Mater. Res. Express6(2019)105703 https://doi.org/10.1088/2053-1591/ab3680
PAPER
Bright green fl uorescence of microwave irradiation-synthesized Cdots as sensitive probe of iron (III)
I W L Lewa1, H Sutanto1,2 , A Subagio1, I Marhaendrajaya1and H Sugito1
1 Department of Physics, Diponegoro University, Jl. Prof. Soedarto, SH, Semarang-50275, Indonesia
2 Central Laboratory of Research and Services Diponegoro University(CORES DU), Jl. Prof. Soedarto, SH, Semarang-50275, Indonesia E-mail:herisutanto@fisika.undip.ac.id
Keywords:Cdots, bio imaging, bright Greenfluorescence, microwave irradiation
Abstract
Carbon nanodots
(Cdots)are very attractive materials due to their
fluorescence and great potential invarious
fields. Onefield that still needs to be developed is heavy metal ion detector. In this study, asimple and cost efficient
fluorescent detector of Fe3+was built. This material was synthesized by microwave irradiation method using citric acid and urea with purified water as the solvent to get the optimal concentration of urea in order to produce bright green
fluorescence. Synthesized Cdots withoptimal concentration of urea emit bright green
fluorescence under UV light radiation. This brightgreen
fluorescence was then used to detect Fe3+. The
fluorescence of this solution was quenched withthe addition of Fe
3+due to transfer charges and exciton recombination. Optical characterization was carried out using UV–vis spectrophotometer, and
fluorescence spectrophotometer. Results showedtwo absorption peaks on Cdots; at 330 nm and 410 nm. Fluorescence analysis was performed using a beam of 532 nm produced emission at a wavelength of 590 nm. In addition, structural characterization was performed using FTIR, SEM and EDX.
Introduction
Carbon nanodots(Cdots)are zero-dimensional carbon material with a dimension of less than 10 nm. Cdots werefirst discovered by Xuet alin 2004 from the purification process of carbon nanotubes[1]. Cdots are spherical[2], non toxic[3]and have high water solubility[4]. Cdots can be used in several application areas such as biomedicine[5], LED[6], energy related applications[7,8], bioimaging[9], and sensors[10]. Cdots are very interesting material with theirfluorescence properties[11]and one of thefields that is being developed using this is sensors. Cdots are the right material choice to replace the commonly used probes. Sensor applications typically use quantum dots semiconductor materials such as CdS[12]. However, CdS is toxic and is not easily degraded in the environment. Cdots are an alternative substitute material that are effective because they are not toxic, compatible, and easily soluble in water[12].
Cdots can be synthesized using several methods that are divided into two categories of top down and bottom up. The top down method breaks large molecules into smaller ones. This method includes laser ablation[13], electrochemistry[14]and arc discharge[15]. Meanwhile, the bottom up method, which is also known as the chemical method, is the preparation of a material from small-sized materials. This method includes pyrolysis [16], hydrothermal[17]and microwave[18]. Microwave irradiation is one of the most widely used methods because it can reach certain heat in shortly, efficiently, fast, easily, and inexpensively. Improving thefluorescence properties of this material is an important aspect that should be considered. Various methods can be used such for this purpose, including surface passivation[19]. Passivation process uses organic materials containing an amine group to improvefluorescence. Fluorescence is caused by a surface energy trap that can be modified to get the properties that are suitable for certain applications. Surface modification method is appropriate for sensing applications[20]. Based on research by Sun and coworkers, the surface passivation process of non-luminescent Cdots derived from polyethylene glycol polymers(PEG)shows strong luminescence[20]. By using microwave
RECEIVED
31 May 2019
REVISED
7 July 2019
ACCEPTED FOR PUBLICATION
29 July 2019
PUBLISHED
7 August 2019
© 2019 IOP Publishing Ltd
irradiation, the passivation process can be carried out in one step, hence, microwave irradiation is the right method to synthesize Cdots[21].
Previous studies have proven that Cdots can be used to detect heavy metals with the turn on/turn off fluorescence mechanism. One study by Zhanget alsynthesizes Cdots from melamine and g-C3N4by heating them for 2 h to detect Pb2+ions[22]. Another study by Kumaret alsynthesizes Cdots using the hydrothermal method for 7 h to detect Hg2+ions[23]. In this research, Cdots synthesis using microwave irradiation was carried out to obtain a material that has strong luminescence and can be used as a detector of Fe3+heavy metal ions. The precursors used here were citric acid as a source of carbon and urea as passivation agents and distilled water as solvent. Variations of urea concentration were made in order tofind the right formula to produce strong luminescence. Sensitivity tests for Fe3+heavy metal ions were also carried out. Further characterization includes using UV–vis spectrophotometer to observe absorbance spectrum,fluorescent spectrometer to observe emission spectrum of Cdots and FTIR to observe functional groups contained in the synthesized material.
Material and methods
A synthesis procedure for Cdots can be seen infigure1. Cdots were made of citric acid and urea using microwave irradiation method. Citric acid was used as a source of carbon, while urea was used as passivation agent with its amine group content. Purified water was used as solvent in the reaction. Two grams of citric acid were mixed with urea with varied concentration of 1 to 7 grams. The solvent for each solution was 60 ml of purified water.
Once the solution is homogenized, it was then irradiated using a microwave with power 450 W for 30 min until a blackish brown crust is formed. The crust was then taken and mashed into powder. Afterwards, the Cdots powder was dissolved in purified water with a concentration 100 ppm. This solution was then characterized and analyzed to determine its properties.
Results and discussion
Cdots synthesis
Thefirst step is synthesizing Cdots using microwave irradiation. The materials used were citric acid as a source of carbon and urea as a surface passivation agent. Physical processes occurring during synthesis are divides into 4 stages of dehydration, polymerization, carbonization and passivation20. Dehydration reaction is the release of water content in the molecules that are reacting. The second step is polymerization. This is where reaction triggers spontaneous nucleation process that is followed by the formation of new longer bonds. The third step is carbonization in which inorganic carbon bonds are derived from citric acid21. Thefinal step is surface
passivation. This is where coating on the surface of Cdots by compounds of the amine functional groups
Figure 1.Sample preparationflowchart.
2
Mater. Res. Express6(2019)105703 I W L Lewaet al
contained in urea takes place. Cdots surface without coating is not only exposed to contaminants, but also powerless because carbon and oxygen are endogenous to be able to react with organic molecules, hence, it can eliminate opto-electronic properties of Cdots. Therefore, coating is very important because it can maintain the stability of Cdots’physical properties and strengthenfluorescence. Surface passivation forms a thin insulating capping layer that can protect Cdots and increase theirfluorescence. There are various types of polymers or organic molecules that can be used as passivation agents as long as they do not contain chromophores from visible light to UV and they do not emit the same wavelength, as this may affect the original luminescence of Cdots[24].
