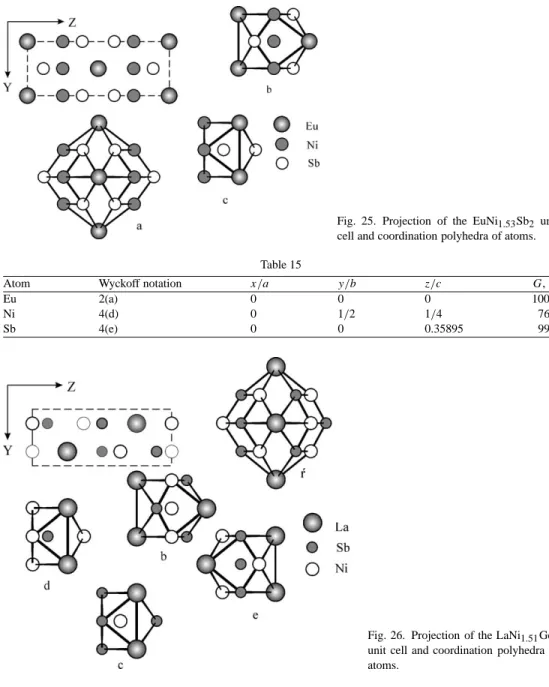
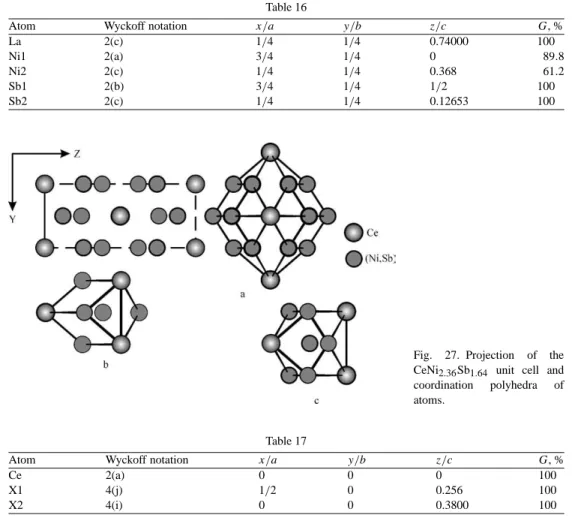
With this volume of the Manual for the physics and chemistry of rare earth Prof. Zuckermann, Transport properties (electrical resistance, thermoelectric power thermal conductivity) of rare earth intermetallic compounds 117.
FILLED SKUTTERUDITES
Introduction
In 1996, some of the lanthanide skutterudites were found to have excellent thermoelectric properties above room temperature (Sales et al., 1996;. In addition to the stoichiometric filled skutterudite compounds of the form RM4X12, a large number of related alloys were also investigated as possible thermoelectric materials.
Structure
This chapter will focus on what is known about the structural, electronic and magnetic properties of the stoichiometric lanthanide-skutterudite compounds of the form RM4X12. Tilting the octahedra in the skutterudite structure reduces the volume of six of these cavities, which become the centers of rectangular pnicogen groups (P4, As4 or Sb4).
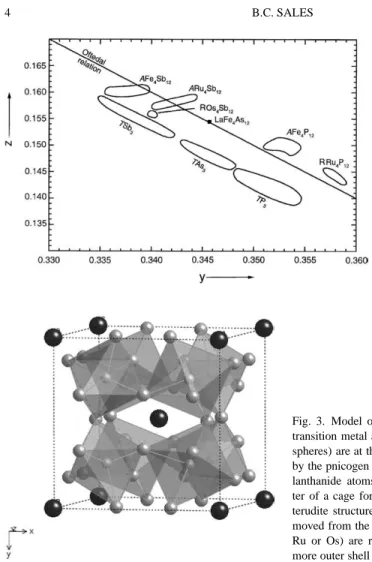
Lanthanide skutterudites as Zintl compounds
The electronic structure of lanthanide-filled skutterudites can also be analyzed using the Zintl picture. This simple argument would suggest that all lanthanide-filled skutterudites should be bad metals.
