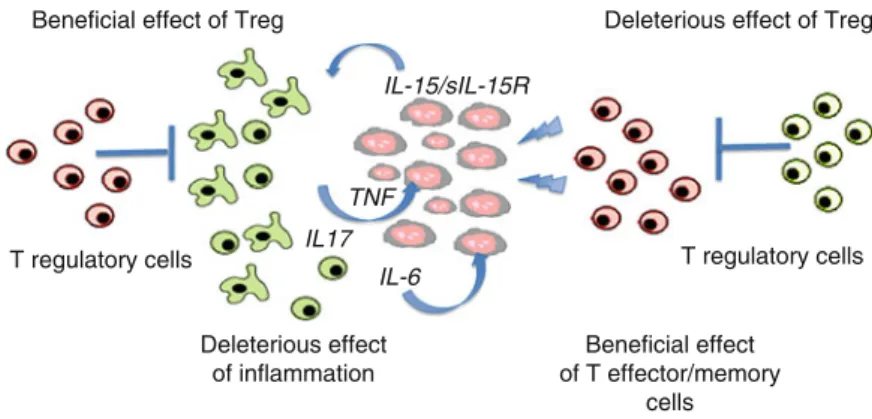
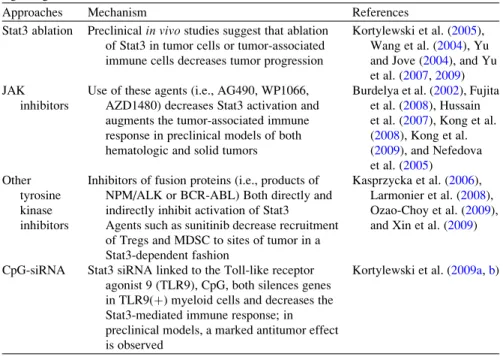
TLRs are pattern recognition receptors for pathogen-associated molecular patterns (PAMPs) and endogenous molecules released from damaged and necrotic cells (DAMPs) (Kumar et al.2009). This, in turn, results in the reversal of immune tolerance induced by Tregs (Kong et al. 2009). Also, the amount of tumor infiltrating Tregs is reduced with imatinib therapy (Larmonier et al. 2008).
Miyara et al.2009), human CD4+ T cells with suppressor function (in vitro) were shown to have two main phenotypes, including Foxp3loCD45RA+ resting Tregs (rTregs) and CD45RA!Foxp3hi-activated Tregs (aTregs). This reagent profoundly depletes Tregs and enhances antitumor vaccines in mice (Litzinger et al. 2007). Furthermore, antigen-presenting cells (APC) such as dendritic cells (DC), macrophages and granulocytes can recognize non-self structures via toll-like receptors (TLR) or C-type lectin receptors (CLRs) (Janeway 1989) Figdor et al . 2002).
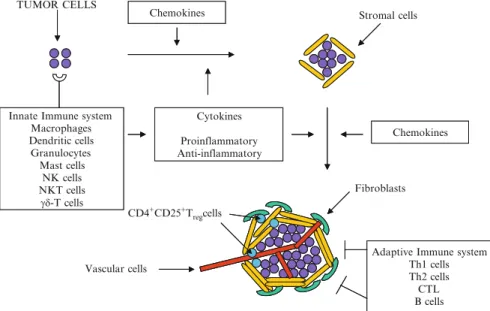
Such self-reactive T cells are regulated by CD4+CD25+T regulatory (Treg) cells (peripheral tolerance) (Vignali et al. 2008). These cells appear to be involved in the recently described innate immunity against tumor cells (Cui et al. 2003). CTLA-4 expression and function can be regulated using anti-CTLA4 antibody therapy (Quezada et al. 2008).
Downregulation of VEGF further prevents disruption of DC formation and maturation (Wang et al. 2004). Several of these therapies have produced significant results (especially ACT), while other strategies have been less successful (cancer vaccines) or have caused significant side effects (severe autoimmunity in patients treated with anti-CTLA-4) (Phan et al 2003). . Furthermore, blockade of VEGF partially reversed thymic atrophy in tumor-bearing mice (Ohm et al. 2003).
ETBR may promote angiogenesis indirectly by upregulating VEGF production in the vasculature (Jesmin et al. 2006). Our laboratory recently demonstrated a novel role for ETBR in tumor immunotherapy (Buckanovich et al. 2008). Bataille et al.1989) because a similar syndrome has been observed in our experiments with neuroblastoma (described below).
