This has resulted in a substantial part of food corn production being shifted in that direction. It is clear that energy storage will become an increasingly important part of the total energy supply and use in the future.

Introduction
An example is the use of hydro pumped storage to capture electricity and feed the electricity back into the grid, as will be described later. It will be seen that there are many different types of energy storage methods and technologies.
Storage in the Fuel Distribution System
The pump hydro technology actually works through a mechanical storage mechanism, as it is based on the gravitational difference between two different water storage reservoirs.
Periodic Storage
Long-Term, or Seasonal, Storage
Daily and Weekly Storage
The Problem of Load Leveling
This tag describes capacity that is not currently connected to the system but can be brought online after only a short delay. In addition, there are additional secondary source technologies that are more flexible, but significantly more expensive, that can be used to handle any need for extra capacity.
Methods That Can Be Used to Reduce the Magnitude of the Variations in Energy Demand
One of these, the so-called spin reserve, is additional generation capacity that can be made available through a relatively simple change in the operating parameters of the large turbines in use. This differs from practices in Europe and Japan, where approximately 10% and 15% of the supplied power is circulated through such storage facilities respectively.

Short-Term Transients
Portable Applications That Require Energy Storage
Storage Methods for Use with Portable Electronic Devices
Energy Use and Storage in Vehicles
The charging of the battery component enables these vehicles to cover a limited range on electricity alone. But in addition, this part of the electrical load will mostly be at night, where the other demands are reduced, and thus it will contribute to load leveling.
Hydrogen Propulsion of Vehicles
These are essentially the same materials as the hydrides used in the negative electrodes of conventional hydride/nickel batteries. The weight of hydride materials is not a problem in the case of submarines, and some of the materials currently known are apparently satisfactory for this purpose.
Temperature Regulation in Buildings
In the BMW case, the hydrogen is stored as a liquid at a low temperature in an insulated tank. When heated, the hydrogen is released so it can be used in the engine.
Improved Lighting Technologies
The Structure of This Book
Introduction
The Mechanical Equivalent of Heat
1Joule ¼ 1Newtonmeter ¼ 1kg m2sec2 ð2:3Þ The amount of heat required to raise the temperature of any material 1C is called its heat capacity or its specific heat. In the latter case, the amount of heat per unit of weight, the dimensions are J kg1K1.
The First Law of Thermodynamics—Conservation of Energy
1 cal ¼ 4.184 Joules ð2:1Þ A thermochemical calorie, a quantitative unit of heat, is defined as the amount of heat that must be added to one gram of water to raise its temperature by 1C.
Enthalpy
On the other hand, if heat is released, ΔHis is negative, the internal energy is reduced and the reaction is called exothermic. This change in the heat content when a reaction takes place is called the latent heat of the reaction.
Entropy
Thermal Entropy
If a system (eg, a material) undergoes a change of state, such as melting or a chemical reaction, there will be a change in enthalpyΔH. This is indicated by the use of the index "0". At a temperature of 298.15 K and a pressure of one bar, the value of H0 is zero for all pure materials.
Configurational Entropy
The Energy Available to Do Work
The Temperature Dependence of G, H, and S
Irreversible and Reversible Storage Modes
The Carnot Limitation
Energy Quality
Introduction
Sensible Heat
An additional factor that may be important in some cases is the thermal conductivity of the storage material, because this affects the rate at which heat can be absorbed or released. Thus, insulation or thermal insulation of the storage material can be quite important, especially if the storage period is considerable.
Latent Heat
Inorganic Phase Change Materials
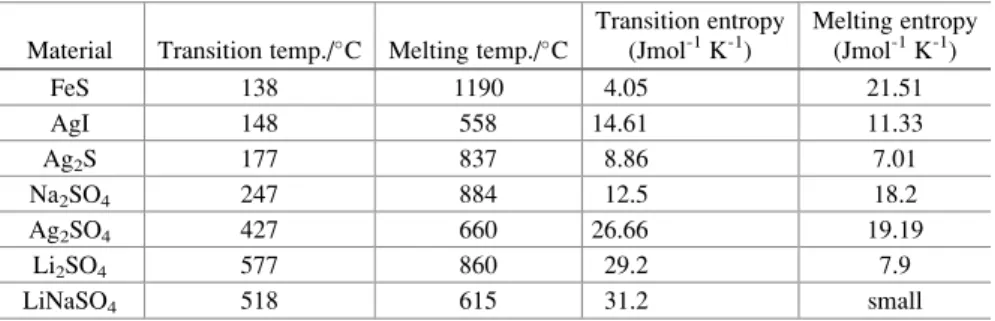
In addition to the phase changes involved in the transformation of solids into liquids, there are a number of cases in which there is a phase change in the solid state. It can be seen in Table 3.3 that the entropy changes associated with the solid state structural changes in the paddle wheel materials are significantly larger than the entropy changes during melting.