Synthesis and crystal growth
A dense single-phase sample of CeFe4As12 was prepared by a similar procedure, followed by the compaction of the powder with a hot press (Watcharapasorn et al., 2002). Single crystals of the antimonides can also be grown using excess antimony as a flux (Chakoumakos et al., 1999;.
La filled skutterudites
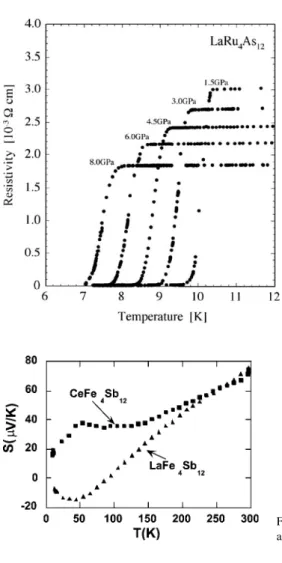
LaRu4As12 is a poor metal with the highest superconducting transition temperature (10.3 K) of all the filled schutterudites. Probably the best interpretation of the susceptibility data was given by Ravot et al. 2001), who were able to account for the nearly Curie–Weiss dependence of the susceptibility in terms of a Stoner band picture.
Ce filled skutterudites
The room temperature values of the Seebeck coefficient and thermal conductivity are +147 µV/K and 10.5 W/m K respectively (Sekine et al., 2001). The resistivity of a polycrystalline sample indicates a small gap of the order of 0.01 eV (Grandjean et al., 1984).
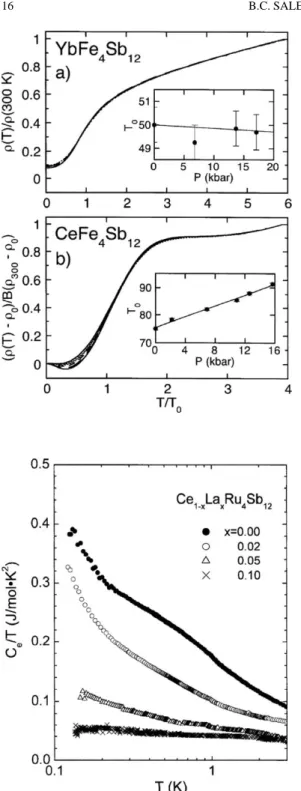
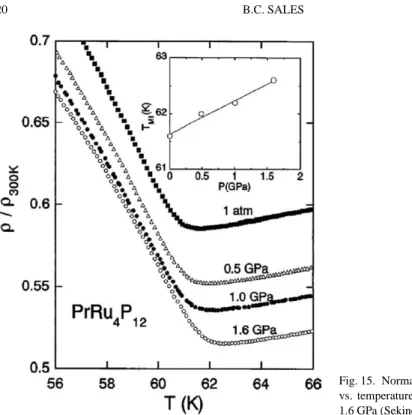
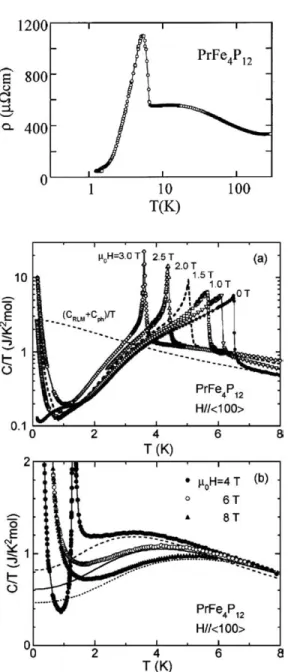
Pr filled skutterudites
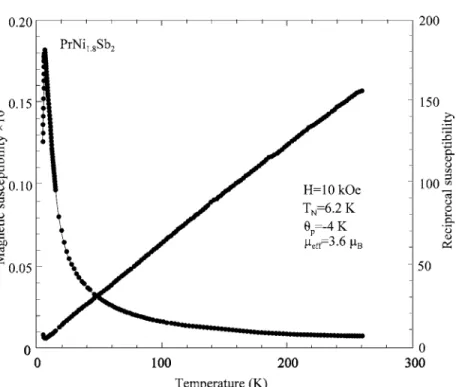
A dHvA branch with a mass of 10 me was also observed in the ordered low-field phase (Sugawara et al., 2001). Magnetization measurements at 2 K indicate a non-magnetic ground state induced by the crystal electric field (Sekine et al., 1997).