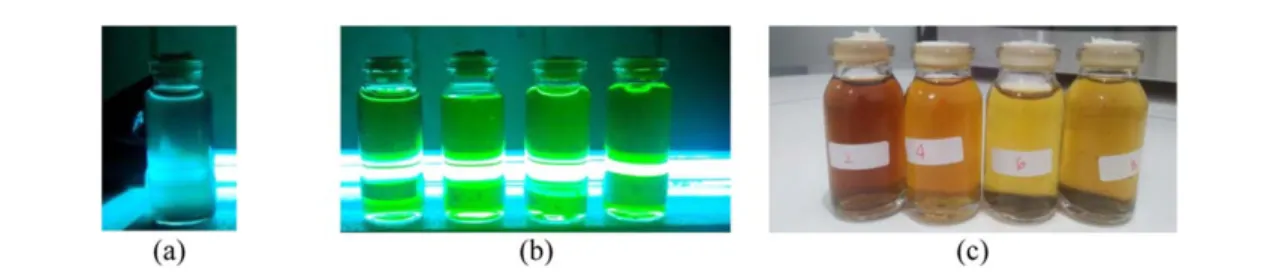
Results obtained in this synthesis process are black solids, which were then dissolved in purified water for further observation. Upon observation using UV radiation with a wavelength of 280–300 nm it appeared that the Cdots emit green luminescence, as shown infigure2below.
Thefigure shows that activated carbon(a)has no green luminescence, while Cdots produce green luminescence(b). This indicates change in optical properties when Cdots are changed into a quantum nano materials dimension.
Optical characterization
Optical characterization of Cdots was performed using UV-visible spectroscopy andfluorescence spectroscopy.
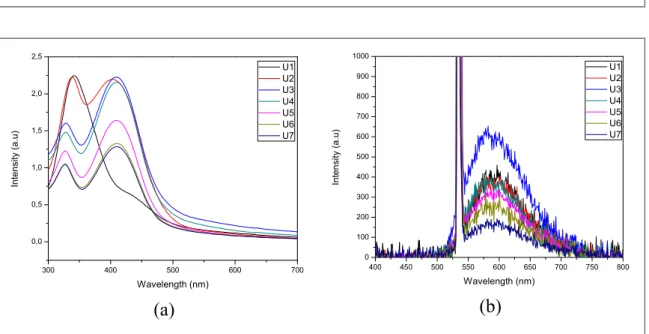
Absorption spectra of synthesized Cdots(figure3(a))showed two absorption peaks; at 330 nm and 410 nm.
These absorption peaks could be associated withπ→π*transition of C=C bond and n→π*transition of C=O bond, respectively. These are transitions of carbonyl and oxygen containing compounds. These results corresponds to several study before[25,26]. Figure3(a)shows the effect of adding urea that causes a decrease in absorption intensity from the sample. This is possible as the more nitrogen levels contained in urea, the less Cdots absorption is because of lower levels of citric acid and more predominant nitrogen levels. Furthermore, surface functional groups also play an important role in determining the absorption wavelength of Cdots.
Thefluorescence spectrum shows a peak at a wavelength of 590 nm. At a concentration of 1 gram, 2 g and 3 g of urea, there is an increase influorescence intensity. Fluorescence that occurs in Cdots is caused by the presence of surface passivation process by passivation agents. The passivation process results in surface energy trap that enables emission stability and hence, improvedfluorescence of Cdots. This is because of the quantum
Figure 2.(a)Activated carbon, and(b)Cdots illuminated by UV radiations, and(c)non-illuminated Cdots.
Figure 3.Optical characterization of Cdots(a)absorbance spectra, and(b)fluorescence spectra.
3
Mater. Res. Express6(2019)105703 I W L Lewaet al
confinement effect of the emission energy trap on particles, where the ratio between the surface and volume of particles affects passive particles[20].Fluorescence intensity of Cdots saturated at a variation of 4 g urea kept on decreasing until the concentration reaches 7 g of urea. The reason is that at a concentration of 4 g, the level of urea is more dominant than citric acid as a carbon source. So it is estimated that the number of carbon bonds decreases and this results in reducedfluorescence intensity. The effect of concentration is very important for the fluorescent properties of Cdots. Emissions from strong surface energy levels are caused by changes in
concentration[27].
Structural characterization
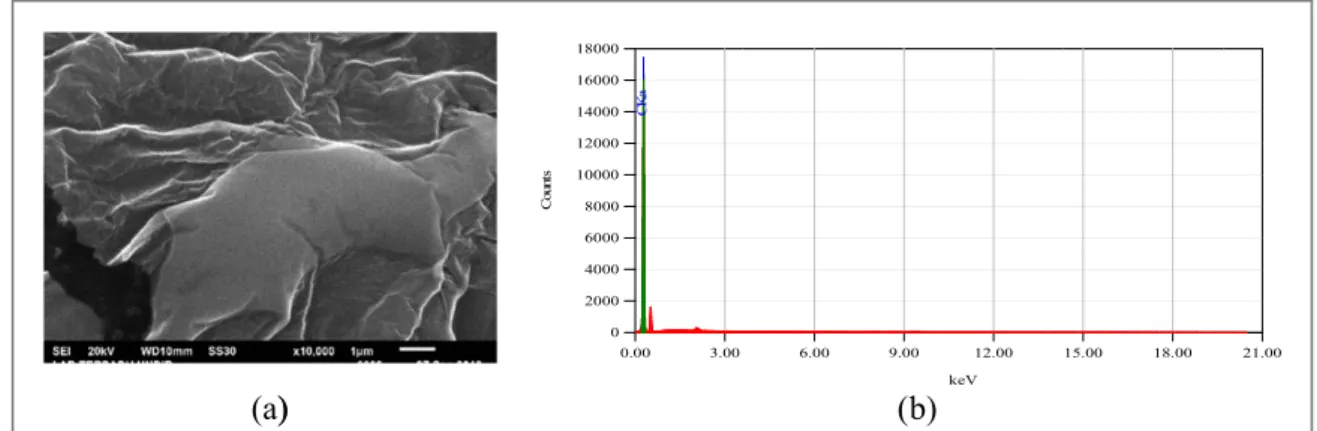
In addition to optical characterization, characterization using SEM, EDX and FTIR were also carried out. SEM is used to see the topography of this material. Fromfigure4(a)we can see that cdots are irregularly shaped with a rough surface. While previous research stated that cdots have spherical shape and uniform size[28]. This is because cdots tend to agglomerate and poses a problem for characterization. EDX used to analyze the elements that exist in the material. Fromfigure4(b)we can see that the material consists of 100% carbon element.