Human Dendritic Cell Subsets
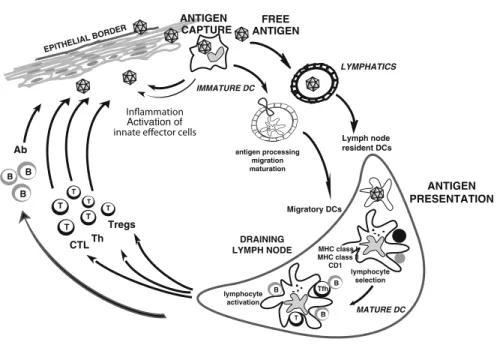
In the steady state, non-activated (immature) DCs present self-antigens to T cells, leading to tolerance (Hawiger et al.2001; Steinman et al. 2003). Once activated (mature), antigen-loaded DCs aim to launch antigen-specific immunity (Finkelman et al. 1996; Brimnes et al. 2003), leading to the proliferation and differentiation of T cells into helper and effector cells. Our recent studies in humans indicate that the acquisition of the Tfh phenotype and function is dependent on IL-12p70 (Schmitt et al.2009).
DCs reside in the tissue where they are ready to capture antigens (Geissmann et al.2010). Antigen can also reach draining lymph nodes without involvement of peripheral tissue DCs and can be captured and presented by DCs resident in the lymph nodes (Itano et al.2003). These findings may explain the modest clinical efficacy of systemic IL-12 administration in cancer patients (Motzer et al.2001; Cheever2008).
LCs induce strong proliferation of naïve allogeneic CD8+T cells compared to CD14+DCs (Klechevsky et al. 2008). Therefore, they are extremely more effective at killing target cells; especially tumor cells expressing low levels of peptide/HLA complexes (Klechevsky et al. 2008). IL-15 could explain the remarkable effects of LC in the development of cytotoxic T lymphocyte (CTL) responses (Mohamadzadeh et al. 2001; Dubsky et al. 2007; Klechevsky et al. 2009).
Plasmacytoid DCs (pDCs) are considered to be the frontline of antiviral immunity because they possess their ability to rapidly produce large amounts of type I interferon (Siegal et al. 1999; Liu 2005). Human pDCs are actually composed of two subsets, characterized by the expression of CD2 (Matsui et al. 2009).
DCs in Tumor Environment
This inhibition appears to be dependent on the binding of ILT7 to the binding of pDCs by BST2 expressed on tumor cells (Cao et al. 2009). Likewise, in ovarian carcinoma, tumor-infiltrating pDCs do not induce CD8+ effector T cell responses, but rather induce the differentiation of IL10+CCR7+CD8+Tregs (Wei et al.2005). Finally, pDCs can promote tumor angiogenesis through the secretion of proangiogenic cytokines (Curiel et al.2004; Coukos et al.2005).
DC can fight back tumors through at least two pathways: an indirect one with the induction of potent CTL responses, and a direct one through DC-dependent tumor cytotoxicity. For example, pDCs appear to contribute directly to the anti-tumor activity of in vivo administered Imiquimod (TLR7 ligand), which is used for the treatment of basal cell carcinoma (Urosevic et al.2005; Panelli et al.2007; Stary et al. al. 2007). Clearly, understanding the functions of DCs in the tumor bed represents an important area of future investigation and exploitation for therapy.
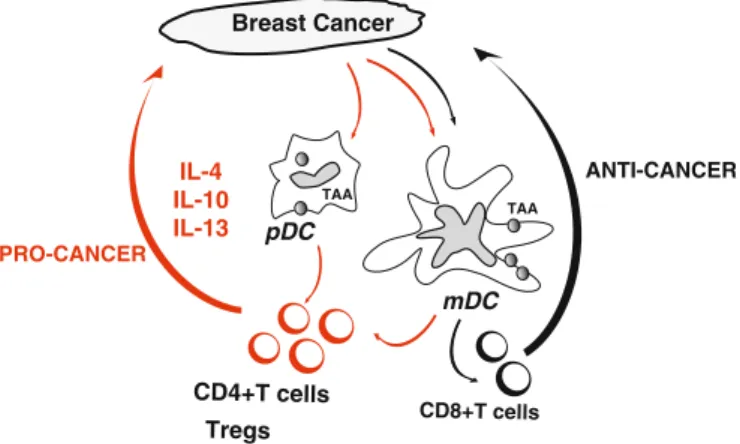
An interesting strategy would be to transform their molecular pathways from “pro-tumor” DCs to “anti-tumor” DCs. Cancer cells attract immature DCs possibly through chemokines such as MIP3 alpha and/or SDF-1. DC can then be either blocked or distorted in their maturation, for example by VEGF, leading to the induction of polarized CD4+T cells that promote the proliferation of cancer cells (pro-cancerous) at the expense of CD8+ T cells that can cause tumor regression (anti-cancer ).
Outcomes of Current DC Vaccination Trials
The Quality of Elicited Antigen-Specific Immune Responses
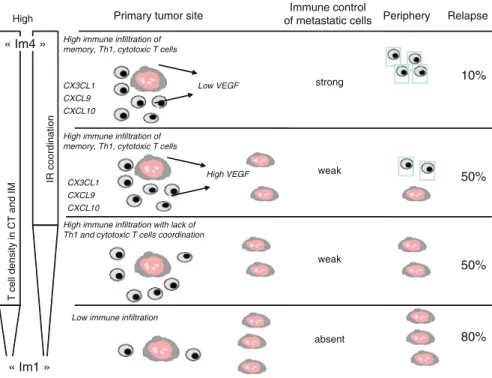
Indeed, the immune responses elicited by the first generation of DC vaccines may not be of a quality to enable the rejection of bulky tumors. In addition, T cells with low avidity may be unable to recognize and/or kill peptide-MHC class I complexes on tumor cells (Appay et al. 2008). Finally, the tumor microenvironment can suppress the functions of effector T cells, for example through the action of myeloid-derived suppressor cells and Tregs, as summarized in recent reviews (Gabrilovich and Nagaraj 2009; Menetrier-Caux et al. 2009).
