The volume concludes with a comprehensive and critical compilation of the thermodynamic properties of all rare earth elements. Rogl, Phase equilibria in ternary and higher order systems with rare earth elements and silicon 1.
INTRODUCTION
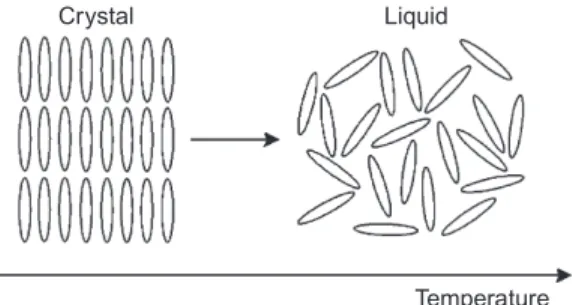
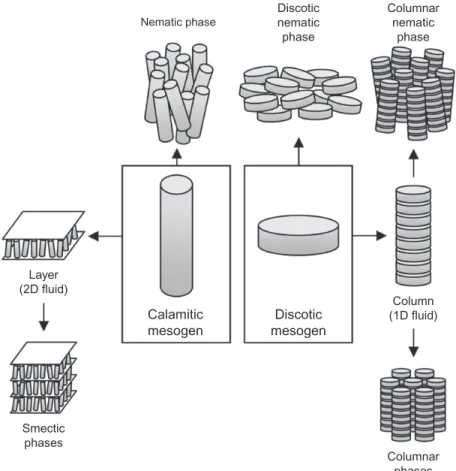
A detailed description of the molecular order in mesophases can be found in the text. Thus, the cylinder represents the average (or effective) shape of the molecule in the liquid crystal structure.
CHARACTERIZATION OF MESOPHASES
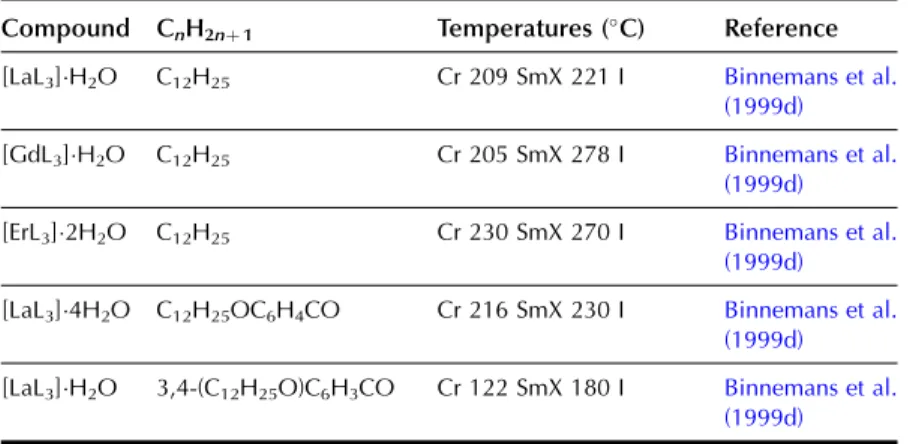
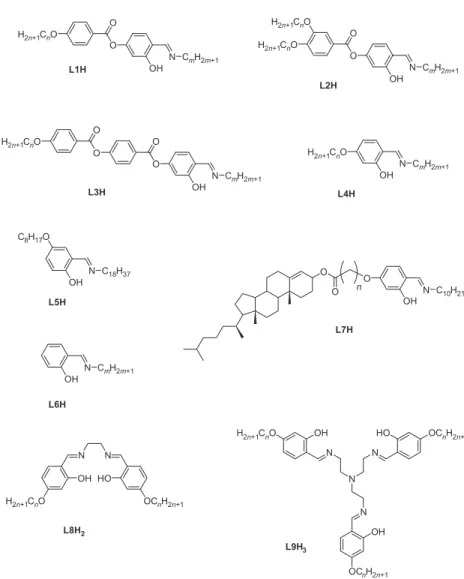
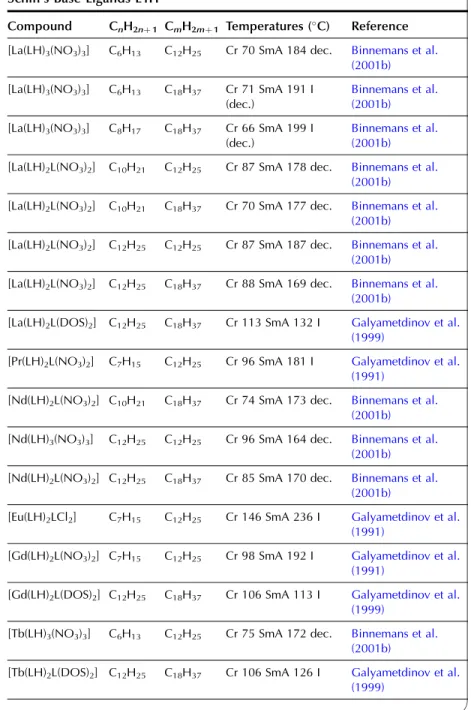
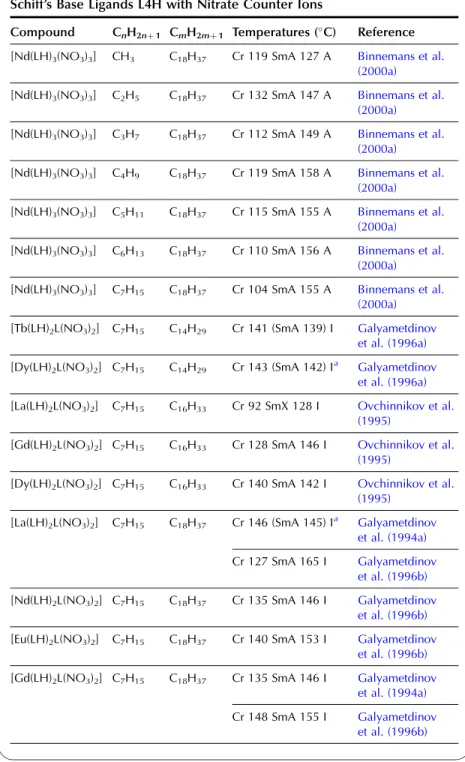
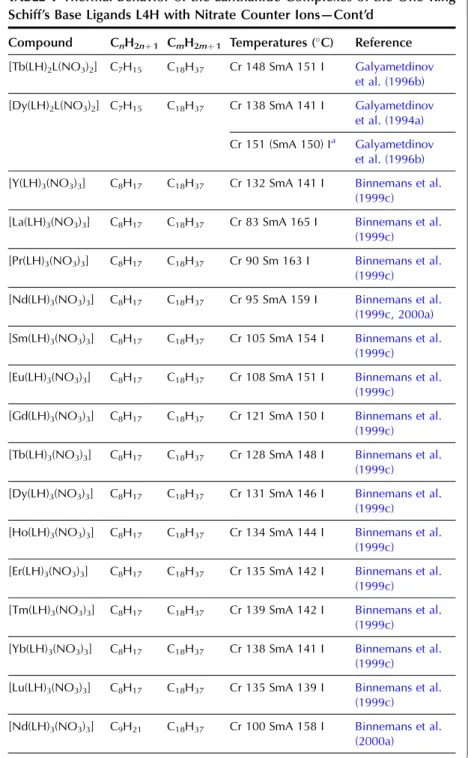
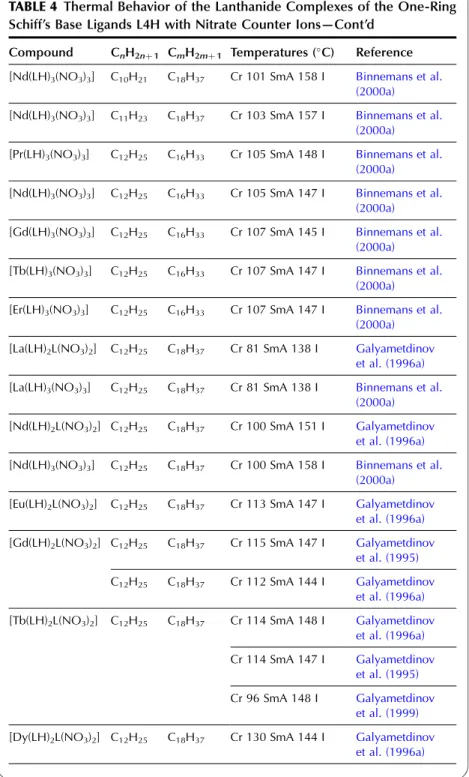
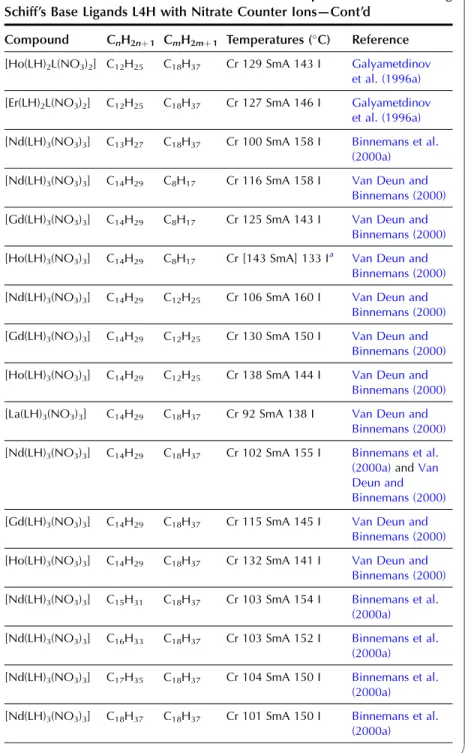
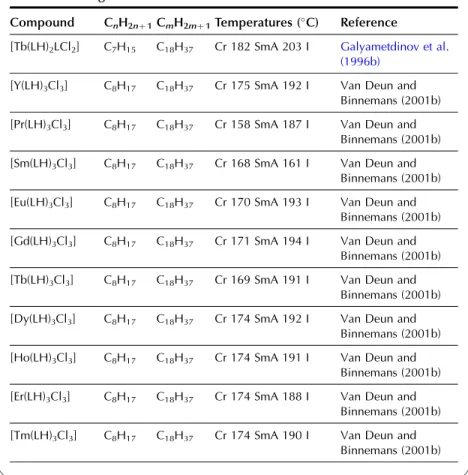
In the wide-angle region of the X-ray pattern, a broad signal is observed at ca. AMAchains. Figure 20 shows a schematic representation of the cross-sectional area of the rigid cores in the SmA (AcoreAchainsAM) and SmC (Acore All the lanthanide complexes of one-ring salicylaldimine Schiff's base ligands exhibit a smectic A phase. Although crystal structures of the non-mesomorphic Schiff's base complexes have been determined (Binnemans et al., 2000a; work on the Schiff's base adducts of the [R(dbm)3] complexes was later extended to all other lanthanide(III) ions, except cerium(III ) and promethium(III) (Binnemans et al., 2003a). The transition temperatures of the benzoyl trifluoroacetonate complexes [R(bta)3(LH)2] were lower than those of the 2-thenoyl trifluoroacetonate complexes [R(tta)3(LH)2]. The transition temperatures of the yttrium(III) complexes are very comparable to those of the dysprosium(III) and holmium(III) complexes. In Table 9, an overview is given of the transition temperatures of the Schiff's base adducts to the lanthanide(III) tris b-diketonate complexes. By substitution in TABLE 10 Thermal behavior of lanthanide complexes of N-Aryl Schiff base ligands L12H. 2010). Only a minor influence of the lanthanide ion on the transition temperatures was found (Kharitonova et