Organic Phase Change Materials
It has been found that these materials can also have unusually high values of ionic conductivity, which differ from those of sodium and silver analogues [6, 7]. Materials that have large values of latent heat associated with phase transitions in the solid state are discussed in [11].
Quasi-Latent Heat
Heat Pumps
Introduction
Types of Non-congruent Chemical Reactions
- Insertion Reactions
- Formation Reactions
- Decomposition Reactions
- Displacement Reactions
ΔG0r ¼ΔG0fð ÞAB ð4:4Þ Values of the standard Gibbs free energy of formation for many materials can be found in a number of sources, for example, [1]. ΔG0r ¼ΔG0fðLi2OÞ ΔG0fðCu2OÞ ð4:9Þ since the standard Gibbs free energy of formation of all elements is zero.
Phase Diagrams
- The Gibbs Phase Rule
- Binary Phase Diagrams
- The Lever Rule
- Three-Phase Reactions in Binary Systems
- Examples of Materials Systems with Peritectic Reactions
- Binary Systems That Contain Eutectic Reactions
The overall composition is of course not limited to the region within the two-phase mixture region of the phase diagram. The discussion so far has assumed that the overall composition of the liquid is the same as the eutectic composition.

Thermal Effects Related to Liquid and Solid Reactions
Thus the effective heat capacity increases, and the cooling rate, the slope of the cooling curve, decreases. When this reaction is complete and there is no more liquid left, the slope of the cooling curve increases again.

Thermal Effects Related to Reversible Gas Phase Reactions
The thermal effects of this reaction can be obtained from information on the standard enthalpies of the species involved. From these data, the temperature dependence of the standard enthalpy change of the reaction can be obtained.

Introduction
Storage of Energy in Living Biomass
The efficiency of using solar radiation to produce stored energy by a number of plant species is shown in Table 5.1[2]. Efficiency is defined as the energy content of the crop divided by the accumulated solar incident energy for a given surface of the earth.

Storage via Animals
For coral reefs and areas where currents bring nutrient-rich water, such as along the Peruvian coast, the conversion efficiency can be 2-3% of the incident solar energy. In many applications, however, only a fraction of the animals' mechanical power is actually used for a useful purpose.
Hard Biomass
If the average food intake is 600 W per animal, the efficiency of converting the food energy to mechanical energy will be 16. For example, there is no useful energy output, although there is energy consumption, when a horse or cow simply walks around.
Synthetic Liquid Fuels
Gaseous Fuels Stored as Liquids
The Energy Content of Various Materials Used as Fuels
Introduction
Potential Energy Storage
In metals and ceramics, Young's modulus is a constant up to a critical value of the stress, called the yield strength. In polymers and rubbers, Young's modulus can vary with the value of the strain due to the effect of different physical processes in their microstructures.

Energy Storage in Pressurized Gas
This is one of the ways in which the hydrogen used as fuel in the fuel cells being developed to propel vehicles is contained. The tanks used to store gaseous hydrogen to power the fuel cells in the cars currently under development can operate up to a pressure of 104psi.
Potential Energy Storage Using Gravity
G is the gravitational constant, 6.671011m3kg1s2, Ms is the mass of the Earth (5.981024 kg), Ms is the moving mass, and is the distance between their centers.
Hydroelectric Power
The gravitational effect of the sun is somewhat smaller and theoretically yields an amplitude of about 25 cm under comparable conditions. Tides rise and fall with a cycle time close to 12 hours, with their magnitudes dependent on the relative positions of the sun and moon.
Pumped-Hydro Storage
Programs have begun to take advantage of large water level fluctuations at several locations by building "lakes" that receive and discharge seawater through turbines. The power generated in this way is of course periodic, related to the time of the tides.

Use of the Kinetic Energy in Moving Water
Kinetic Energy in Mechanical Systems
Linear Kinetic Energy
Rotational Kinetic Energy
The strength of the material from which the flywheel is made must be taken into account, as it must be able to withstand the centrifugal force. 3R30σmax ð6:17Þ for the case when the strength of the material from which the shaft is made is the same as the strength of the flywheel material.

Internal Structural Energy Storage
Introduction
One involves the use of electrical devices and systems where energy is stored in materials and configurations that exhibit capacitor-like properties.
Energy Storage in Capacitors
Energy in a Parallel Plate Capacitor
The capacity C for this configuration is given by. where ε0 is the relative permittivity of the material, and ε0 is the permittivity of a vacuum F per meters. It is interesting that the amount of energy stored in such a capacitor is inversely proportional to the volume of the dielectric material between the plates of the capacitor.