Nd filled skutterudites
Neutron scattering measurements have confirmed the ferromagnetic ordering of Nd moments below 2 K (Keller et al., 2001). Low temperature heat capacity data confirm the bulk nature of the magnetic transition (Takeda and Ishikawa, 2000b).
Sm filled skutterudites
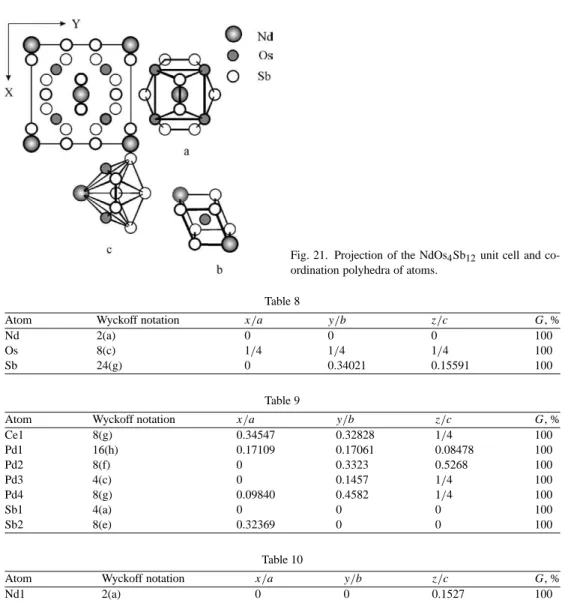
De Hass–van Alfen measurements on NdFe4P12 crystals showed a Fermi surface similar to that of LaFe4P12 except for the splitting of the dHvA branches due to the ferromagnetic exchange interaction (Sugawara et al., 2000). NdOs4Sb12 can undergo a displacement-type phase transition at –86 ◦C in which the Nd atoms freeze off-center (Evers et al., 1995).
Eu filled skutterudites
The value of the Eu isomer shift in EuFe4P12 is −6 mm/s and the isomer shift is independent of temperature from 4 K to 300 K. The value of the Eu isomer shift in EuRu4P12 is −9.3 mm/s, which is compatible with Eu +2in a metallic compound.
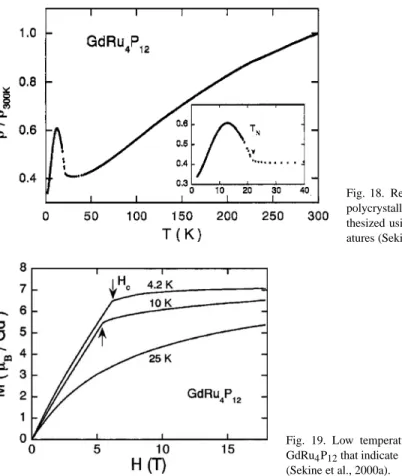
Gd filled skutterudites
XANES measurements would be useful to decide whether the Eu valence is intermediate in this material. The saturation moment is about 10% smaller than the Eu+2 value of 7µB, which may imply an intermediate Eu valence or an incomplete filling of the lanthanide site.