al., 1996). There is a similarity between the molecular arrangement of phthalocyanine complexes in a columnar mesophase and a 1D conducting organic crystal. The yield of the reaction decreased from 50% to 30% for the octyloxymethyl to octadecyloctomethyl chain. Compared to metal-free phthalocyanines, the enthalpy changes of [(CnH2nþ1OCH2)8Pc]2Lu complexes are large (Piechocki, 1985). The ordered nature of the mesophase is evidenced by a rather narrow peak found at large angles in the XRD patterns. An increase in chain length in the series [(CnH2nþ1O)8Pc]2Er (n seemed to influence the clearing temperatures only slightly. In the large angular region of the XRD diffractograms, two peaks due to the stacking distance h1(ca. Complexes containing tetra-alkylated terphenyl groups at the 5,15 position of the parent porphyrin show only a hexagonal columnar phase (Colh) with one stacking distance. Based on the extinction rules for 2D lattices, the rectangular columnar phase was found to have P2/a symmetry. The differences in mesomorphism were attributed to differences in the steric hindrance of the side chains to rotation along the axis connecting the center of the three porphyrins. In addition to the mesomorphic properties, the electrochemical and spectroscopic properties of the ternary complexes have also been reported. Also similar alkyl chain length complexes between hexyloxy and hexadecyloxy formed a hexagonal columnar phase (Qi and Liu, 2003). Hydroxo complexes of tetra [(p-alkyloxy-m-ethyloxy)phenyl]porphyrins L38 with lanthanide(III) ions exhibit a hexagonal columnar mesophase (Yu et al., 2005). By changing the stoichiometric ratio it is possible to obtain mixed compounds of the type [RClx(CnH2nþ1COO)3x].Misra et al. 1987) prepared lanthanide(III) alkanoates with three different alkyl chains within one and the same compound by stepwise substitution of the isopropoxide groups in lanthanide(III) isopropoxides. Also, the coordination of water can help saturate the coordination sphere of the lanthanide(III) ion. Mesomorphism of the lanthanide(III) soaps was first observed by Burrows and colleagues in a series of cerium(III) alkanoates (Marques et al., 