Introduction
Oxidative Stress-Associated Carbonylation in Breast Cancer
Introduction
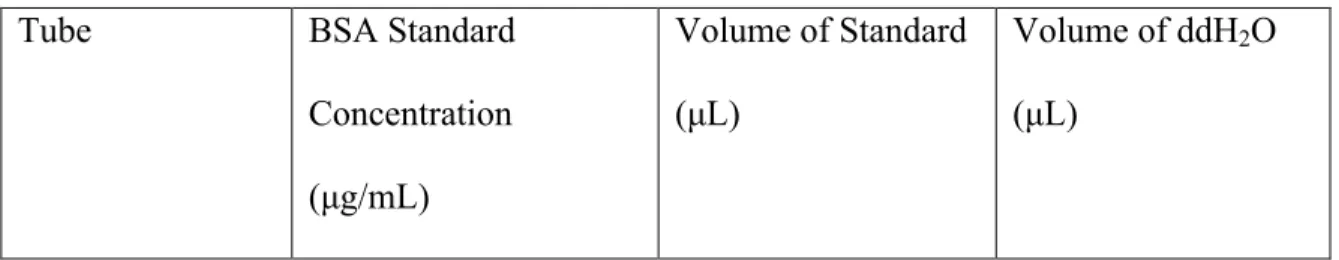
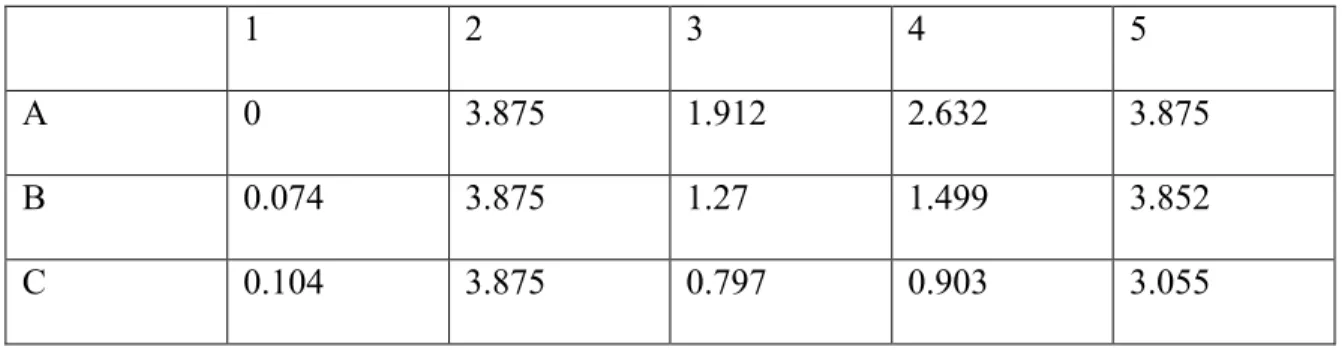
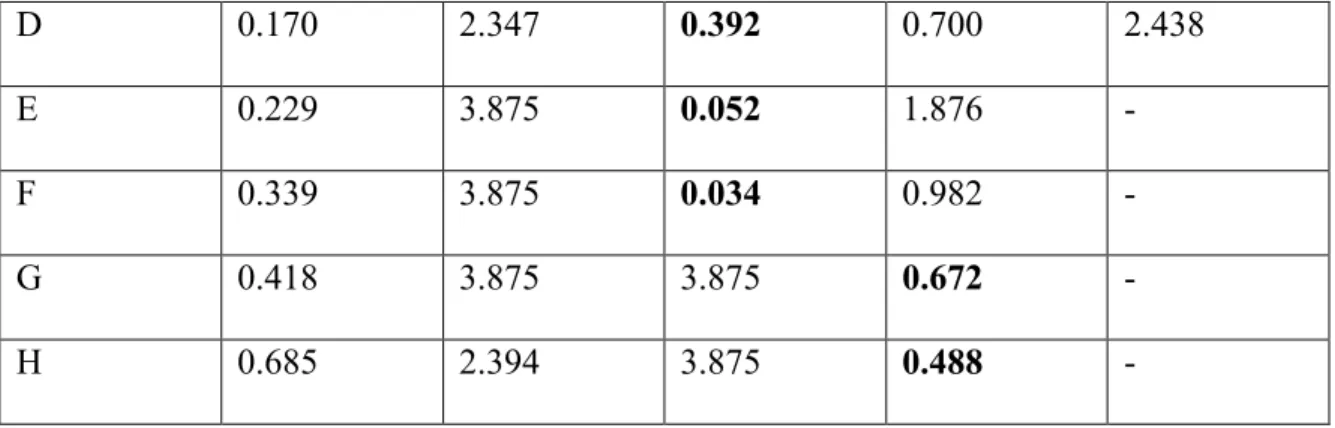
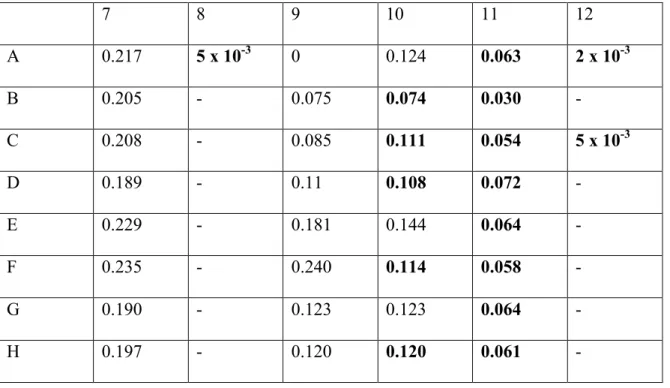
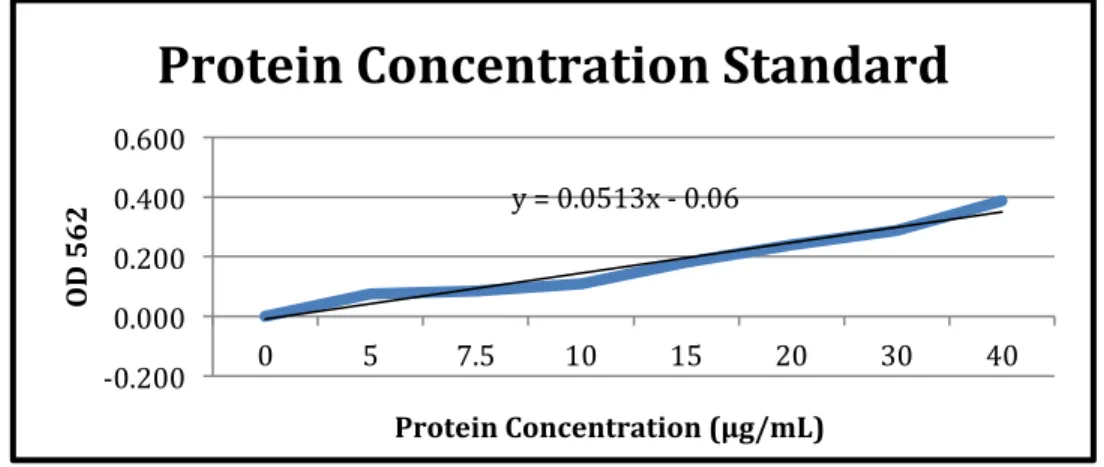
The observed concentration readings after normalization for background interference (620 nm) can be seen in the results section of Table 5. The graph and associated trend line can also be seen in the results section of Figure 1. For the second experiment of BCA protein assay, BCA Standards and their dilutions were performed in the same method as shown in Table 2, and the working solution for this round was again made using the three primary solutions provided.