Meanwhile, FTIR was used to determine the functional group of synthesized material.
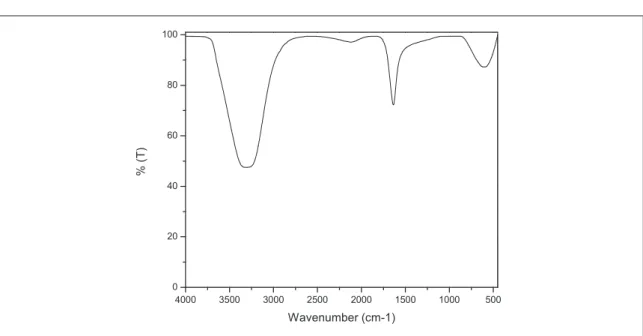
FTIR analysis is used to determine the functional groups contained in Cdots[29]. Fromfigure5we can see that cdots shows transmitance band at 3307 cm−1correspond to O–H bond , C≡C bond at 2111 cm−1and transmitance band 1635 cm−1correspond to C=O and C=C bonds . These results show the same results according to previous research explain that the functional group of Cdots contain O–H at wave numbers 3100–3400 cm−1and function group C=O at 1600–1770 cm−1[30]. Results of FTIR measurement show no significant differences with urea variation. Cdots will be more stable and hidrofility increases if there are O–H and N–H groups[31].
Figure 4.(a)SEM image from the sample produced at 10,000 magnification, and(b)EDX results showing 100% carbon atoms.
Figure 5.FTIR measurement from citric acid and urea.
4
Mater. Res. Express6(2019)105703 I W L Lewaet al
Fe3+ions detection
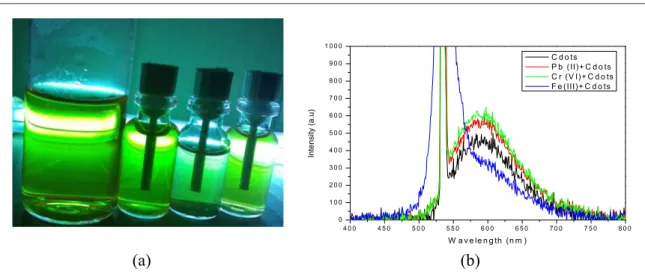
The Fe3+ions detection test was carried out tofind out changes influorescence properties of Cdots when some heavy metal solutions were added. Observations show that Cdotsfluorescence changes with the addition of heavy metals ions solution, as can be seen infigure6.
It can be seen infigure6(a)that when given a standard solution of Cr6+and Pb2+fluorescence of cdots remained constant. But it was different when Cdots were given a solution of Fe3+, as thefluorescence began to quench. This can be proven by observing PL spectrum shown infigure6(b). It is easy to see in the spectrum that when compared to other metal ions, Fe3+shows more quenched effect with decrease in the emission spectrum.
Therefore, we can conclude that Cdots synthesized from citric acid and urea are sensitive to Fe3+ion solutions.
Quenchedfluorescence of cdots are caused by several functional groups on the their surface. These include hydroxyl and carboxyl groups that are capable to selectively respond to Fe3+ions. The mechanism of this fluorescence turning off stems from charge transfer and controlled exciton recombination[26,32].
Conclusions
A synthesis of Cdots has been successfully carried out using the microwave irradiation method of citric acid and urea via microwave irradiation process. The synthesized Cdots show bright greenfluorescence and are sensitive to Fe3+ions. Therefore, it can be concluded that the addition of urea increasesfluorecence. Nonetheless, Cdots saturate at some point and have reduced the intensity. The most appropriate concentration of citric acid-urea that produce the strongestfluorescence is at a concentration 2:3. Cdotsfluorescence is quenched upon administration of a standard solution of Fe3+ion. This quenching mechanism is caused by the respons of carboxyl and hydroxyl functional groups with Fe3+ions that reside on the surface of Cdots.
Acknowledgments
The authors would like to thank Diponegoro University for funding this research in 2019.
ORCID iDs
H Sutanto https://orcid.org/0000-0003-3404-0337
References
[1]Xiaoyou Xuet al2004 Electrophoretic analysis and purification offluorescent single-walled carbon nanotube fragmentsJ. Am. Chem.
Soc.12612736–7
[2]De B and Karak N 2013 A green and facile approach for the synthesis of water solublefluorescent carbon dots from banana juiceRSC Adv.38286–90
[3]Bhamore J R, Jha S, Park T J and Kailasa S K 2019 Green synthesis of multi-color emissive carbon dots from Manilkara zapota fruits for bioimaging of bacterial and fungal cellsJ. Photochem. Photobiol. B Biol.191150–5
Figure 6.(a)Sensitivity test of Cdots against heavy metalsfluorescence spectrum of Fe3+under UV Lamp(b)PL Spectrum of cdots and cdots with several heavy metal ions.
5
Mater. Res. Express6(2019)105703 I W L Lewaet al
[4]Zheng L, Chi Y, Dong Y, Lin J and Wang B 2009 Electrochemiluminescence of water-soluble carbon nanocrystals released electrochemically from graphiteJ. Am. Chem. Soc.1314564–5
[5]Jaleel J A and Pramod K 2018 Artful and multifaceted applications of carbon dot in biomedicineJ. Control. Release269302–21 [6]Cui Bet al2017 The use of carbon quantum dots asfluorescent materials in white LEDsXinxing Tan Cailiao/New Carbon Mater.32
385–401
[7]Genc Ret al2017 High-Capacitance hybrid supercapacitor based on multi-coloredfluorescent carbon-dotsSci. Rep.71–13 [8]Li L, Wu G, Yang G, Peng J, Zhao J and Zhu J J 2013 Focusing on luminescent graphene quantum dots: current status and future
perspectivesNanoscale54015–39
[9]Zhang J and Yu S H 2016 Carbon dots: large-scale synthesis, sensing and bioimagingMater. Today19382–93 [10]Ng S M 2019Carbon Dots as Optical Nanoprobes for Biosensors.(Elsevier Inc)
[11]Qu S, Wang X, Lu Q, Liu X and Wang L 2012 A biocompatiblefluorescent ink based on water-soluble luminescent carbon nanodots Angew. Chemie - Int. Ed.5112215–8
[12]Das A and Snee P T 2016 Synthetic developments of nontoxic quantum dotsChem. Phys. Chem.17598–617
[13]Li X, Wang H, Shimizu Y, Pyatenko A, Kawaguchi K and Koshizaki N 2011 Preparation of carbon quantum dots with tunable photoluminescence by rapid laser passivation in ordinary organic solventsChem. Commun.47932–4
[14]Ming Het al2012 Large scale electrochemical synthesis of high quality carbon nanodots and their photocatalytic propertyDalt. Trans.