1998). It is not easy to determine whether some of the lanthanide(III) alkanoates form a mesophase or not. The thermal history and hydration history of the lanthanide(III) alkanoates do not appear to affect first-order transition temperatures, but rather the heat changes and temperature range of second-order (or weakly first-order) transitions that continue the main first-order transition (melting effects). The corresponding compounds of the heavier lanthanides (R¼Nd, Sm, Eu) are not liquid crystalline. Singh et al. By creating a hydrophobic shell around the polyoxometalate cluster, water molecules are excluded from the immediate environment of the POM. Faul and co-workers obtained LCs at room temperature by complexing a compressed europium-containing polyoxometalate [Eu(SiW9Mo2O39)2]13 with a series of cationic surfactants (Zhang et al., 2005). Stable smectic mesophases have been observed in materials based on the surfactant N-[12-(4-carboxylphenoxy)dodecyl]-N-dodecyl-N,N-dimethylammonium, a benzoic acid-terminated surfactant (Yin et al., 2009). ). Eu(BW11O39)2]15 was combined with cationic surfactants containing biphenyl groups in their chains (Li et al., 2008a). Thermotropic mesophases were observed for combinations of the europium-exchanged derivative of a Pressler-type polyoxometalate, [EuP5W30O110]12, in combination with hydrophobic cations such as dihexadecyldimethylammonium, dioctadecyldimethylammonium, and trioctadecylmethylammonium (Zhang et al., 2009b). ). The [EuP5W30O110]12 anion exhibits strong luminescence and exhibits electrochromism (that is, the color of solutions turns blue upon reduction). A synthetic route was proposed in which a rare earth ion promotes the decomposition of the Habbeic ligand to a symmetrical azine. The transition temperatures changed as a function of the lanthanide ion: the stability region of the mesophase became narrower as the size of the lanthanide ion decreased. The introduction of a lanthanide ion into the macrocyclic cavity results in the solidification of the macrocyclic system and promotes the formation of the mesophase. Although complexes of ligands with only one or two alkyl chains in the aryl