Electrochemical Charge Storage Mechanisms
- Electrostatic Energy Storage in the Electrical Double-Layer in the Vicinity of an Electrolyte/
- Underpotential Faradaic Two-Dimensional Adsorption on the Surface of a Solid Electrode
- Faradaic Deposition that Results in the Three- Dimensional Absorption of the Electroactive Species
- Faradaically-Driven Reconstitution Reactions
This results in the occupation of specific crystallographic sites on the surface of the solid electrode by species from the electrolyte. It was also found [16] that the amount of charge that can be stored in hydrated RuO2 is independent of the surface area but proportional to the total mass.
![Fig. 7.2 Apparent capacitance of RuO 2 hydrate as a function of surface area [16]](https://thumb-ap.123doks.com/thumbv2/123dok/10274018.0/112.659.134.524.609.870/fig-apparent-capacitance-ruo-hydrate-function-surface-area.webp)
Comparative Magnitudes of Energy Storage
This means that all intensive variables, including the electrode potential, are independent of the overall composition, and thus independent of the amount of charge delivered. The actual amount of energy available will of course depend on the power level, due to unavoidable losses, such as those due to the unavoidable internal resistance of the system.
Importance of the Quality of the Stored Energy
As stated above, the maximum amount of energy available in any case is the area under the V/Q curve. We see that the maximum amount of energy that can be stored in an electrode behaving like a capacitor is 1/2 (Vmax)(Qmax).
Transient Behavior of a Capacitor
The rate at which energy can be stored - or supplied - is determined by the time constant. But it is also clear that if the capacitance C is large, the time constant will be large.

Modeling Transient Behavior of Electrochemical Systems Containing Capacitive Components Using
Introduction
Use of Laplace Transform Techniques
Simple Examples
Upon imposing a step in potential F0, the time dependence of the current i(t) is given by. For the case of a step in current i0, the time dependence of the electrode potential F(t) is given by.

Energy Storage in Magnetic Systems
Energy in a Material in a Magnetic Field
Miss called the magnetization, and μ0Mis the additional induced magnetic field due to the properties of the solid. It turns out that the presence of material with a high sensitivity value amplifies the external field.

Energy Storage in Superconducting Magnetic Systems
In this case, it is assumed that there is very little hysteresis, so that the data measured when the field is increased is essentially the same as that when the field is decreased. There is a serious potential danger if either the temperature or the field becomes too high, so that the material is no longer superconducting.
Superconductive Materials
Nb–Ti alloys, which have a ductile BCC crystal structure, and can be formed into wires and made into coils, are commonly used for this purpose. Fibers of this material can be embedded in an aluminum or copper matrix for structural purposes.

Introduction
However, the greatest use of hydrogen at present is not as an energy carrier, but as a reactant in a number of important large-scale chemical processes.
The Production of Hydrogen
- The Steam Reforming Process
- The Reaction of Steam with Carbon
- Electrolytic Production of Hydrogen
- Thermal Decomposition of Water to Produce Hydrogen
- Chemical Extraction of Hydrogen from Water
- Additional Approaches to the Production of Hydrogen
The temperature dependence of the standard Gibbs free energies of these two reactions is shown in Fig. Using values from [1], the temperature dependence of the standard Gibbs free energy of water formation is shown in Fig.8.5.
Governmental Promotion of the Use of Hydrogen
Another is the amount of hydrogen that would be needed if a significant number of vehicles were converted to hydrogen propulsion. It has been estimated [12] that one million fuel cell vehicles would consume about 0.4 million tons of hydrogen per year.

Current On-Board Hydrogen Storage Alternatives
Storage of Gaseous Hydrogen in High-Pressure Tanks
Storage of Liquid Hydrogen in Insulated Tanks
Storage of Hydrogen as Protons in Solids
Other Approaches to Hydrogen Storage
Hydrogen from the Decomposition of Materials Containing Hydride Anions
But then there must also be a system for transporting the used and renewed materials. The result was that the discharged battery electrodes had to be sent to a chemical plant to convert the ZnO product back into elemental zinc.
Ammonia and Related Materials as Hydrogen Storage Media
Another concept is to use a hydrogen-containing material that could react with another material and release hydrogen as one of the reaction products. The values of the standard Gibbs free energy of formation for the corresponding phases in the lithium-hydrogen-nitrogen system at 298 K [17] are included in Table 8.8.

Storage of Hydrogen in Reversible Organic Liquids
An example of the use of this concept [19] involves installation in a 17-ton truck in Switzerland. An additional interesting feature of the reversible liquid hydrogen systems has to do with their potential use for transferring energy over long distances using simple, low-cost pipelines.