Recent advances in immunomonitoring of specific immune responses in the blood and at the tumor site should help us address these questions (Palucka et al. 2006; Vence et al. 2007; Butterfield et al. 2008; Janetzki et al. 2009; Tahara et al others 2009). Modern approaches involving polychromatic flow cytometry instead of single cytokine analysis (e.g. IFN-gELISPOT) and/or the frequency of tetramer positive cells will contribute to a better assessment of the quality of immune responses elicited in patients (Kammula et al. 1999; Lee et al. .1999). Indeed, several studies, mostly conducted in the context of HIV vaccines, have led to the conclusion that measuring the frequency of IFN-g-secreting CD8+ T cells alone is insufficient to assess the quality of vaccine-induced immunity (Wille-Reece et al. 2006; Appay et al. 2008; Seder et al. 2008).
Optimal DCs
Studies in mice show that specific targeting of antigen to DCs in vivo results in a significant enhancement of antigen-specific CD4+ and CD8+ T cell immunity when the DC maturation signal is provided (Hawiger et al.). For example, TLR2 ligation promotes the induction of Tregs rather than Th1 or Th17 cells (Manicassamy et al. 2009), does not appear to be a preferred option for cancer vaccines. These groundbreaking studies have already been extended to demonstrate targeting of tumor antigens to DCs (Caminschi et al.2009) and Langerhans cells (LCs) in animal models (Flacher et al. and generation of antitumor immunity (Wei et al.2009) .
Candidate tumor antigens include: (a) unique (mutated) antigens; and (b) shared self-antigens including cancer/testis antigens and tissue differentiation antigens (Gilboa1999; Vlad et al.2004; Boon et al.2006; Parmiani et al.2007). Mutated antigens are postulated to have several advantages, for example their specific T-cell repertoire should not be deleted as they are not recognized as "self" by immune cells (Parmiani et al.2007). It has now been shown that the disease is due to the development of cdr-2 specific CD8+CTL (Albert et al. 1998).
The list of onconeural antigens is growing and in addition to cdr2, two other antigens such as Nova and amphiphysin are emerging as potential targets of the immune system (Floyd et al.1998; Rosin et al.1998). A major shift in antigen target selection could be brought about by the identification of cancer stem cells (Jordan et al.2006; Polyak and Hahn2006; Rossi et al.2008). The importance of stem cell-associated antigens in malignancies is best illustrated by the presence of SOX-2-specific immunity in patients with monoclonal gammopathy (Spisek et al.2007).
So far, all antigenic targets are protein antigens whose peptides can be presented on the cell surface in the form of complexes with classical MHC molecules (Townsend et al. 1985). However, tumors express altered lipids and sugars that can be bound by CD1 molecules on APCs and presented to NKT cells as well as T cells (Beckman et al.1994; Fujii et al.2002; Hava et al.2005).
Combining DC Vaccines with Other Therapies
The discovery of onconeural antibodies led to the proposal that paraneoplastic cerebellar degeneration (PCD), associated with breast and ovarian cancer, is an autoimmune disease mediated by the humoral arm of the immune system. These antibodies allowed the cloning of the cdr2 antigen, a protein with a coil/leucine zipper domain. Although most studies have focused on eliminating mature cancer cells with limited proliferation capacity, it seems more efficient to target the self-renewing cancer stem cells.
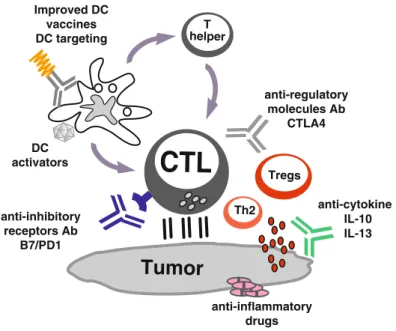
Ideal target genes would be those shared between cancer cells and germ cells, which are necessary for the survival of cancer cells, but which are not expressed in adult stem cells (Dhodapkar2010). In particular, antibodies, or other soluble antagonists such as engineered receptors, can be used to block suppressive cytokines in the tumor microenvironment such as IL-10 (Moore et al. 2001), IL-13 (Terabe et al. 2000) , TGF-b(Li et al.2005; Terabe et al.2009) and VEGF (Gabrilovich2004; Rabinovich et al.2007). However, the clinical efficacy of current approaches is limited, possibly because tumors invade the immune system by myeloid-derived suppressor cells, type 2 inflammatory T cells, and regulatory T cells (Tregs).