group were not liquid crystalline, some of the complexes of a ligand with three tetradecyloxy chains were mesomorphic. Light lanthanide complexes (R¼La, Pr, Nd, Sm, Eu) formed a cubic mesophase, while heavy lanthanide complexes (R¼Gd, Tb, Dy, Ho) did not show any thermotropic mesomorphism. The solid phase transformation of the 1:2 complex to the 1:1 complex occurred when the compound was heated to 183–200 K. The widest mesophase region was observed for a mixture consisting of 65 wt% lanthanum(III) dodecyl sulfate and 35 wt% ethylene glycol. The mesophase behavior of C12EO10:La(NO3)36H2O/water can be modified by adding the polymer poly(ethylene nime) (Zakharova et al., 2007). A stable mesophase was observed within the La(NO3)36H2O concentration was between 3.2 and 40 wt%, whereas the samples crystallized out at higher salt concentrations. The nematic phase occurs for the following composition: 45 wt% C12DMAO/lanthanum(III) complex, 50 wt% water and 5 wt% dodecanol. Of note is the fact that the nematic phase is already observed at temperatures as low as 5°C. Clear point was found to increase with increasing concentration of surfactants in the system. At gadolinium(III) oleate concentrations below 1% by weight, phase separation occurred with the formation of a lamellar phase rich in gadolinium(III) oleate and a cubic phase. A review of the luminescence of metallomesogens (including lanthanide mesogens) in the liquid crystalline state is published by Binnemans (2009). These differences were explained by the orientational effects of the chelate molecules in the LC solvent. The photoluminescence intensity of europium (III) and terbium (III) complexes dissolved in nematic LC 4-(isothiocyanotophenyl)-1-(trans-4-hexyl) cyclohexane (6CHBT) strongly depends on the strength of the applied electric field ( Palewska et al. , 2007). Interestingly, the emission color of the europium(III)-containing LC mixture could be tuned from blue (emission color of the host matrix) to red (emission color of Eu3þ), depending on the excitation wavelength and the counterion of europium(III). The magnetic alignment arises from the anisotropic magnetic energy of the LCs (Yamaguchi