www.elsevier.com / locate / livprodsci
Immunohistochemical examination of myogenesis and
expression pattern of myogenic regulatory proteins
(myogenin and myf-3) in pigs
a ,
*
b b cM. Christensen
, N. Oksbjerg , P. Henckel , P.F. Jørgensen
a
The Royal Veterinary and Agricultural University, Department of Dairy and Food Science, Rolighedsvej 30, DK-1958 Frederiksberg C, Denmark
b
Danish Institute of Agricultural Sciences, P.O. Box 50, DK-8830 Tjele, Denmark
c
The Royal Veterinary and Agricultural University, Department of Anatomy and Physiology, Groennegaardsvej 7, DK-1870 Frederiksberg C, Denmark
Abstract
An experiment was conducted to immunohistochemically investigate myogenesis and the expression pattern of two myogenic regulatory proteins, myf-3 and myogenin, during formation of secondary fibres in pigs. Muscle fibres develop as two distinct populations in the foetal pig. Our data in foetal pigs suggest that formation of secondary fibres occurs from about day 60 to 80 of gestation and that myogenesis is concluded at approximately day 80 of gestation. The frequency of nuclei expressing myf-3 did not change, while that of nuclei expressing myogenin decreased from day 60 to 100 of gestation. Thus, myf-3 and myogenin appear to exhibit different expression patterns during myogenesis. The significance of decline in the frequency of nuclei expressing myogenin during secondary muscle fibre formation is not clear. During postnatal muscle growth no clear relationships between the expression pattern of myf-3 and myogenin with different fibre types were established. 2000 Elsevier Science B.V. All rights reserved.
Keywords: Myogenesis; Myogenin; Myf-3; Pig; Muscle growth
1. Introduction per unit of cross-sectional area is of a higher quality than meat with a small number of fibres. According Muscle mass is determined by muscle fibre num- to Dwyer et al. (1993), a high muscle fibre number ber and muscle fibre size. Animals with more muscle was correlated with faster and more efficient growth. fibres are known to produce more lean meat at In the pig, the number of muscle fibres is fixed from slaughter (Staun, 1963). Furthermore, Staun (1963) the time of birth (Staun, 1963). Understanding the found that meat with a large number of muscle fibres prenatal determination and regulation of muscle fibre number (myogenesis) will therefore elucidate how postnatal growth potential can be influenced.
*Corresponding author. Tel.:145-3528-3202; fax: 1
45-3528-Myogenesis is a function of myoblast
determi-3341.
E-mail address: [email protected] (M. Christensen). nation, proliferation and differentiation. Muscle
cific genes of the MyoD family encode proteins to establish the postnatal relationships between myf-which are thought to initiate myogenesis and regulate 3 and myogenin with different fibre types.
the transcription of muscle-specific genes (Buckin-gham, 1992). The rodent MyoD family consists of
four members, myoD (Davis et al., 1987), myogenin 2. Materials and methods (Wright et al., 1989), myf-5 (Braun et al., 1989) and
mrf-4 (Braun et al., 1990). Homologue genes have A total of six pregnant gilts (Landrace3Yorkshire been found in humans and are called myf-3, myf-4, crossbreed) were stunned with CO and exsanguin-2
myf-5 and myf-6, respectively (Braun et al., 1989). ated. Four foetuses (two males and two females) In mice, the gene products myf-5 and myoD are were removed, immediately after slaughter, from two thought to play an important role at the level of gilts at each days of 60, 80 and 100 of gestation, myoblast determination and proliferation, whereas respectively. Days of gestation were considered as myogenin and mrf-4 intervene at the level of myob- the number of days between the day of mating and last differentiation (Buckingham, 1994). the day of slaughter. The animals were only served According to Swatland and Casses (1973), two once. The study comprised a total of 24 foetuses. populations of myofibres are formed in the foetal Musculus longissimus dorsi (LD) and M. trapezius pig. These myofibres are called primary and sec- (TRP) were excised from all foetuses. Furthermore, ondary fibres. The first type of foetal myofibres to be LD from eight female pigs (Landrace3Yorkshire found in appreciable numbers is the primary fibres crossbreed) were excised immediately after slaugh-which tend to lie centrally within the fasciculi of ter, one at each days of 11, 30, 65, 84, 94, 106, 140 muscles in older foetuses. Secondary fibres are and 201 of age.
