저작자표시-비영리-변경금지 2.0 대한민국 이용자는 아래의 조건을 따르는 경우에 한하여 자유롭게
l 이 저작물을 복제, 배포, 전송, 전시, 공연 및 방송할 수 있습니다. 다음과 같은 조건을 따라야 합니다:
l 귀하는, 이 저작물의 재이용이나 배포의 경우, 이 저작물에 적용된 이용허락조건 을 명확하게 나타내어야 합니다.
l 저작권자로부터 별도의 허가를 받으면 이러한 조건들은 적용되지 않습니다.
저작권법에 따른 이용자의 권리는 위의 내용에 의하여 영향을 받지 않습니다. 이것은 이용허락규약(Legal Code)을 이해하기 쉽게 요약한 것입니다.
Disclaimer
저작자표시. 귀하는 원저작자를 표시하여야 합니다.
비영리. 귀하는 이 저작물을 영리 목적으로 이용할 수 없습니다.
변경금지. 귀하는 이 저작물을 개작, 변형 또는 가공할 수 없습니다.
치의과학 석사학위논문
Human Dental Epithelial Cells Induce Odontogenic Differentiation of SHED
사람 치아 상피세포에 의한 치수 줄기세포의 상아모세포 분화 유도
2019년 2월
서울대학교 대학원 치의과학과 분자유전학 전공
채 근 영
Abstract
Human Dental Epithelial Cells Induce Odontogenic Differentiation of SHED
Geun Young Chae
Molecular Genetics Major
Graduate School, Seoul National University
(Directed by Prof. Gene Lee)
Tooth organogenesis and regeneration occur through reciprocal interaction between epithelial and ectomesenchymal stem cells. This cell-to-cell communication is also a key regulator in the differentiation of ameloblasts and odontoblasts which secrete enamel and dentin, respectively. In studying tooth regeneration, the dental pulp has been widely used as the source of mesenchymal stem cells. On the contrary, most of the epithelium is lost after tooth eruption and root completion, and thus have not been vastly explored.
Recently, prior research has characterized dental epithelial cells known as Hertwig’s epithelial root sheath/epithelial rests of Malassez (HERS/ERM) extracted from human periodontium and its cell line was established.
However, the epithelial-mesenchymal signaling capacity of HERS/ERM has
yet to be elucidated. Thus, this study was conducted to elucidate the effect of HERS/ERM conditioned medium (CM) on the odontogenic differentiation of stem cells from human exfoliated deciduous teeth (SHED). To simulate the effect of epithelial paracrine actions on mesenchyme, SHED were cultured in differentiation medium supplemented with varying proportions of CM derived from the HERS/ERM cell line. The CM collected was freeze-dried for further concentration of the solution, and the potency of CM was tested at 1X (differentiation medium supplemented with 10 v/v% CM), 4X and 8X concentration factors, using freeze-dried CM for the 4X and 8X treatments.
To determine the effects of the varying concentration of CM, Alizarin red S staining, RT-qPCR, Western blot, and DAPI nuclear staining were assessed and the results were compared to SHED treated with the freeze-dried basal media of CM. Alizarin red S staining revealed that increasing the concentration of CM treatment had a distinctive impact on the amount of calcium nodule formed in the differentiated SHED. Expression levels of mineralization-related markers, RUNX2, BSP, DMP1, ON, OC, and MEPE also confirmed the enhanced odontogenic effect of CM concentration on day 8 and 12 as CM concentration was increased. In contrast, the addition of freeze-dried basal media exhibited a lack of calcium nodule formation and no significant changes in the mineralization-related mRNA expression levels.
The expression of dentin phosphoprotein, an odontoblast marker observed by Western blot, was also more prominent in CM-treated SHED than in basal media-treated SHED on both day 16 and 20. Also, long-term culture of SHED
with CM exhibited cell death, whereas the basal media control group did not.
The DAPI nuclear staining revealed a phenomenon similar to the terminal differentiation of cells. The data indicate that CM from human HERS/ERM cell line has odontogenic differentiation capabilities that are concentration- dependent, and further investigations may contribute to the discovery of specific growth factors and cytokines at play. This study is the first report of odontogenic induction potential of concentrated CM derived from the human dental epithelial cell line.
