SOURCE: CAC/RCP 1-1969, amended — The definition has been amended and note 1 to the text has been added.]. SOURCE: CAC/RCP 1-1969, amended — The phrase “or condition of” has been deleted from the definition and notes on the term have been added.]. SOURCE: CODEX STAN 1, amended — The reference to “and/or processed and/or packaged” is included in the definition and comments have been added to the term.].
Understanding the organization and its context
Understanding the needs and expectations of interested parties
Determining the scope of the food safety management system
Food safety management system
Leadership and commitment
Policy
Establishing the food safety policy
Communicating the food safety policy
Organizational roles, responsibilities and authorities
Actions to address risks and opportunities
Objectives of the food safety management system and planning to achieve them
Planning of changes
Resources
- General
- People
- Infrastructure
- Work environment
- Externally developed elements of the food safety management system
- Control of externally provided processes, products or services
When an organization establishes, maintains, updates and continuously improves its FSMS using externally developed elements of an FSMS, including prerequisite programs, the hazard analysis and the hazard management plan (see 8.5.4), the organization shall ensure that the elements provided: .. a) developed in accordance with the requirements of this document;.
Competence
Awareness
Communication
General
External communication
Internal communication
The food safety team will ensure that this information is included when the FSMS is updated (see 4.4 and 10.3). Top management will ensure that relevant information is included as input to the management review (see 9.3).
Documented information
General
Creating and updating
Top management ensures that relevant information is included as input for management review (see 9.3). c) examination and approval of suitability and appropriateness.
Control of documented information
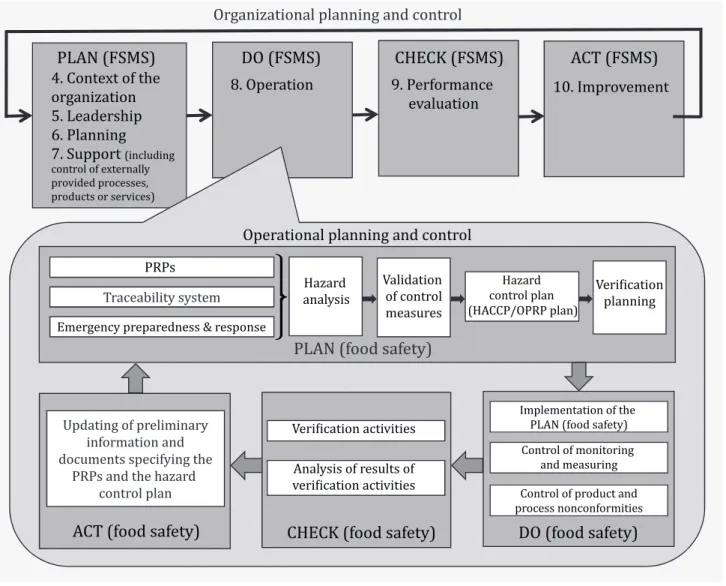
Operational planning and control
Prerequisite programmes (PRPs)
Traceability system
Documented information as evidence of the traceability system must be retained for a specified period, indicating at least the shelf life of the product. NOTE Where applicable, verification of the system is expected to include matching of finished product quantities to ingredient quantities as evidence of effectiveness.
Emergency preparedness and response
General
Handling of emergencies and incidents
Hazard control
- Preliminary steps to enable hazard analysis
- Hazard analysis
- Validation of control measure(s) and combinations of control measures
- Hazard control plan (HACCP/OPRP plan)
Groups of consumers/users known to be particularly vulnerable to specific food safety hazards must be identified. Flow charts must be clear, accurate and sufficiently detailed to the extent necessary to carry out the hazard analysis. The food safety team will confirm the accuracy of the flowcharts on site, update the flowcharts where appropriate and retain as documented information.
The degree of control should ensure food safety and, where appropriate, a combination of control measures should be used. The Codex Alimentarius Commission defines FSOs as "the maximum frequency and/or concentration of a hazard in a food at the time of consumption that provides or contributes to an adequate level of protection (ALOP)". 8.5.2.2.3 The organization shall determine the acceptable level in the final product of any identified food safety hazards, whenever possible.
Provided by IHS Markit under license from ANSI Licensee=Hong Kong Polytechnic University User=Siu, Kit Hang .. b) the severity of adverse health effects in relation to the intended use (see 8.5.1.4). The methodology used should be described and the result of the hazard assessment kept as documented information. 8.5.2.4.1 Based on the hazard assessment, the organization shall select an appropriate control measure or combination of controls capable of preventing or reducing the identified significant food safety hazards to defined acceptable levels.