As before, the concentration readings observed after normalization for background interference can be seen in the results section of Table 6. The graph and associated trend line can also be seen in the results section of Figure 2. The readings observed in the table are the concentration ( µg/ mL) of protein samples and the purpose of obtaining these concentrations was so that the amount of protein in each sample could be normalized for the oxyblot experiment.
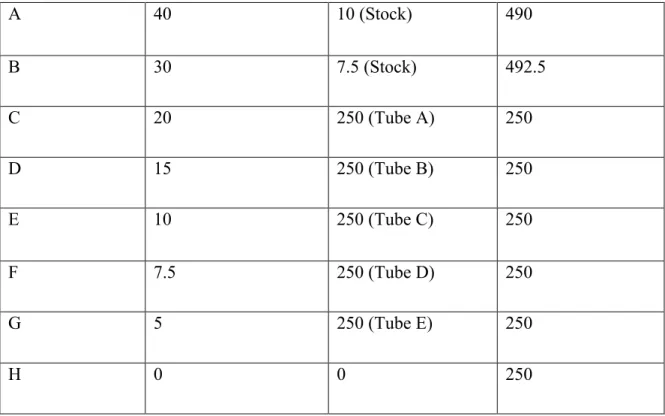
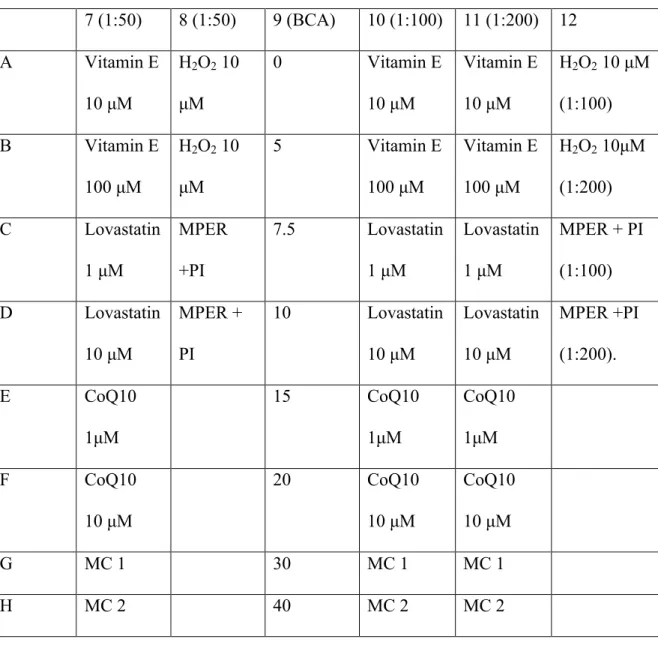
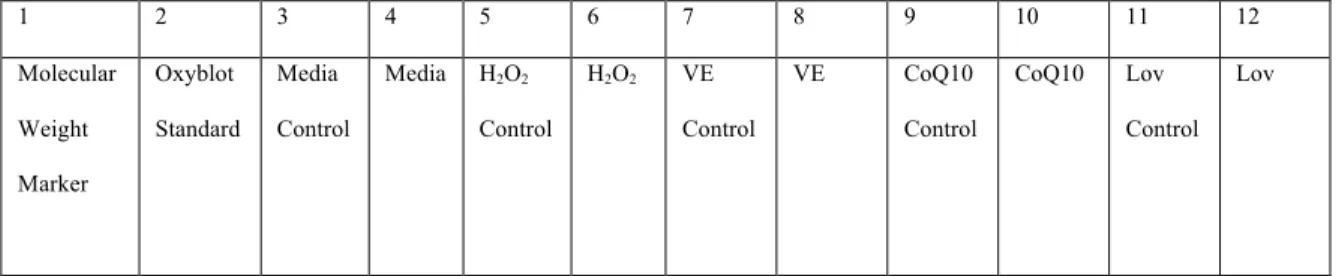
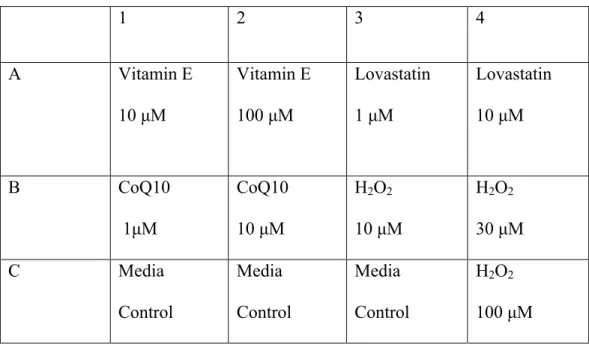
Because the number of lanes in the SDS-PAGE gel limited us to twelve, the highest concentration of each sample that decreased. By using the highest available concentration of each sample, the effects of the compounds could be better observed in the oxyblot experiment. Two rounds of oxyblotting were performed and the first step in the process is the derivatization reaction.