………
Keywords : Hertwig’s epithelial root sheath/epithelial rests of Malassez (HERS/ERM), stem cells from human exfoliated deciduous teeth (SHED), epithelial-mesenchymal interaction, conditioned medium, tooth
development and regeneration, odontogenic differentiation Student Number : 2017-24083
4
Tables of Contents
Introduction ... 5
Literature Review ... 7
Materials and Methods ... 14
Results ... 20
Figures and Table ... 23
Discussion ... 33
References ... 38
Abstracts (Korean) ... 50
5
Introduction
Tooth development is dependent on both morphogenesis and differentiation of related cells highly regulated by epithelial-mesenchymal interaction [1]. Tooth organogenesis is normally divided into bud, cap, and bell stages, depending on the changes in epithelial-mesenchymal morphology, position and function mediated by signaling centers such as the placode during the bud stage and enamel knots during the last two stages [2]. Some important signaling molecules include BMPs, FGFs, SHHs, WNTs, as well as TGF-β and TNF proteins [2, 3]. Single molecules have been tested to demonstrate their role in odontogenesis by investigating the defects resulting from their absence [4-6]. However, further examinations are needed as the molecules are never found alone at work in a developmental setting [7].
The underlying mechanism of odontogenesis is commonly investigated through co-cultures of two or more cell types in vitro [8, 9], while recent advances have shifted their focus towards inducing odontogenic responses with conditioned medium (CM) [10]. Most references highlight the role of CM derived from animal cell sources, such as mouse and rat [11-13], which are the most commonly used model organisms for human odontogenesis.
Much research has gained insight into their cell properties and mechanisms;
however, rodents retain stem cells that continuously renew their teeth [14], whereas humans have two sets of dentition, one of which is permanent [15].
CM from human cell cultures lacks both the foundation and affirmative data
6
for odontogenesis. Also, there is a need to evaluate a range of CM concentrations to find the optimal level for odontogenic differentiation. This study is the first report of odontogenic induction potential of concentrated CM derived from the human dental epithelial cell line.
In this study, I examined the effect of CM from a stable epithelial cell line established from Hertwig’s Epithelial Root Sheath/Epithelial Rests of Malassez (HERS/ERM), which originate from human molars [16, 17], on stem cells from human exfoliated deciduous teeth (SHED). Also, different concentrations of CM were tested for comparison. The effects of human cell- derived CM were analyzed by CCK-8 assay, Alizarin red S and DAPI staining, real-time qPCR, and Western blotting. The results showed that CM increased the number of mineralized nodules in differentiated SHED culture, and thus enhanced the odontogenic potential of SHED in vitro in a concentration- dependent manner.
7
Literature Review
I. Odontogenesis and related signaling factors
Odontogenesis, the mechanism of tooth development in organisms, entails an organized series of queues that outlines different stages of tooth morphogenesis. Tooth formation results from epithelial and mesenchymal stem cells interacting with one another through extracellular signaling molecules of transforming necrosis factors (TNFs), bone morphogenetic proteins (BMPs), fibroblast growth factors (FGFs), wingless/integrated (WNTs), and sonic hedgehog (SHH) families to generate an inductive environment [3]. The signaling pathway and regulatory molecules involved are known to be conserved in embryonic development, and the specific epithelial-mesenchymal interactions are well identified for each step of tooth organogenesis [18]. These communications are mediated by several signaling centers during tooth development. For instance, the placode, formed by the thickening of ectoderm, signals the underlying neural-crest mesenchyme to condense around the epithelial bud [2], and the primary and secondary enamel knots regulate morphogenesis of the crown during bud and cap stage [19].
Tooth crown is finalized in bell stage when the epithelial and mesenchymal cells differentiate into ameloblasts and odontoblasts at the interface and deposit hard tissues, enamel and dentin matrices, respectively [2].
8
i. Conditioned medium in vitro and in vivo
Odontogenesis has been explored in vitro and in vivo through co-cultures of epithelium and mesenchymal cells [8], in vivo therapy [6], induction by conditioned medium (CM) [11, 13, 20], and so on. However, considering the difficulty in obtaining and maintaining stem cells from human sources as well as transplant rejection or engraftment issues and risk of developing cancer in clinic applications, CM seems to be the most viable option.