separated into primary or secondary fibres and the The relative distribution of fibre types, fibre mean postnatal muscle fibres into ST or FT fibres. cross-sectional areas and the frequency of nuclei Immunohistochemical staining for the myf-3 / expressing myf-3 and myogenin on a total of 200 myoD protein included fixation of the cryostat fibres and 300 nuclei (sum of myonuclei and satellite sections with Zambonis fixative (NovoCastra, UK) cell nuclei), were calculated using a computerised for 10 min at room temperature. Non-specific bind- image analysis system (TEMA, Scan Beam A / S) ing sites were inhibited by incubating with normal (Henckel et al., 1998).
rabbit serum (Dako) for 10 min at room temperature. Prenatal data were statistically analysed using the The sections were incubated with a purified mouse mixed procedure in SAS (SAS, 1988). The model anti-myf-3 monoclonal antibody (dilution 1:80) included the fixed effects of muscle, sex, age and (NovoCastra) overnight at 48C. In this study, the their appropriate interactions. Sow was included as a protein which the myf-3 antibody labels is called random effect. Postnatal data were analysed by myf-3, the human homologue to rodent myoD. The regression analyses using the General Linear Model biotinylated rabbit anti-mouse IgG secondary anti- procedure in SAS.
body was added for 45 min at 378C. HRP-labelled streptavidin was added for 15 min at 378C followed
by incubation with DAB for 5 min at room tempera- 3. Results ture. In between each step, the sections were rinsed
with TBS. No statistically significant sex by muscle by age When staining for the myogenin protein, acetone interactions were found for any of the traits mea-fixation was used. The sections were then incubated sured on foetuses.
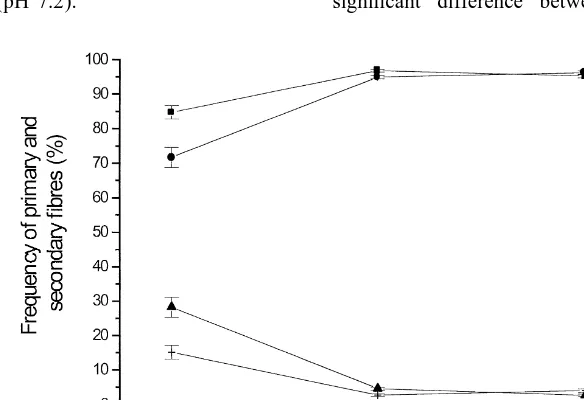
with purified mouse anti-myogenin monoclonal anti- From day 60 to 80 of gestation, the frequency of body (diluted 1:100) (Pharmingen, USA) for 1 h at primary fibres decreased (P,0.001) and the fre-room temperature. Subsequent steps were identical to quency of secondary fibres increased (P,0.001) the staining procedure for myf-3. In between each simultaneously in LD and TRP, with no further step the sections were rinsed with phosphate-buf- changes until day 100 of gestation (Fig. 1). No fered saline (PBS) (pH 7.2). significant difference between male and female
foetuses were found. From day 11 to 201 after birth, of nuclei expressing myogenin remained constant the frequency of ST and FT fibres did not change (Fig. 3b).