The Question of Safety
Introduction
Simple Chemical and Electrochemical Reactions
The chemical reaction continues with the transport of A+ ions through the electrolyte and electrons in the external circuit from the left side (A) of the cell to the right side. It is also possible, of course, for the ions in the electrolyte to be negatively charged.

Major Types of Reaction Mechanisms in Electrochemical Cells
Formation Reactions
By the time the overall composition reaches the lower Li limit of the "LiAl" region, no more Alsat is present. Adding more lithium causes the overall composition to shift into the "LiAl" phase range.
Displacement Reactions
For example, the LiCl phase can result from the reaction of lithium with chlorine gas, and ZnO can form as a result of the reaction of zinc with oxygen in the air. LiþCu2O¼Li2OþCu ð9:11Þ in which the reaction of lithium with Cu2O results in the formation of two new phases, Li2O and elemental copper.
Insertion Reactions
A change in the chemical state in the electrode naturally leads to a change in its electrical potential. In some cases, the new element or phase formed by such an interstitial displacement process is crystalline, while in other cases it may be beamorphic.
Important Practical Parameters
- The Operating Voltage and the Concept of Energy Quality
- The Charge Capacity
- The Maximum Theoretical Specific Energy (MTSE)
- Variation of the Voltage as Batteries Are Discharged and Recharged
- Cycling Behavior
- Self-Discharge
The energy in an electrochemical system is the integral of the voltage multiplied by the charge capacity, i.e. the amount of available charge. These are shown here to represent cell voltage as a function of state of charge parameter.

General Equivalent Circuit of an Electrochemical Cell
- Influence of Impedances to the Transport of Ionic and Atomic Species Within the Cell
- Influence of Electronic Leakage Through the Electrolyte
- Transference Numbers of Individual Species in an Electrochemical Cell
- Relation Between the Output Voltage and the Values of the Ionic and Electronic Transference Numbers
- Joule Heating Due to Self-Discharge in Electrochemical Cells
- What If Current Is Drawn from the Cell?
There will always be some impedance to the transport of the electroactive ions and the related atomic species across the cell. It is clear that even without external current, there is an internal current related to the transport of the electronic species through the electrolyte.

Introduction
Thermodynamic Properties of Individual Species
Thus, gradients in the chemical potential of species i produce chemical forces that cause i to tend to move in the direction of lower μi. Consider an electrochemical cell in which the activity of species i is different in two electrodes, ai() in the negative electrode and ai(+) in the positive electrode.
A Simple Example: The Lithium/Iodine Cell
Calculation of the Maximum Theoretical Specific Energy
From this information and the weights of the reactants, the value of the maximum theoretical specific energy of a Li/I2 cell can now be calculated. However, the lack of chargeability, the cost of the ingredients and the low discharge rate unfortunately limit the application area of Li/I2 cells.
The Temperature Dependence of the Cell Voltage
The data in Table 10.1 show that the entropy values of simple solids are significantly lower than those of liquids, which, in turn, are lower than gases. The resulting variation of cell voltage with temperature from about room temperature to operating temperature of high temperature oxide-electrolyte fuel cells is shown in Fig.10.3.
The Shape of Discharge Curves and the Gibbs Phase Rule
That is, the amount of lithium changes with the state of charge of the Li/I2 battery. This means that the potential of electrode I2 does not change with its state of charge.

The Coulometric Titration Technique
Since the electrode of pure A has a unit activity, this relation can be written as. This method can be used to make very small changes in the composition of the electrode material.
![Fig. 10.10 Changes in unit cell dimensions as a function of composition in Li x Mn 2 O 4 [6]](https://thumb-ap.123doks.com/thumbv2/123dok/10274018.0/194.659.133.525.85.782/fig-changes-unit-cell-dimensions-function-composition-li.webp)
Introduction
Relationship Between Phase Diagrams and Electrical Potentials in Binary Systems
As an example, Figure 11.3 shows a version of the phase diagram presented in Figure 11.1. The corresponding change in electric potential with composition is shown schematically in fig.
A Real Example, the Lithium–Antimony System
When the overall composition reaches composition B, all of the α phase will have been consumed and only the β phase will be present. At composition C, the upper limit of βphase composition at that temperature, the overall composition again enters a two-phase interval (βandγ) and the potential is again independent of composition.




![Fig. 1.4 Short-time transients superimposed upon the load curve. After [2]](https://thumb-ap.123doks.com/thumbv2/123dok/10274018.0/46.659.108.551.657.873/fig-short-time-transients-superimposed-load-curve.webp)