CM has been studied in cell cultures with diverse cells or media types and culture or disease conditions for a wide range of applications [10]. Few disease conditions include injuries in liver [21, 22], lung [23, 24], spinal cord [25] and brain [26, 27], in which CM improved the circumstances. CM composition also varied between cell monolayer and spheroids [28]; it has been reported that spheroid conditions produced higher concentrations of growth factors and cytokines in CM than monolayer conditions [29]. Hypoxic conditions also enriched paracrine factors in CM, subsequently enhancing its effect on proliferation and tubular formation of cells [30]. Other variations in culture duration, basal medium and supplements also affected CM configuration [10]. Due to such a vast range of possibilities and deviations, it is open to accommodate a large variety of in vitro and in vivo experiments in numerous fields of study.
In vitro studies have used the paracrine factors within CM to promote growth [31], repair [30] or differentiation [8, 32] of cells. Cellular factors, such as cytokines and signaling molecules, have been investigated to enhance
9
cell performance [5, 33]. Previous studies have determined single factors that influenced cell fate in vitro [34, 35], but their mode of action when combined is uncertain. Thus, others have analyzed the composition of CM to help narrow down the combination of elements at work [25, 36-38]. Tooth formation is also governed by a network of growth and transcription factors that shift the odontogenic potential back and forth between epithelium and mesenchyme at E12.5 [3]. Likewise, tooth germ cell-derived CM, which is thought to contain regulators from both epithelium and the surrounding mesenchyme, was used to differentiate adult stem cells into odontoblast-like cells in vitro [13]. In other words, CM is able to reproduce the temporospatial effect of an odontogenic microenvironment of in vivo state required for cellular transformation [13, 39]. However, the regulation of these molecules are complex and the specific roles for each are difficult to assign because most have multiple roles, some are redundant, and together they have synergistic effects [7].
CM also received attention as a novel therapeutic method to overcome the limitations of stem cell therapy [36]. This cell-free system has been functionally verified in in vivo angiogenesis [36, 40], liver regeneration [22], and odontogenesis [12] to name a few. Another study inserted preameloblast- derived CM in a hollow root canal space in mice with human pulp cells, which successfully restored pulp tissue [11]. Moreover, a study comparing porcine and human tooth germ cell-derived CM showed that both had similar effects on the odontogenic induction of human dental pulp stem cells in vitro and in
10
vivo as indicated by morphological and genetic changes [20], raising hopes for practical application of xenogeneic CM. Additionally, CM from cell lines have been used as a tool for discovery of new biomarkers for diseases, and analyses of various types of CM revealed cell-specific secretome, including signal peptides, growth factors, enzymes and other soluble factors [41], which may help identify specific factors for enhancement of clinical effect. Also, unlike osteogenesis and mineralization, odontogenic-specific markers are not well known, and thus CM may provide a list of potential biomarkers explicit in odontogenesis.
ii. Conditioned medium contains extracellular vesicles
Like the odontogenic environment of tooth formation in vivo, the key premise of CM is that it provides a microenvironment that is similar to the actual odontogenic conditions, allowing cells within it to be reprogrammed.
For instance, tooth germ cell-derived CM contains biological factors such as TGF-β and BMP molecules [7, 20] that play a role in cytodifferentiation and directing cell fate [42, 43]. CM is also known to contain exosomes, a type of extracellular vesicles gaining attention in recent years. Although the mechanism of cellular communication in tooth development remains obscure, molecules of sizes larger than 100 nm seem to be involved in the polarization or differentiation of cells [44]. Exosomes, also within this boundary, were able to induce amelogenesis or dentinogenesis depending on whether mesenchymal exosomes were used on epithelium or vice versa. [45].