Tb filled skutterudites
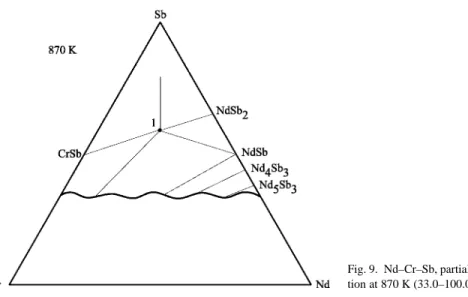
Low temperature magnetization curves for GdRu4P12 indicating a field-induced phase transition (Sekine et al., 2000a). The magnetization data also indicate a field-induced phase transition at low temperatures for magnetic fields≈5–6 T (fig. 19).
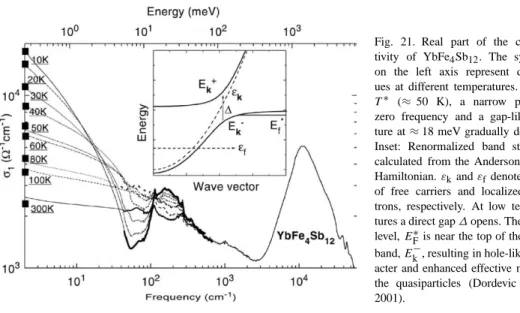
Yb filled skutterudites
Filled skutterudite thermoelectrics
Electrical transport in charged skutterudites is altered by the presence of rattles. The high carrier concentrations in filled skutterudites are mostly due to the proportion of lanthanide sites that remain.
Concluding remarks
Only at temperatures in the temperature range of 600–900 K are the thermoelectric properties of the filled skutterudite antimonides of interest for use in power generation applications. The filled skutterudite antimonides have demonstrated the validity of the "electron-crystal, phonon-glass" idea in the design of new thermoelectric materials for operation at elevated temperatures.
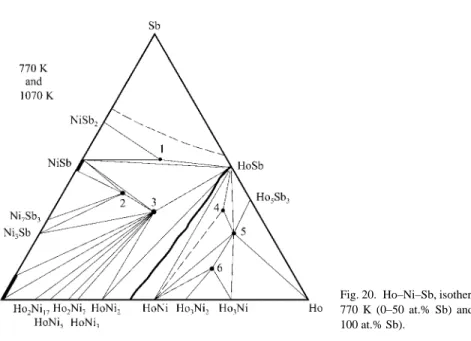
RARE EARTH – ANTIMONY SYSTEMS
Binary systems
The crystal structure of the La3TiSb5 compound was studied by Bollore et al. Files of the lanthanum were prepared under argon and annealed with the corresponding amounts of antimony in evacuated sealed silica tubes for two days at 723 K, followed by five days at 1023 K. The resulting antimonide LaSb was ground together with appropriate amounts of Fe and Sb, pressed to pellets and sealed in evacuated silica tubes. The alloys were obtained via a powder metallurgical reaction. the sintering of Zn and Sb powders with fine shavings of neodymium.
The crystal structure of the ternary phase ~NdZn2Sb2 (1) has not yet been determined (Sologub and Salamakha, 1999). Samarium coils were prepared under argon and annealed with appropriate amounts of antimony in sealed evacuated silica tubes for two days at 723 K, followed by five days at 1023 K. The resulting SmSb antimonide was milled together with appropriate amts. of Ru and Sb. pressed into pellets and sealed in evacuated silica tubes. Morozkin and Sviridov (2001) investigated the crystal structure of the LuZrSb compound using X-ray powder diffraction.
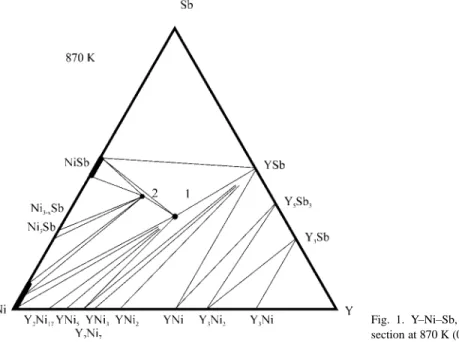
Quaternary systems
Structure types of the ternary antimonides
Crystallographic data for alloys EuCu1.75As2–EuCu2Sb2 Composition Type of structure Lattice parameters, nm.