419526–31
[15]Tuerhong M, XU Y and YIN X B 2017 Review on carbon dots and their applicationsChinese J. Anal. Chem.45139–50
[16]Marpongahtun S, Gea Y, Muis, Andriayani T, Novita and Piliang A F 2018 Synthesis of carbon nanodots from cellulose nanocrystals oil palm empty fruit by pyrolysis methodJ. Phys. Conf. Ser.11201–6
[17]Zhao Set al2015 Green synthesis of bifunctionalfluorescent carbon dots from garlic for cellular imaging and free radical scavenging ACS Appl. Mater. Interfaces717054–60
[18]Liu Xet al2017 Carbon nanodots as afluorescence sensor for rapid and sensitive detection of Cr(VI)and their multifunctional applicationsTalanta165216–22
[19]Zhai Xet al2012 Supporting information - highly luminescent carbon nanodots by microwave-assisted pyrolysisChem. Commun.48 7955–7
[20]Sun Y Pet al2006 Quantum-sized carbon dots for bright and colorful photoluminescenceJ. Am. Chem. Soc.1287756–7 [21]Guo C, Zhang X, Wang C, Lu J and Zhou X 2016 Rapid microwave synthesis of N-doped carbon nanodots with highfluorescence
brightness for cell imaging and sensitive detection of iron(III)Opt. Mater. (Amst).641–8 [22]Zhang H, Huang Y, Zheng Y and Zhou J 2019SC
[23]Kumar G G, Kim A R, Annaraj J, Xavier S S J, Siva G and Yoo D J 2017 Sensitive and selective turn-off-onfluorescence detection of Hg2+and cysteine using nitrogen doped carbon nanodots derived from citron and urineSensors Actuators B Chem.2591133–43 [24]Dimos K 2015 Carbon quantum dots: surface passivation and functionalizationCurr. Org. Chem.20682–95
[25]Zhang Met al2012 Facile synthesis of water-soluble, highlyfluorescent graphene quantum dots as a robust biological label for stem cellsJ. Mater. Chem.227461–7
[26]Liu Yet al2019 Nitrogen doped graphene quantum dots as afluorescent probe for mercury(II)ionsMicrochim. Acta186
[27]Jusuf B N, Sambudi N S, Isnaeni I and Samsuri S 2018 Microwave-assisted synthesis of carbon dots from eggshell membrane ashes by using sodium hydroxide and their usage for degradation of methylene blueJ. Environ. Chem. Eng.67426–33
[28]Devi S, Kaur A, Sarkar S, Vohra S and Tyagi S 2018 Synthesis and characterization of highly luminescent N-doped carbon quantum dots for metal ion sensingIntegr. Ferroelectr.18632–9
[29]Hu B, Wang K, Wu L, Yu S H, Antonietti M and Titirici M M 2010 Engineering carbon materials from the hydrothermal carbonization process of biomassAdv. Mater.22813–28
[30]Liu H, Ye T and Mao C 2007 Fluorescent carbon nanoparticles derived from candle sootAngew. Chemie - Int. Ed.466473–5 [31](Saunders Golden Sunburst Series)Donald L. Pavia, Gary M. Lampman, George S. Kriz-Introduction to Spectroscopy -Brooks Cole
(2000).pdf.’, Washington(US): Thomson Learning, Inc
[32]Zhang Y Let al2013 Graphitic carbon quantum dots as afluorescent sensing platform for highly efficient detection of Fe3+ionsRSC Adv.33733–8
6
Mater. Res. Express6(2019)105703 I W L Lewaet al
Bright green fluorescence of microwave irradiation-
synthesized
by Agus Subagio
Submission date: 12-Nov-2019 06:52PM (UTC+0700) Submission ID: 1212180536
File name: ight_green_fluorescence_of_microwave_irradiation-synthesized.pdf (842.95K) Word count: 3136
Character count: 16454
6 %
SIMILARITY INDEX
3 %
INTERNET SOURCES
5 %
PUBLICATIONS
4 %
STUDENT PAPERS
1 2 %
2 1 %
3 1 %
4 < 1 %
5 < 1 %
Bright green fluorescence of microwave irradiation-synthesized
ORIGINALITY REPORT
PRIMARY SOURCES
link.springer.com
Internet Source
Sachin Kadian, Gaurav Manik, Ashish Kalkal, Manjinder Singh, Rishi Pal Chauhan. "Effect of sulfur doping on fluorescence and quantum yield of graphene quantum dots: an
experimental and theoretical investigation", Nanotechnology, 2019
Publication
Fu-Gen Wu, Xiaodong Zhang, Xiaokai Chen, Wei Sun, Yan-Wen Bao, Xian-Wu Hua, Ge Gao, Hao-Ran Jia. "Chapter 3 Quantum Dots for
Cancer Therapy and Bioimaging", Springer Nature, 2018
Publication
Submitted to Thammasat University
Student Paper
nanoscalereslett.springeropen.com
Internet Source
Li, Panpan, Lei Huang, Youjie Lin, Leo Shen, Qi
6 < 1 %
7 < 1 %
8 < 1 %
9 < 1 %
10 < 1 %
Chen, and Wangzhou Shi. "Printable
temperature-responsive hybrid hydrogels with photoluminescent carbon nanodots",
Nanotechnology, 2014.
Publication
Submitted to University of Wisconsin - Stout
Student Paper
Hongzhen Liu, Xin Zhao, Fei Wang, Yunpeng Wang, Liang Guo, Jingjing Mei, Cancan Tian, Xiaotian Yang, Dongxu Zhao. "High-Efficient Excitation-Independent Blue Luminescent Carbon Dots", Nanoscale Research Letters, 2017
Publication
Submitted to Queen's University of Belfast
Student Paper
Anil Kumar, M R, H P Nagaswarupa, K S
Anantharaju, K Gurushantha, C Pratapkumar, S C Prashantha, T R Shashishekar, H
Nagabhushana, S C Sharma, Y S Vidya, B Daruka Prasad, C S Vivek Babu, and K R
Vishnu Mahesh. "Banyan latex: a facile fuel for the multifunctional properties of MgO
nanoparticles prepared via auto ignited
combustion route", Materials Research Express, 2015.