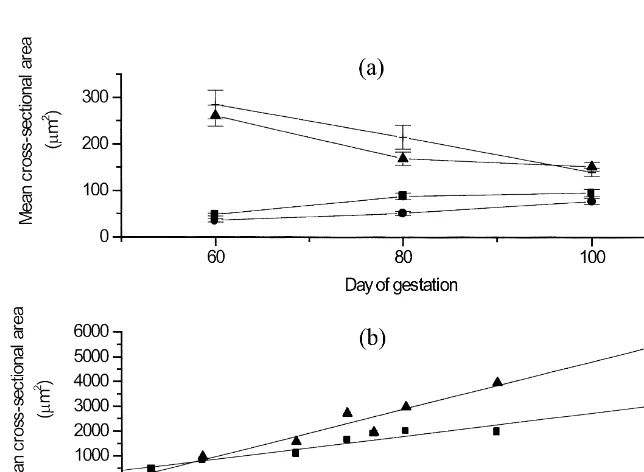
(P.0.05) (data not shown). A greater (P,0.001) expression of myf-3 in ST From day 60 to 100 of gestation, the mean cross- fibres than in FT fibres was present (Fig. 4), whereas sectional area of primary fibres tended to decrease no relationships between the number of nuclei (P,0.06), whereas the mean cross-sectional area of expressing myogenin and different muscle fibre types secondary fibres did increase (P,0.05) (Fig. 2a). were found in this study (data not shown).
No significant differences between male and female foetuses were found. After birth, the mean
cross-2
sectional area of ST ( y513.2 mm / day1412.1 4. Discussion
2 2 2
mm ) and FT ( y527.2mm / day121.2mm ) fibres
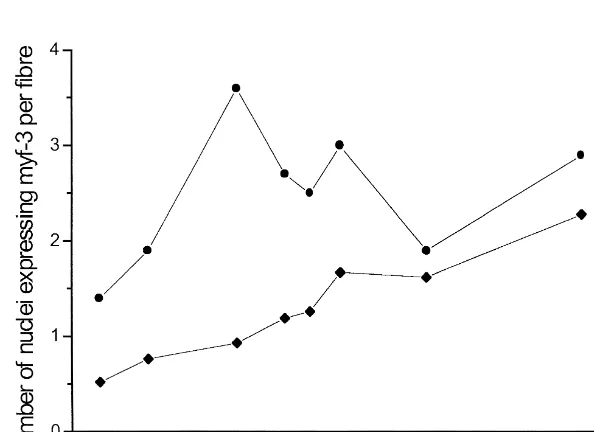
both increased (P,0.001) (Fig. 2b). According to From day 60 to 80 of gestation, the frequency of the statistical test, 95.3% and 96.7% of the increase primary fibres decreased in LD and TRP, while the in the mean cross-sectional area of ST and FT fibres, frequency of secondary fibres increased. The fre-respectively, could be explained by age. quency of primary fibres did not change markedly As shown in Fig. 3a, the frequency of nuclei after day 80 of gestation; therefore, it may be expressing myf-3 did not change (P.0.05), while hypothesised that muscle fibre formation had that of nuclei expressing myogenin decreased (P, stopped. By day 100 of gestation, LD comprised 0.05) from day 60 to 100 of gestation. No significant approximately 3% primary and 97% secondary fibres effects of sex and muscle were found. From day 11 and TRP approximately 4% primary and 96% sec-to 106 of age, the frequency of nuclei expressing ondary fibres. An attempt was made to determine the myf-3 increased (P,0.05), whereas the frequency expression of embryonic, developmental and foetal
Fig. 3. The frequency of nuclei expressing myf-3 (j) and myogenin (d) in relation to (a) age of gestation (n52) and (b) age after birth (n51). The values are given as means6S.E.