and Tanimoto, 2006). This decrease can be attributed to the melting of the alkylthio chains with a resulting increase in disorder in the columns. The ion pairing effects are responsible for the shift of the reduction potentials of these compounds. Four or more ligands (or two or three bidentate ligands) can bind to the uranyl ion in the equatorial plane. The uranyl ion is chemically a very stable oxocation and it is very difficult to remove one or both oxygen atoms. Extending the chain length leads to a wider range of mesophase stability, but otherwise the properties of these complexes are similar (Elliott et al., 2002). The uranyl complexes were not photoluminescent in their pure form due to auto-quenching, but luminescence in the visible spectral region could be observed after dilution of the complex in an ionic liquid. Decreasing the concentration of acetone led to a narrowing of the temperature range of the existence of the nematic mesophase. It has been criticized that the liquid-crystalline behavior of the lanthanide mesogens only marginally affects their magnetic and photophysical properties (Gimenez et al., 2002). The relationship between the size of the lanthanide ion and the thermal properties of lanthanide mesogens is not yet well understood. Although light lanthanides (La to Eu) are generally more abundant in the earth's crust, and find large-scale practical use, all the rare earth elements are of critical importance to consumers. Rare earths recycle rare earths from the internal scrap (scrap created during production processes), while rare earths from the external scrap (scrap from industrial products) are almost never recycled (Goonan, 2011; JOGMEC, 2010). While details of rare earth recycling practiced by various companies are generally owned by companies, a significant number of academic papers and reports have been published to develop the technologies for recycling both the internal and external wastes. Given that studies on rare earth recycling have increased dramatically recently, this is a good opportunity to thoroughly review the current state of rare earth recycling. To this end, there is a need not only for the