Publication
11 < 1 %
Exclude quotes Off Exclude bibliography On
Exclude matches Off
Jing-Hui Zhang, Aping Niu, Jing Li, Jian-Wei Fu, Qun Xu, De-Sheng Pei. "In vivo characterization of hair and skin derived carbon quantum dots with high quantum yield as long-term bioprobes in zebrafish", Scientific Reports, 2016
Publication
FINAL GRADE
/0
Bright green fluorescence of microwave irradiation-synthesized
GRADEMARK REPORT
GENERAL COMMENTS
Instructor
PAGE 1 PAGE 2 PAGE 3 PAGE 4 PAGE 5 PAGE 6
Document details
5 of 30
Bright green fluorescence of microwave irradiation-synthesized Cdots as sensitive probe of iron (III)
(Article), , , ,
Department of Physics, Diponegoro University, Jl. Prof. Soedarto, SH, Semarang, 50275, Indonesia
Central Laboratory of Research and Services, Diponegoro University (CORES DU), Jl. Prof. Soedarto, SH, Semarang, 50275, Indonesia
Abstract
Carbon nanodots (Cdots) are very attractive materials due to their fluorescence and great potential in various fields.
One field that still needs to be developed is heavy metal ion detector. In this study, a simple and cost efficient fluorescent detector of Fe was built. This material was synthesized by microwave irradiation method using citric acid and urea with purified water as the solvent to get the optimal concentration of urea in order to produce bright green fluorescence. Synthesized Cdots with optimal concentration of urea emit bright green fluorescence under UV light radiation. This bright green fluorescence was then used to detect Fe . The fluorescence of this solution was quenched with the addition of Fe due to transfer charges and exciton recombination. Optical characterization was carried out using UV-vis spectrophotometer, and fluorescence spectrophotometer. Results showed two absorption peaks on Cdots; at 330 nm and 410 nm. Fluorescence analysis was performed using a beam of 532 nm produced emission at a wavelength of 590 nm. In addition, structural characterization was performed using FTIR, SEM and EDX. © 2019 IOP Publishing Ltd.
SciVal Topic Prominence
Topic:
Prominence percentile: 99.978
Author keywords
bio imaging bright Green fluorescence Cdots microwave irradiation
Indexed keywords
Engineering controlled terms:
Heavy metals Iron compounds Irradiation Metabolism Metal ions Meteorological instruments Microwave irradiation Spectrophotometers Urea
Engineering uncontrolled terms
Bio-imaging Cdots Fluorescence spectrophotometer Green fluorescence Optical characterization Optimal concentration Structural characterization UV-Vis spectrophotometers
Engineering main heading:
Fluorescence
◅ Back to results ◅ Previous Next ▻
Export Download Print E-mail Save to PDF ⋆ Add to List More...▻
View at Publisher Materials Research Express
Volume 6, Issue 10, 7 August 2019, Article number 105703
Lewa, I.W.L.a Sutanto, H.a,b Subagio, A.a Marhaendrajaya, I.a Sugito, H.a
a b
View references (32)
3+
3+
3+
Semiconductor quantum dots | Carbon | Dots GQDs
PlumX Metrics
Usage, Captures, Mentions, Social Media and Citations beyond Scopus.
Metrics
Cited by 0 documents
Inform me when this document is cited in Scopus:
Related documents
, ,
(2012) Materials Letters
, ,
(2018) AIP Conference Proceedings
, ,
(2014) Green Chemistry
Find more related documents in Scopus based on:
❓ View all metrics ▻
Set citation alert ▻ ▻ Set citation feed
Facile synthesis of fluorescent carbon dots using watermelon peel as a carbon source Zhou, J. Sheng, Z. Han, H.
Carbon-electroluminescence: An organic approach to lighting Kumari, S. Chaudhary, T.
Chandran, V.
Electrochemical synthesis of photoluminescent carbon nanodots from glycine for highly sensitive detection of
hemoglobin
Wang, C.-I. Wu, W.-C.
Periasamy, A.P.
View all related documents based on references
▻
Authors Keywords ▻
Brought to you by Universitas Diponegoro
Search Sources Lists SciVal ↗ Create account Sign in
References (32)
Xu, X., Ray, R., Gu, Y., Ploehn, H.J., Gearheart, L., Raker, K., Scrivens, W.A.
(2004) Journal of the American Chemical Society, 126 (40), pp. 12736-12737. . doi: 10.1021/ja040082h
De, B., Karak, N.
(2013) RSC Advances, 3 (22), pp. 8286-8290. . doi: 10.1039/c3ra00088e
Bhamore, J.R., Jha, S., Park, T.J., Kailasa, S.K.
(2019) Journal of Photochemistry and Photobiology B: Biology, 191, pp. 150-155. . doi: 10.1016/j.jphotobiol.2018.12.023
Zheng, L., Chi, Y., Dong, Y., Lin, J., Wang, B.
(2009) Journal of the American Chemical Society, 131 (13), pp. 4564-4565. . doi: 10.1021/ja809073f
Jaleel, J.A., Pramod, K.
(2018) Journal of Controlled Release, 269, pp. 302-321. . doi: 10.1016/j.jconrel.2017.11.027
Cui, B., Feng, X.-T., Zhang, F., Wang, Y.-L., Liu, X.-G., Yang, Y.-Z., Jia, H.-S.
(2017) Xinxing Tan Cailiao/New Carbon Materials, 32 (5), pp. 385-401. . doi: 10.1016/S1872-5805(17)60130-6
ISSN: 20531591 Source Type: Journal Original language: English
DOI: 10.1088/2053-1591/ab3680 Document Type: Article
Publisher: Institute of Physics Publishing
▻ View in search results format All Export Print E-mail Save to PDF Create bibliography
1
Electrophoretic analysis and purification of fluorescent single-walled carbon nanotube fragments
Cited 1559 times http://pubs.acs.org/journal/jacsat
View at Publisher
2
A green and facile approach for the synthesis of water soluble fluorescent carbon dots from banana juice
Cited 356 times http://pubs.rsc.org/en/journals/journalissues
View at Publisher
3
Green synthesis of multi-color emissive carbon dots from Manilkara zapota fruits for bioimaging of bacterial and fungal cells
Cited 14 times www.elsevier.com/locate/jphotobiol
View at Publisher
4
Electrochemiluminescence of water-soluble carbon nanocrystals released electrochemically from graphite
Cited 595 times http://pubs.acs.org/doi/pdfplus/10.1021/ja809073f
View at Publisher
5
Artful and multifaceted applications of carbon dot in biomedicine
Cited 25 times www.elsevier.com/locate/jconrel
View at Publisher
6
The use of carbon quantum dots as fluorescent materials in white LEDs
Cited 6 times http://xxtcl.periodicals.net.cn/default.html
View at Publisher
Genc, R., Alas, M.O., Harputlu, E., Repp, S., Kremer, N., Castellano, M., Colak, S.G., (...), Erdem, E.