The resistivity also shows a broad maximum at 113 K, followed by a rapid rise below 21 K. The latter indicates the formation of holes in the electronic density of states. The magnetic behavior of the RMnSb2 compounds is characterized by the onset of ferromagnetism of the Mn sublattice in the temperature range between 100 and 520 K. The temperature dependence of the magnetic susceptibilityχ (T) for R3Cu3Sb4,R=Ce, Pr, Nd, Gd , Tb , Dy, Er can be described by the Curie-Weiss law with.
1998) reported on measurements of magnetic susceptibility, electrical resistance and magnetoresistance for the Ce3Rh3Sb4 in the temperature range from 2 K to 300 K. The compound was found to order antiferromagnetic at 22 K. The magnetic resistance shows a broad maximum at about 50 K. Magnetic measurements were performed up to the temperature of liquid helium by Sologub et al.
Peculiarities of the interaction of the rare earths and antimony 1. Binary systems
As can be seen from the previous sections, the interaction between the components of the R-M-Sb ternary systems has not been sufficiently investigated. The compounds of the Y3Au3Sb4 type have not been observed for the systems with silver. The structure of the LaSnSb2 compound (Cmcm space group) is related to the structure of YbSb2 (ZrSi2structure type, Cmcm space group).
Interaction of the components of ternary systems Ce–(Si, Ge)–(C, Sn, Sb, Bi), phase equilibria and crystal structure of compounds.
THERMODYNAMIC PROPERTIES OF THE LANTHANIDE(III) HALIDES
Polymorphism 1. LnF 3
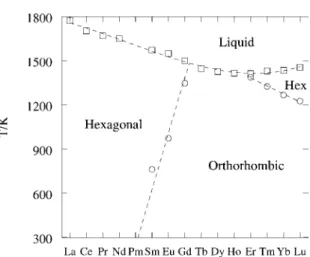
Four crystallographic modifications have been reported for the lanthanide trifluorides (Greis and Haschke, 1982; Meyer and Wickleder, 2000). The minimum in the melting curve of the lanthanide trichlorides occurs at TbCl3, where there is a change in the structure of the LnCl3, as was the case for the trifluorides. The reported melting points for the tribromides are in good agreement as shown in Table A.3 of Annex A.
The minimum in the melting curve of the lanthanide tribromides occurs in SmBr3 or possibly PmBr3.
Low-temperature heat capacity and standard entropy of the solid trihalides
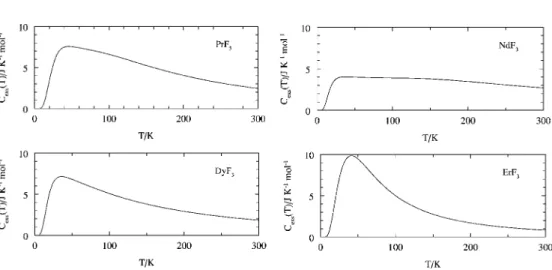
These crystal field states have been identified for most of the lanthanide trifluorides and are summarized in Table 2. This is evident from a plot of Cp values at 298.15 K as a function of atomic number as shown in fig. 8 Experimental entropies for the lanthanide trifluorides are plotted as a function of atomic number.
In the case of PmF3, spectroscopic data are missing and the gender is calculated from the degeneracy of the ground state of the lanthanide ion.