Once all the samples and standards were prepared, they were ready to be loaded onto the gel. The membrane was incubated in the solution for approximately one hour at room temperature with gentle shaking. For the second round of oxyblot, the protocol was performed in the same manner as previously described, but different chemicals were used.
Although hydrogen peroxide is known to cause an increase in oxidative stress levels, no protein carbonylation was present in the oxyblot. Protein carbonylation levels in the media control sample were expected and used as a baseline of normal cancer cell levels of oxidative stress within cancer cells. For CoQ10 and lovastatin, the samples did not experience a significant increase or decrease in the level of protein carbonylation compared to the media control.
In light of the literature on the efficacy of vitamin E as an antioxidant, it was interesting to note that cells treated with vitamin E expressed higher levels of protein carbonylation than were present in the medium control. Therefore, the pro-oxidative properties induced by vitamin E could lead to an increase in oxidative species in the cell, resulting in the increased level of protein carbonylation observed in Figure 4. V.) Conclusion.
Materials/Methods
Results
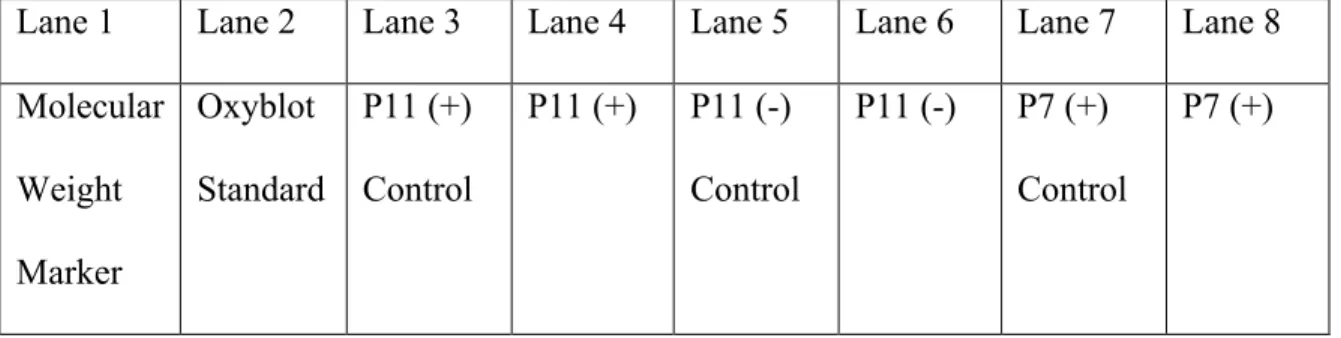
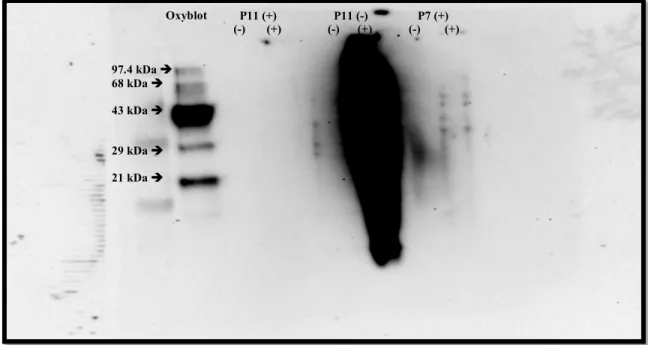
The results consist of Figure 3 and Figure 4, which show the gels obtained from the oxyblot experiments performed. The primary purpose of this experiment was to perfect the oxyblot protocol in addition to observing the effects of adding β-mercaptoethanol to the samples. It was observed that samples containing β-mercaptoethanol did not show results for protein carbonylation, but the opposite was observed for the sample without β-mercaptoethanol, where it blurred into the surrounding lanes.