(Open Access)
(2017) Scientific Reports, 7 (1), art. no. 11222. . doi: 10.1038/s41598-017-11347-1
Li, L., Wu, G., Yang, G., Peng, J., Zhao, J., Zhu, J.-J.
(2013) Nanoscale, 5 (10), pp. 4015-4039. . doi: 10.1039/c3nr33849e
Zhang, J., Yu, S.-H.
(Open Access) (2016) Materials Today, 19 (7), pp. 382-393. .
doi: 10.1016/j.mattod.2015.11.008
Ng, S.M.
(2019) Carbon Dots As Optical Nanoprobes for Biosensors., p. 269.
Qu, S., Wang, X., Lu, Q., Liu, X., Wang, L.
(2012) Angewandte Chemie - International Edition, 51 (49), pp. 12215-12218. . doi: 10.1002/anie.201206791
Das, A., Snee, P.T.
(2016) ChemPhysChem, 17 (5), pp. 598-617. . doi: 10.1002/cphc.201500837
Li, X., Wang, H., Shimizu, Y., Pyatenko, A., Kawaguchi, K., Koshizaki, N.
(2011) Chemical Communications, 47 (3), pp. 932-934. . doi: 10.1039/c0cc03552a
Ming, H., Ma, Z., Liu, Y., Pan, K., Yu, H., Wang, F., Kang, Z.
(2012) Dalton Transactions, 41 (31), pp. 9526-9531. . doi: 10.1039/c2dt30985h
7
High-Capacitance Hybrid Supercapacitor Based on Multi-Colored Fluorescent Carbon-Dots
Cited 122 times www.nature.com/srep/index.html
View at Publisher
8
Focusing on luminescent graphene quantum dots: Current status and future perspectives
Cited 846 times http://www.rsc.org/publishing/journals/NR/Index.asp
View at Publisher
9
Carbon dots: large-scale synthesis, sensing and bioimaging
Cited 199 times http://www.journals.elsevier.com/materials-today/
View at Publisher
10
11
A biocompatible fluorescent ink based on water-soluble luminescent carbon nanodots
Cited 660 times
View at Publisher
12
Synthetic Developments of Nontoxic Quantum Dots
Cited 37 times http://onlinelibrary.wiley.com/journal/10.1002/(ISSN)1439-7641
View at Publisher
13
Preparation of carbon quantum dots with tunable photoluminescence by rapid laser passivation in ordinary organic solvents
Cited 272 times View at Publisher
14
Large scale electrochemical synthesis of high quality carbon nanodots and their photocatalytic property
Cited 417 times
View at Publisher
Tuerhong, M., XU, Y., YIN, X.-B.
(2017) Chinese Journal of Analytical Chemistry, 45 (1), pp. 139-150. . doi: 10.1016/S1872-2040(16)60990-8
Marpongahtun, Gea, S., Muis, Y., Andriayani, Novita, T., Piliang, A.F.
(Open Access)
(2018) Journal of Physics: Conference Series, 1120 (1), art. no. 012071.
doi: 10.1088/1742-6596/1120/1/012071
Zhao, S., Lan, M., Zhu, X., Xue, H., Ng, T.-W., Meng, X., Lee, C.-S., (...), Zhang, W.
(2015) ACS Applied Materials and Interfaces, 7 (31), pp. 17054-17060. . doi: 10.1021/acsami.5b03228
Liu, X., Li, T., Wu, Q., Yan, X., Wu, C., Chen, X., Zhang, G.
(2017) Talanta, 165, pp. 216-222. . doi: 10.1016/j.talanta.2016.12.037
Zhai, X., Zhang, P., Liu, C., Bai, T., Li, W., Dai, L., Liu, W.
(2012) Chemical Communications, 48 (64), pp. 7955-7957. . doi: 10.1039/c2cc33869f
Sun, Y.-P., Zhou, B., Lin, Y., Wang, W., Fernando, K.A.S., Pathak, P., Meziani, M.J., (...), Xie, S.-Y.
(2006) Journal of the American Chemical Society, 128 (24), pp. 7756-7757. . doi: 10.1021/ja062677d
Zhang, X., Lu, J., Zhou, X., Guo, C., Wang, C.
(2017) Optical Materials, 64, pp. 1-8. . doi: 10.1016/j.optmat.2016.11.026
Zhang, H., Huang, Y., Zheng, Y., Zhou, J.
(2019) SC 15
Review on Carbon Dots and Their Applications
Cited 64 times https://www.journals.elsevier.com/chinese-journal-of-analytical-chemistry
View at Publisher
16
Synthesis of Carbon Nanodots from Cellulose Nanocrystals Oil Palm Empty Fruit by Pyrolysis Method
http://iopscience.iop.org/journal/1742-6596
View at Publisher
17
Green Synthesis of Bifunctional Fluorescent Carbon Dots from Garlic for Cellular Imaging and Free Radical Scavenging
Cited 175 times http://pubs.acs.org/journal/aamick
View at Publisher
18
Carbon nanodots as a fluorescence sensor for rapid and sensitive detection of Cr(VI) and their multifunctional applications
Cited 29 times https://www.journals.elsevier.com/talanta
View at Publisher
19
Highly luminescent carbon nanodots by microwave-assisted pyrolysis
Cited 541 times
View at Publisher
20
Quantum-sized carbon dots for bright and colorful photoluminescence
Cited 2441 times
View at Publisher
21
Rapid microwave synthesis of N-doped carbon nanodots with high fluorescence brightness for cell imaging and sensitive detection of iron (III)
Cited 8 times
View at Publisher
22
Xavier, S.S.J., Siva, G., Annaraj, J., Kim, A.R., Yoo, D.J., kumar, G.G.
(2018) Sensors and Actuators, B: Chemical, 259, pp. 1133-1143. . doi: 10.1016/j.snb.2017.12.046
Dimos, K.
(2016) Current Organic Chemistry, 20 (6), pp. 682-695. . doi: 10.2174/1385272819666150730220948
Zhang, M., Bai, L., Shang, W., Xie, W., Ma, H., Fu, Y., Fang, D., (...), Yang, S.
(2012) Journal of Materials Chemistry, 22 (15), pp. 7461-7467. . doi: 10.1039/c2jm16835a
Liu, Y., Tang, X., Deng, M., Cao, Y., Li, Y., Zheng, H., Li, F., (...), Qiu, F.