establishment of a social system to recover rare earth products at the end of their useful life, but also for the development of highly efficient recycling technologies. An advantage of recycling rare earths over processing natural ores is that wastes containing rare earth products generally have much simpler chemical compositions than the natural ores, eliminating or greatly simplifying the separation process. Another advantage is that the problem of dealing with radioactive waste, which is almost always present in rare earth mining, is no longer present in the recovery of rare earths from waste. Natural rare earth ores often contain significant amounts of thorium, which must be separated and safely disposed of during processing. Since industrial products do not contain thorium, the recovery of rare earths from waste does not cause such a problem. From these viewpoints, we review the recent literature on the recycling of rare earths from various waste rare earth materials such as permanent magnets, phosphors, secondary nickel metal hydride batteries, polishing powders, optical glass, and catalysts.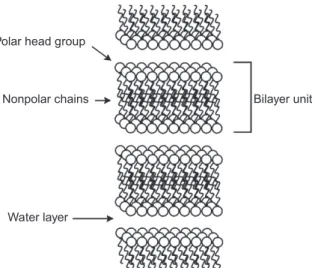
SCHIFF’S BASE COMPLEXES
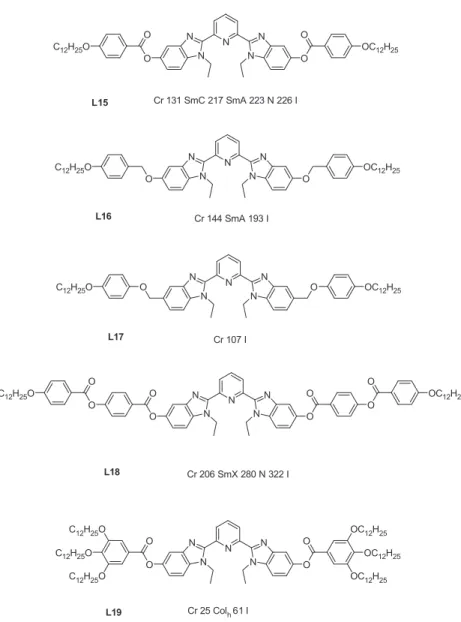
BIS(BENZIMIDAZOLYL)PYRIDINE COMPLEXES
PORPHYRIN COMPLEXES
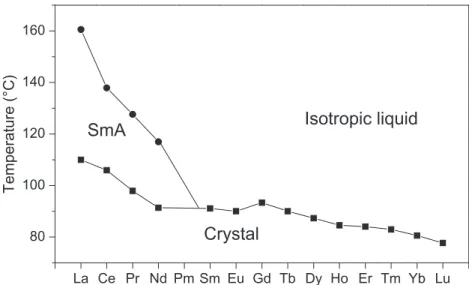
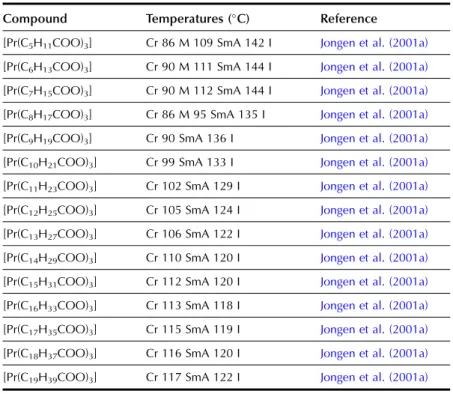
LANTHANIDE ALKANOATES
POLYOXOMETALATE-SURFACTANT COMPLEXES
MISCELLANEOUS THERMOTROPIC LANTHANIDOMESOGENS
LYOTROPIC LANTHANIDOMESOGENS
PHYSICAL PROPERTIES 1. Luminescence
![FIGURE 62 Luminescence spectra of [Eu(tta) 3 L 2 ] (gray curve) and [Eu(bta) 3 L 2 ] (black curve) as a thin film in the mesophase at 25 C](https://thumb-ap.123doks.com/thumbv2/123dok/10272262.0/158.648.158.492.104.399/figure-luminescence-spectra-gray-curve-black-curve-mesophase.webp)
ACTINIDOMESOGENS
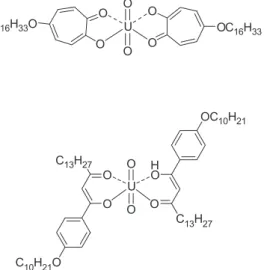
CONCLUSIONS AND OUTLOOK
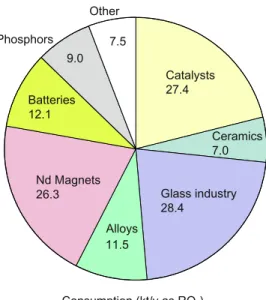
PERMANENT MAGNETS
![FIGURE 30 DSC trace (second heating and cooling run) of the complex [Er(LH) 3 (DOS) 3 ].](https://thumb-ap.123doks.com/thumbv2/123dok/10272262.0/85.648.132.510.458.715/figure-dsc-trace-second-heating-cooling-complex-dos.webp)