The decision-making process and the results of the selection and categorization of the control measures must be kept as documented information. The food safety team must validate that the selected control measures are capable of achieving the intended control of the significant food safety hazard(s). The food safety team must maintain the validation methodology and evidence of the ability of the control measure(s) to achieve the intended control as documented information.
A monitoring system is set up at each CCP for each control measure or combination of control measure(s) to detect any shortcomings within the critical limits. For each OPRP, a monitoring system must be established for the control measure or combination of control measure(s) to detect failure to meet the action criterion. The organization must implement and maintain the hazard management plan and retain evidence of the implementation as documented information.
Updating the information specifying the PRPs and the hazard control plan
At each CCP, the monitoring method and frequency shall be capable of timely detection of any failure to remain within critical limits, to enable timely isolation and evaluation of the product (see 8.9.4). For each OPRP, the monitoring method and frequency will be proportional to the probability of failure and the severity of the consequences. When the monitoring of an OPRP is based on subjective data from observations (eg visual inspection), the method must be supported by instructions or specifications.
Control of monitoring and measuring
Calibration of all equipment must be traceable to international or national measurement standards; where no standards exist, the basis used for calibration or verification shall be kept as documented information. The organization shall evaluate the validity of previous measurement results when the equipment or process environment is found to be non-compliant. The organization must take appropriate action regarding the equipment or process environment and any products affected by the nonconformity.
Software used in monitoring and measuring within the FSMS must be validated by the organization, software vendor or third party prior to use. Documented information on validation activities must be maintained by the organization and the software must be updated in a timely manner. When changes occur, including software configuration/modifications to commercial off-the-shelf software, they must be authorized, documented and validated before implementation.
NOTE: Commercial standard software in general use within the intended scope of use may be considered sufficiently validated.
Verification related to PRPs and the hazard control plan
Verification
Analysis of results of verification activities
Control of product and process nonconformities
- General
- Corrections
- Corrective actions
- Handling of potentially unsafe products
- Withdrawal/recall
The organization will keep products that have been identified as potentially unsafe under its control until the products have been evaluated and disposition determined. If products that have left the organization's control are subsequently determined to be unsafe, the organization must notify the relevant stakeholders and initiate a recall/withdrawal (see 8.9.5). Controls and related responses from relevant stakeholders and authorization to handle potentially unsafe products will be kept as documented information.
Products affected by failure to remain within critical limits at CCPs shall not be released but shall be handled in accordance with 8.9.4.3. Products not meeting the action criterion for OPRPs shall only be released as safe if one of the following conditions applies: .. (a) evidence other than the monitoring system demonstrates that the control measures have been effective; Documented information regarding the disposal of non-conforming products, including identification of the person(s) with approval authority, must be retained. The organization should be able to ensure the timely withdrawal/recall of many end products identified as potentially unsafe by designating competent person(s) with the authority to initiate and carry out the withdrawal/recall.
Returned/recalled products and finished products remaining in stock shall be secured or kept under organizational control until managed in accordance with 8.9.4.3. The cause, extent and outcome of a withdrawal/recall will be retained as documented information and reported to top management as input to management review (see 9.3). The organization must verify the implementation and effectiveness of withdrawals/recalls using appropriate techniques (e.g., mock withdrawals/recalls or practice withdrawals/recalls) and maintain documented information.
Monitoring, measurement, analysis and evaluation
General
Analysis and evaluation
The analysis will be carried out: .. a) to confirm that the overall performance of the system complies with the planned arrangements and the FSMS requirements established by the organization;. The results of the analysis and the resulting activities will be retained as documented information. The results will be reported to top management and used as input for the management review (see 9.3) and the updating of the FSBS (see 10.3).
Internal audit
Management review
General
Management review input
Management review output
Nonconformity and corrective action
Continual improvement
Update of the food safety management system
Determine the corrective actions to be taken when monitoring indicates that a particular CCP is not under control. Provided by IHS Markit under license with ANSI Licensee=Hong Kong Polytechnic University User=Siu, Kit Hang. 5] ISO/TS 22003, Food safety management systems — Requirements for bodies providing audit and certification of food safety management systems.
6] ISO 22005, Traceability in the food and feed chain — General principles and basic requirements for system design and implementation. 8] CAC/GL 60-2006, Principles for traceability / Product traceability as a tool within the food inspection and certification system. Provided by IHS Markit under license with ANSI Licensee=Hong Kong Polytechnic University User=Siu, Kit Hang.