(2019) Microchimica Acta, 186 (3), art. no. 140. . doi: 10.1007/s00604-019-3249-4
Jusuf, B.N., Sambudi, N.S., Isnaeni, I., Samsuri, S.
(2018) Journal of Environmental Chemical Engineering, 6 (6), pp. 7426-7433. . doi: 10.1016/j.jece.2018.10.032
Devi, S., Kaur, A., Sarkar, S., Vohra, S., Tyagi, S.
(2018) Integrated Ferroelectrics, 186 (1), pp. 32-39. . doi: 10.1080/10584587.2017.1369322
Hu, B., Wang, K., Wu, L., Yu, S.-H., Antonietti, M., Titirici, M.-M.
(2010) Advanced Materials, 22 (7), pp. 813-828. . doi: 10.1002/adma.200902812
23
Sensitive and selective turn-off-on fluorescence detection of Hg and cysteine using nitrogen doped carbon nanodots derived from citron and urine
2+
Cited 23 times View at Publisher
24
Carbon quantum dots: Surface passivation and functionalization
Cited 51 times http://www.benthamdirect.org/pages/all_b_bypublication.php
View at Publisher
25
Facile synthesis of water-soluble, highly fluorescent graphene quantum dots as a robust biological label for stem cells
Cited 461 times
View at Publisher
26
Nitrogen doped graphene quantum dots as a fluorescent probe for mercury(II) ions
Cited 15 times http://www.springer/at/mca
View at Publisher
27
Microwave-assisted synthesis of carbon dots from eggshell membrane ashes by using sodium hydroxide and their usage for degradation of methylene blue
Cited 2 times http://www.journals.elsevier.com/journal-of-environmental-chemical-engineering/
View at Publisher
28
Synthesis and characterization of highly luminescent N-doped carbon quantum dots for metal ion sensing
Cited 9 times www.tandf.co.uk/journals/titles/10584587.asp
View at Publisher
29
Engineering carbon materials from the hydrothermal carbonization process of biomass
Cited 971 times
http://www3.interscience.wiley.com/cgi-bin/fulltext/123240973/PDFSTART View at Publisher
5 of 30 Liu, H., Ye, T., Mao, C.
(2007) Angewandte Chemie - International Edition, 46 (34), pp. 6473-6475. . doi: 10.1002/anie.200701271
Pavia, D.L., Lampman, G.M., George, S.
(2000) Kriz-Introduction to Spectroscopy -Brooks Cole. . pdf.rsquo;, Washington (US): Thomson Learning, Inc
Zhang, Y.-L., Wang, L., Zhang, H.-C., Liu, Y., Wang, H.-Y., Kang, Z.-H., Lee, S.-T.
(2013) RSC Advances, 3 (11), pp. 3733-3738. . doi: 10.1039/c3ra23410j
© Copyright 2019 Elsevier B.V., All rights reserved.
30
Fluorescent carbon nanoparticles derived from candle soot
Cited 1165 times
View at Publisher
31
Cited 137 times
32
Graphitic carbon quantum dots as a fluorescent sensing platform for highly efficient detection of Fe ions3+
Cited 166 times http://pubs.rsc.org/en/journals/journal/ra
View at Publisher
◅ Back to results ◅ Previous Next ▻ Top of page
About Scopus
What is Scopus Content coverage Scopus blog Scopus API Privacy matters
Language
⽇本語に切り替える 切换到简体中文 切換到繁體中文 Русский язык
Customer Service
Help Contact us
Copyright © . All rights reserved. Scopus® is a registered trademark of Elsevier B.V.
We use cookies to help provide and enhance our service and tailor content. By continuing, you agree to the .
Terms and conditions ↗ Privacy policy ↗ Elsevier B.V ↗
use of cookies
Source details
Materials Research Express
Scopus coverage years: from 2014 to 2019 Publisher: Institute of Physics Publishing
E-ISSN: 2053-1591
Subject area: Materials Science: Metals and Alloys Materials Science: Polymers and Plastics
Materials Science: Surfaces, Coatings and Films Materials Science: Electronic, Optical and Magnetic Materials Materials Science: Biomaterials
View all documents ▻ Save to source list Journal Homepage
CiteScore 2018
1.33
SJR 2018
0.353
SNIP 2018
0.501
CiteScore CiteScore rank & trend CiteScore presets Scopus content coverage
Calculated using data from 30 April, 2019
CiteScore
*CiteScore includes all available document types
1.33
= Citation Count 2018 Documents 2015 -2017*
=
Metrics displaying this icon are compiled according to , a collaboration between industry and academia.
2018
▻ 2,973 Citations
▻ 2,230 Documents
▻
View CiteScore methodology CiteScore FAQ▻
Last updated on 08 December, 2019
CiteScoreTracker 2019
1.76
= Citation Count 2019 Documents 2016 - 2018=
Updated monthly
▻ 6,406 Citations to date 3,631 Documents to date▻
↗
Snowball Metrics
CiteScore rank
Category Rank Percentile
Materials Science
#46/148 69th
Materials Science
#71/147 51st
Metals and Alloys
Polymers and Plastics
▻
View CiteScore trends
🔗
Add CiteScore to your site
About Scopus
What is Scopus Content coverage Scopus blog Scopus API Privacy matters
Language
⽇本語に切り替える 切换到简体中文 切換到繁體中文 Русский язык
Customer Service
Help Contact us
Copyright © . All rights reserved. Scopus® is a registered trademark of Elsevier B.V.
We use cookies to help provide and enhance our service and tailor content. By continuing, you agree to the .
Terms and conditions ↗ Privacy policy ↗ Elsevier B.V ↗
use of cookies
Brought to you by Universitas Diponegoro
Search Sources Lists SciVal ↗ Create account Sign in
10/16/2019 Materials Research Express
https://www.scimagojr.com/journalsearch.php?q=21100432452&tip=sid&clean=0 1/6
also developed by scimago: SCIMAGO INSTITUTIONS RANKINGS Scimago Journal & Country Rank
Home Journal Rankings Country Rankings Viz Tools Help About Us
Materials Research Express
Country United Kingdom - SIR Ranking of United Kingdom
21
H Index Subject Area and
Category Materials Science Biomaterials
Electronic, Optical and Magnetic Materials Metals and Alloys
Polymers and Plastics Surfaces, Coatings and Films Publisher IOP Publishing Ltd.
