed in many species (Sedgley and Griffin 1989). We observed differences among families in their propensity to flower that could not be attributed solely to differences in mean tree size (cf. Figure 2); however, we used too few members per family in each treatment combination to test for differences among families in flowering. Even though family differences in flowering were evident, overall treatment responses were clear, indicating that the results should be generally applicable to other genotypes.
We conclude that if A. rubra stands are being managed for seed production, the trees should be widely spaced: ≥ 2 m at the time of the first seed crop and wider spacings as trees get larger. Because crown recession and competition-related mor-tality occur very rapidly in dense stands of A. rubra, use of spacings narrower than 2 m at planting is only feasible if roguing is done in the first 2 to 3 years. Establishment on dry sites may be ideal if supplemental irrigation is minimal during June and July to promote flowering and is limited to amounts needed to maintain long-term survival and health during the rest of the year.
Acknowledgments
This research was supported in part by funding from US Dept. of Energy, Biofuels Feedstock Development Program, Interagency Re-search Agreement DE-AI05-810R20914. We thank J. Hawks, S. Bailey and M. Paschke for assistance in conducting the study and personnel at the Washington State Department of Natural Resources, Meridian Seed Orchard for their cooperation.
References
Ager, A., Y. Tanaka and J. McGrath. 1994. Biology, ecology, and utilization of red alder seed. In The Biology and Management of Red Alder. Eds. D.E. Hibbs, D.S. DeBell and R.F. Tarrant. Oregon State Univ. Press, Corvallis, OR, pp 159--169.
Brown, S.M. 1986. Sexual allocation patterns in red alder (Alnus rubra Bong.) along three elevational transects. M.S. Thesis. Univ. Wash-ington, 242 p.
DeBell, D.S., C.A. Harrington and G.W. Clendenen. 1991. Increasing the productivity of biomass plantations of alder and cottonwood in the Pacific Northwest. USDA For. Serv., Pac. NW Res. Stn. and US Dept. Energy, Woody Crops Program, Interagency Agreement DE-AI05-810R20914, Annu. Tech. Rep., 60 p.
DeBell, D.S. and P.A. Giordano. 1994. Growth patterns of red alder. In The Biology and Management of Red Alder. Eds. D.E. Hibbs, D.S. DeBell and R.F. Tarrant. Oregon State Univ. Press, Corvallis, OR, pp 116--130.
Furlow, J.J. 1979. The systematics of the American species of Alnus (Betulaceae). Rhodora 81:1--21.
Harrington, C.A., J.C. Zasada and E.A. Allen. 1994. Biology of red alder (Alnus rubra Bong.). In The Biology and Management of Red Alder. Eds. D.E. Hibbs, D.S. DeBell and R.F. Tarrant. Oregon State Univ. Press, Corvallis, OR, pp 3--22.
Hibbs, D.E. and A.A. Ager. 1989. Red alder: guidelines for seed collection, handling, and storage. For. Res. Lab Spec. Pub. 18, Col. For., Oregon State Univ. Press, Corvallis, OR, 6 p.
Hibbs, D.E., D.S. DeBell and R.F. Tarrant. 1994. Biology and manage-ment of red alder. Oregon State Univ. Press, Corvallis, OR, 256 p. LaBastide, J.G.A. and C.L.H. van Vredenburch. 1970. The influence
of weather conditions on the seed production of some forest trees in the Netherlands. Mededeling, Stichting Bosbouwproefstation ‘De Dorschkamp,’ Wageningen, No. 102, 12 p.
Longman, K.A. 1984. Physiological studies in birch. Proc. Roy. Soc. Edinburgh 85B:97--113.
Longman, K.A. 1985. Variability in flower initiation in forest trees. In Attributes of Trees as Crop Plants. Eds. M.G.R. Cannell and J.E. Jackson. Inst. Terrestrial Ecol., Nat. Environ. Res. Council, Hun-tingdon, U.K., pp 398--408.
Matthews, J.D. 1963. Factors affecting the production of seed by forest trees. For. Abstr. 24:1--13.
McVean, D.N. 1955. Ecology of Alnus glutinosa (L.) Gaertn. I. Fruit formation. J. Ecol. 44:219--222.
O’Reilly, C. and J.N. Owens. 1988. Reproductive growth and devel-opment in seven provenances of lodgepole pine. Can. J. For. Res. 18:43--53.
Owens, J.N. 1991. Flowering and seed set. In Physiology of Trees. Ed. A.S. Raghavendra. John Wiley and Sons Inc., New York, pp 247--271.
Radwan, M.S. and D.S. DeBell. 1994. Fertilization and nutrition of red alder. In The Biology and Management of Red Alder. Eds. D.E. Hibbs, D.S. DeBell and R.F. Tarrant. Oregon State Univ. Press, Corvallis, OR, pp 216--228.
Ross, S.D. and R.P. Pharis. 1985. Promotion of flowering in tree corps: different mechanisms and techniques, with special reference to conifers. In Attributes of Trees as Crop Plants. Eds. M.G.R. Cannell and J.E. Jackson. Inst. Terrestrial Ecol., Nat. Environ. Res. Council, Huntingdon, U.K., pp 383--397.
Sedgley, M. and A.R. Griffin. 1989. Sexual reproduction of tree crops. Academic Press, London, 378 p.
Snedecor, G.W. and W.G. Cochran. 1980. Statistical methods, 7th Edn. The Iowa State University Press, Ames, Iowa, pp 325--329. Stettler, R.F. 1978. Biological aspects of red alder pertinent to
poten-tial breeding programs. In Utilization and Management of Alder. Eds. D.G. Briggs, D.S. DeBell and W.A. Atkinson. USDA For. Serv., Pac. NW For. and Range Exp. Stn., Portland, OR, Gen. Tech. Rep. PNW-70, pp 209--222.
Swartz, D. 1971. Collegiate dictionary of botany. Ronald Press Co., New York, 520 p.