Braun, T., Bober, E., Buschhausen-Denker, G., Kotz, S.,
Grzes-found predominantly in muscles exhibiting a FT
chik, K.-H., Arnold, H., 1989. Differential expression of
phenotype.
myogenic determination genes in muscle cells: possible
au-The method used to determine the expression toactivation by the Myf gene products. EMBO J. 8, 3617– pattern of the two myogenic regulatory proteins, 3625.
myf-3 and myogenin, is semi-quantitative. Therefore, Braun, T., Bober, E., Winter, B., Rosenthal, N., Arnold, H.H., 1990. Myf-6, a new member of the human gene family of
it is impossible to predict how the actual amounts of
myogenic determination factors: evidence for a gene cluster on
the regulatory proteins change in respect to prenatal
chromosome 12. EMBO J. 9, 821–831.
muscle fibre development and postnatal muscle Brooke, M.H., Kaiser, K.K., 1970. Muscle fiber types: how many growth. It would therefore be interesting in future and what kind? Arch. Neurol. 23, 369–379.
studies to examine the changes in the myogenic Buckingham, M., 1992. Making muscle in mammals. Trends in Genet. 8, 144–148.
regulatory proteins by western blotting techniques
Buckingham, M., 1994. Which myogenic factors make muscle?
which would allow for a quantification of the two
Curr. Biol. 4, 61–63.
proteins. Davis, R.L., Weintraub, H., Lassar, A.B., 1987. Expression of a
single transfected cDNA converts fibroblasts to myoblasts. Cell 51, 987–1000.
Dwyer, C.M., Fletcher, J.M., Stickland, N.C., 1993. Muscle
5. Conclusion
cellularity and postnatal growth in the pig. J. Anim. Sci. 71, 3339–3343.
The present study revealed that myogenesis is Garlic, P.J., Maltin, C.A., Baillie, A.G.S., Delday, M.I., Gruble, concluded at approximately day 80 of gestation in D.A., 1989. Fiber-type composition of nine rat muscles II.
pig LD and TRP. The myogenic regulatory proteins, Relationship to protein turnover. Am. J. Physiol. 257, 828– 832.
myf-3 and myogenin, appear to exhibit different
Henckel, P., Ducro, B., Oksbjerg, N., Hassing, L., 1998.
Objectivi-expression patterns during myogenesis and postnatal
ty of two methods of differentiating fibre types and
repeatabili-muscle growth. The significance of decline in the ty of measurements by application of the TEMA image frequency of nuclei expressing myogenin during analysis system. Eur. J. Histochem. 42, 49–62.
secondary muscle fibre formation is not clear. Final- Petersen, J.S., Henckel, P., Oksbjerg, N., Sørensen, M.T., 1998. Adaptation in muscle fibre characteristics induced by physical
ly, it was not possible to establish any clear
relation-activity in pigs. Anim. Sci. 66, 733–740.
ships between the expression pattern of myf-3 and
SAS, 1988. SAS / STAT User’s Guide, Release 6.03 Edition, SAS
myogenin gene products with fibre types during Institute, Cary, NC.
postnatal muscle growth. Swatland, H.J., Casses, R.G., 1973. Prenatal development, his-tochemistry and innervation of porcine muscle. J. Anim. Sci. 36, 343–354.
Staun, H., 1963. Various factors affecting number and size of
Acknowledgements
muscle fibers in the pig. Acta Agric. Scand. 3, 293–322. Suzuki, A., Cassens, R.G., 1980. A histochemical study of
The authors thank Dr. J.S. Petersen and secretary myofiber types in muscle of the growing pig. J. Anim. Sci. 51,
A. Sørensen for fruitful discussions and secretarial 1449–1461.
Voytik, S.L., Przyborski, M., Badylak, S.F., Konieczny, S.F.,
assistance during the preparation of this paper.
1993. Differential expression of muscle regulatory factor genes in normal and denervated adult rat hindlimb muscles. Dev. Dyn. 198, 214–224.
References Wigmore, P.M.C., Stickland, N.C., 1983. Muscle development in large and small pig fetuses. J. Anat. 137, 235–245.
Wright, W.E., Sassoon, D.A., Lin, V.K., 1989. Myogenin, a factor Ashmore, C.R., Tompkins, G., Doerr, L., 1972. Postnatal
develop-regulating myogenesis, has a domain homologous to MyoD. ment of muscle fibre types in domestic animals. J. Anim. Sci.