Publication type Journals ISSN 20531591 Coverage 2014-ongoing
Scope Materials Research Express (MRX) is a multidisciplinary journal devoted to publishing new experimental and theoretical research on the properties, characterization, design and fabrication of all classes of materials, and on their technological applications. Characterized by article length exibility and a fast-track peer review process, areas of particular interest include: - Materials characterization -Material properties -Computational materials science and modelling -Material applications -Synthesis and fabrication -Engineering applications of materials listed below Material classes within the journal scope: -Biomaterials -Carbon allotropes and 2D materials -Electronic materials (including semiconductors) -Glasses and ceramics -Magnetic materials -Metals and alloys -Nanomaterials and nanostructures -Photonic materials and metamaterials -Polymers and organic compounds -Smart materials -Soft matter - Superconducting materials -Surfaces, interfaces and thin lms
Homepage
How to publish in this journal Contact
Join the conversation about this journal
Enter Journal Title, ISSN or Publisher Name
Quartiles
The set of journals have been ranked according to their SJR and divided into four equal groups, four quartiles. Q1 (green) comprises the quarter of the journals with the highest values, Q2 (yellow) the second highest values, Q3 (orange) the third highest values and Q4 (red) the lowest values.
Category Year Quartile
Biomaterials 2015 Q3
Biomaterials 2016 Q4
Biomaterials 2017 Q1
Biomaterials 2018 Q3
2015 2016 2017 2018
Biomaterials Electronic, Optical and Magnetic Materials Metals and Alloys Polymers and Plastics Surfaces, Coatings and Films
10/16/2019 Materials Research Express
https://www.scimagojr.com/journalsearch.php?q=21100432452&tip=sid&clean=0 2/6
SJR
The SJR is a size-independent prestige indicator that ranks journals by their 'average prestige per article'. It is based on the idea that 'all citations are not created equal'. SJR is a measure of scienti c in uence of journals that accounts for both the number of citations received by a journal and the importance or prestige of the journals where such citations come from It measures the scienti c in uence of the average article in a journal it expresses how central to the global
Citations per document
This indicator counts the number of citations received by documents from a journal and divides them by the total number of documents published in that journal. The chart shows the evolution of the average number of times documents published in a journal in the past two, three and four years have been cited in the current year.
The two years line is equivalent to journal impact factor
™ (Thomson Reuters) metric.
Cites per document Year Value Cites / Doc. (4 years) 2014 0.000 Cites / Doc. (4 years) 2015 1.269 Cites / Doc. (4 years) 2016 1.066 Cites / Doc. (4 years) 2017 1.245 Cites / Doc. (4 years) 2018 1.369 Cites / Doc. (3 years) 2014 0.000 Cites / Doc. (3 years) 2015 1.269 Cites / Doc. (3 years) 2016 1.066 Cites / Doc. (3 years) 2017 1.245 Cites / Doc. (3 years) 2018 1.332 Total Cites Self-Cites
Evolution of the total number of citations and journal's self-citations received by a journal's published documents during the three previous years.
Journal Self-citation is de ned as the number of citation from a journal citing article to articles published by the same journal.
Cites Year Value
S lf Cit 2014 0
External Cites per Doc Cites per Doc
Evolution of the number of total citation per document and external citation per document (i.e. journal self- citations removed) received by a journal's published documents during the three previous years. External citations are calculated by subtracting the number of self-citations from the total number of citations received by the journal’s documents.
Cit Y V l
% International Collaboration
International Collaboration accounts for the articles that have been produced by researchers from several countries. The chart shows the ratio of a journal's documents signed by researchers from more than one country; that is including more than one country address.
Year International Collaboration 2014 26.75
2015 25 39 Citable documents Non-citable documents
Not every article in a journal is considered primary research and therefore "citable", this chart shows the ratio of a journal's articles including substantial research (research articles, conference papers and reviews) in three year windows vs. those documents other than research articles, reviews and conference papers.
Documents Year Value
N it bl d t 2014 0
Cited documents Uncited documents
Ratio of a journal's items, grouped in three years windows, that have been cited at least once vs. those not cited during the following year.
Documents Year Value
Uncited documents 2014 0 Uncited documents 2015 117 Uncited documents 2016 391 Uncited documents 2017 627
←Show this widget in your own website Just copy the code below and paste within your html code:
<a href="https://www.scimag
2015 2016 2017 2018
0 0.6 1.2 1.8
Cites / Doc. (4 years) Cites / Doc. (3 years) Cites / Doc. (2 years)
2014 2015 2016 2017 2018
0 0.3 0.6 0.9 1.2 1.5
2014 2015 2016 2017 2018
0 2k 4k
2014 2015 2016 2017 2018
0 0.7 1.4
2014 2015 2016 2017 2018
12 18 24 30
2014 2015 2016 2017 2018
0 2k 4k
2014 2015 2016 2017 2018
0 2k 4k
An open access, rapid peer-review journal publishing high quality research on the design, fabrication, properties and applications of all classes of materials.
From 1 October 2019 MRX changed to a fully gold open access journal – read the news
announcement.
25% DISCOUNT TO THE ARTICLE PUBLICATION CHARGE UNTIL 2021
MEDIAN TIME TO FIRST DECISION
14 days
2018 IMPACT FACTOR
1.449
Number 1, January 2020 Go
Vol 7, 2020 Go
Current volume
Journal archive
This site uses cookies. By continuing to use this site you agree to our use of cookies. To find out
more, see our Privacy and Cookies policy.
Submit an article Track my article
Most read Most cited Latest articles Review articles Accepted manuscripts Trending
View all abstracts
OPEN ACCESS
Cactus material-based adsorbents for the removal of heavy metals and dyes: a review
Abdelfattah Amari et al 2020 Mater. Res. Express 7 012002 View article PDF
View abstract
JOURNAL LINKS Submit an article About the journal Editorial Board Author guidelines Review for this journal Publication charges
OPEN ACCESS
Adsorption of Congo red with hydrothermal treated shiitake mushroom
Kai Yang et al 2020 Mater. Res. Express 7 015103 View article PDF View abstract
OPEN ACCESS
Strengthening of β polymorph in PVDF/FLG and PVDF/GO nanocomposites
Atif Islam et al 2020 Mater. Res. Express 7 015017 View article PDF View abstract
OPEN ACCESS
Hydrothermal synthesis of SnS /MoS Nanospheres for enhanced adsorption capacity of organic dyes
Bowen Cui et al 2020 Mater. Res. Express 7 015016
2 2
View article PDF View abstract
OPEN ACCESS
Thermal plasma synthesis and electrochemical properties of high-voltage LiNi Mn O nanoparticles
Hirotaka Sone et al 2020 Mater. Res. Express 7 015015
0.5 1.5 4
View article PDF View abstract
News and editorial Awards
Journal collections Pricing and ordering Contact us
JOURNAL HISTORY
2014-present Materials Research Express doi:10.1088/issn.2053-1591
Online ISSN: 2053-1591