The lanes labeled (-) were the negative control samples, and the (+) labeled lanes are the samples that should show protein carbonylation. Observation of Figure 4 shows low levels of protein carbonylation at 29 kDa and 63 kDa molecular weight proteins in the media, CoQ10 and lovastatin samples, but nothing particularly significant. This is most likely because the cells have been severely degraded by the compound treatment, resulting in most of them dying.
However, the vitamin E sample in Figure 4 shows high levels of protein carbonylation on these proteins, indicative of increased oxidative stress in the cells.
Discussion
The most intriguing finding from the experiments presented is the level of vitamin E-induced protein carbonylation in breast cancer cells. Vitamin E has been used as an antioxidant for several experiments, and as a result, there is literature evaluating its effectiveness as an antioxidant. In some cases, vitamin E has been shown to reduce oxidative stress within cells, thereby reducing the level of protein carbonylation.
When vitamin E is present with lipids in the cell membrane, it works to inhibit cancer formation by neutralizing ROS. A direct relationship has also been established between vitamin E deficiencies and lipid peroxide production (Nathan et al. 2011). In addition, vitamin E and epicatechin treatment appeared to ameliorate the effects of nicotine-induced toxic oxidative stress by scavenging free radicals and enhancing the effects of antioxidant enzymes (Al-Malki and Moselhy 2013).
1994 also suggests the involvement of tocopherols, such as vitamin E, in the prevention of oxidative damage by circulating proteins. Although there is no definitive answer to this, one possible reason is that vitamin E, in high doses, can exhibit prooxidative properties (Kodentsova et al. 2013). Therefore, the prooxidative properties induced by vitamin E may lead to the increase of oxidative species within the cell, which will lead to the increased level of protein carbonylation observed in Figure 4.
Conclusion
Although the results from these experiments are not conclusive, they provide another perspective on the compounds used and their ability to reduce oxidative stress and lower protein carbonylation levels. 34; Protective effect of vitamin E and epicatechin against nicotine-induced oxidative stress in rats." Toxicology and Industrial Health, vol. Oxidative stress: its effects on the growth, metastatic potential, and response to treatment of breast cancer." Breast cancer research: BCR vol.
34;Relationships between the levels of protein carbonyls, retinol and tocopherols in human plasma." Biochemistry and Molecular Biology International, vol. 34;Lipid droplets induced by secreted phospholipase A2 and unsaturated fatty acids protect breast cancer cells from nutritional and lipotoxic stress.". 34; The effect of lovastatin on oxidative stress and antioxidant enzymes in hydrogen peroxide-intoxicated rats." Food and Chemical Toxicology, vol.
34;Coenzyme Q10 supplementation reduces oxidative stress and increases antioxidant enzyme activity in patients with coronary artery disease." The role of biomarkers of oxidative stress in breast cancer risk and prognosis: a systematic review of the epidemiologic literature." Journal of Women's Health (2002) vol. Breast cancer: conventional diagnosis and treatment modalities and recent patents and technologies. Breast Cancer: Basic and Clinical Research vol.