High-temperature heat capacity of the solid trihalides 1. LnF 3
THERMODYNAMIC PROPERTIES OF THE LANTHANIDES(III) HALIDES 165 Table 8. Recommended high temperature heat capacity functions for the solid lanthanide trifluorides. only nominal comparisons, tend to deviate at low temperatures in several of their measurements. In analogy to the approach described in the section on the low-temperature heat capacity, the high-temperature heat capacity of the LnX3 compounds can be described as the sum of the lattice and excess contributions (cf. Also the results of Gaune-Escard et al 1996) agree well, although they indicate a somewhat different slope of the curve.
For this connection, the results of Gaune-Escard et al. 1996) is significantly lower than in the other two studies, which agree very well.
Enthalpy of formation of the solid trihalides 1. LnF 3
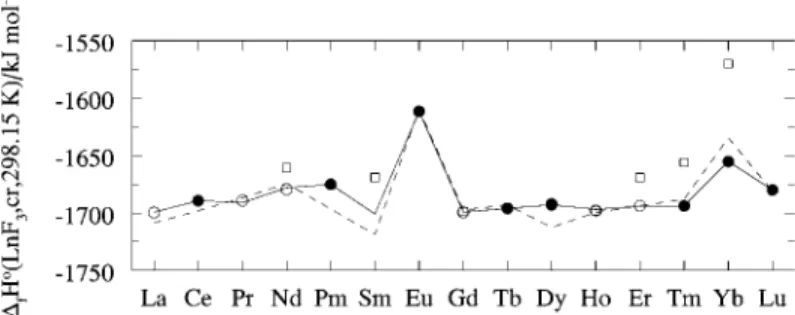
Only in the case where the enthalpy of formation of the sesquioxide is based on the combustion of the lanthanide metal are the two schemes truly independent. For the calculation of the formation enthalpies of the tribromides and triiodides, the same reaction cycles were used as for the trichlorides. The variation of the formation enthalpies of the trichlorides, tribromides and triiodides is shown in Fig.
Enthalpy of formation of lanthanide trichlorides, tribromides and triiodides as a function of atomic number.
Heat capacity of the liquid trihalides 1. LnF 3
Fusion entropies (◦) and sum of transition and fusion entropies (•) of LnF3 compounds. Heat capacity of liquid lanthanide trichlorides as a function of volume change V / Vcr. Enthalpy of fusion and liquid phase heat capacity for lanthanide tribromides and triiodides.
The heat capacity of the liquid tribromides is assumed to show a slight trend, those of the triiodides being approximately constant (table 15).
Heat capacity of the gaseous trihalides 1. LnX 3 monomers
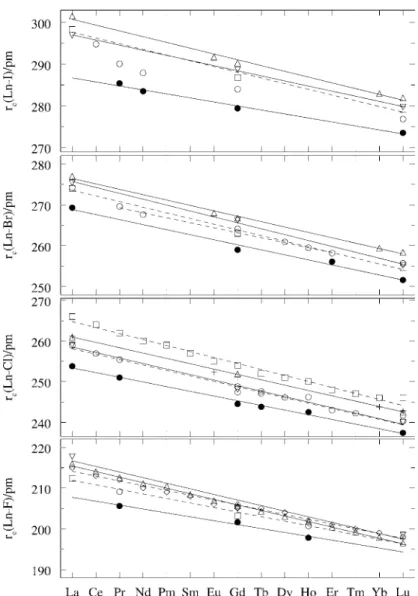
However, this compound undergoes a solid-state transition, as previously discussed, and the sum of the entropies of transition and fusion is close to (54 J·K−1·mol−1). For the orthorhombic tribromides, no experimental data are available, and we have assumed that the entropy of fusion is close to that of the isostructural triiodides. The bond length of the lanthanide trihalides;•, experimental values from ED (re);◦, experimental values from ED (rg);, Adamo and Maldivi (1998) for the BP-DS/TZ,TZd level;♦Joubert et al. 1991) at the CISD+Q/ECPDf,ECPDdlevel;Adamo and Maldivi.
The structure and vibrational spectra of the lanthanide trihalides have been extensively studied over the past decade.