11
Exosomes in osteogenic conditions also increased mineralization nodules and corresponding matrix proteins, and the effect was reported to be dose- dependent [45-47]. Moreover, exosome treatment amplified DPP and DMP1 expression at the junction of dentin and soft tissue of exosome-treated samples, indicating augmented matrix mineralization [46]. Similarly, co- culture of two cell types with a permeable barrier in between allowed transmission of cytokines, resulting in mineralized nodules [48]. Moreover, Shapiro et al. (2015) claims that exosomes are related to matrix vesicles [49], the initiating point of mineralization in bone and other mineralizing tissues [50-53], despite the fact that the former is a non-adherent extracellular vesicle, while the latter is always found attached to the extracellular matrix components [49, 54]. They argued that the size of matrix vesicles, from 0.02 to 2.0 μm [55], were within range of the diameter of exosomes varying from 0.04 to 1.0 μm [56], and the structure and formation of matrix vesicles appeared similar to the budding of exosomes [57, 58]. If matrix vesicles are indeed a type of exosomes, the study of CM may contribute greatly to unveiling the mechanisms behind osteo- and odontogenic mineralization.
iii. Limitations of conditioned medium
Unfortunately, CM also needs to overcome a couple of limitations for clinic use. First, the media used for CM collection contains elements that are not suitable for humans such as HEPES; and secondly, the concentration of secretome from cell culture is too low to have a significant effect on humans
12
[29]. Also, the specific content of CM must be reviewed for each type or batch and method of CM collection standardized before clinical use in the future for consistency and safety issues. Nonetheless, clinic efforts have been made recently to treat multiple sclerosis [59], hair loss [60], and aging of skin [61], all of which presented a positive outlook on the potential uses of CM. Further research is needed to test more cases, but overall CM appears to be a durable solution in the long term.
II. Mineralization, apoptosis and terminal differentiation
In organisms, apoptosis is known to play a significant role in embryonic development as well as in tooth organogenesis [62, 63]. The shaping of the tooth, silencing of signaling centers, and reduction of cell numbers are primary examples of dental apoptosis [63]. For instance, as the dentin matrix begins to occupy more space after secondary dentin deposition, the number of odontoblasts are reduced by half through apoptosis [64]. Thus, a defect in apoptosis may affect the overall dental development, influencing the number or the size of teeth [65, 66].
A recent study reported the effect of calcium and mineralization of dental pulp cells on apoptosis [67]. Although the reasons for cell death is unclear, the higher calcium ion concentration of 5.4 and 9.0 mM increased apoptotic and necrotic markers [67]. Moreover, proinflammatory cytokines also induce odontogenic differentiation of dental pulp stem cells [68, 69]. This may be closely related to apoptotic bodies’ potential role as nucleation sites for
13
calcification as it is with matrix vesicles [70-72]. Terminal differentiation of growth plate chondrocytes is also associated with apoptosis [73, 74], and a factor regulating differentiation and apoptosis, activating transcription factor 2 (ATF-2), was statistically more expressed in terminally differentiated odontoblasts than in pulpal fibroblasts [75]. Still, not much is known about the correlation between mineralization, cell death and terminal differentiation in odontogenesis, and thus requires further investigation to make a statement.
14
Materials and Methods
Primary cell culture
To culture SHED, human deciduous teeth were obtained in Hank’s balanced salt solution (HBSS; Welgene, Daegu, Korea) supplemented with 3% antibiotics-antimycotics (Gibco, Carlsbad, CA, USA) from patients who gave informed consent. Dental pulp tissues were extracted with fine forceps and digested in 1 mg/ml of Collagenase Type I (Gibco) and 2.4 mg/ ml of Dispase (Gibco) at 37°C. After one hour of incubation, the Collagenase/Dispase enzyme solution was inactivated by Dulbecco’s modified Eagle’s medium (DMEM; Welgene) supplemented with 10% fetal bovine serum (FBS; Hyclone, Logan, UT, USA) and 1% antibiotics- antimycotics (Gibco). Single cell suspensions were seeded and maintained in minimum essential medium alpha (αMEM; Hyclone) supplemented with 10%
FBS and 1% antibiotics-antimycotics. The medium was changed every two days. Cells were sub-cultured at 70% confluence.
HERS/ERM cells were isolated and cultured as described by Nam et al.
[1]. Briefly, human third molars were obtained in HBSS (Welgene) supplemented with 3% antibiotics-antimycotics as explained above.
Periodontal ligament tissues, gently separated from the surface of the root, were incubated in Collagenase/Dispase enzyme solution at 37°C. After one hour of incubation, the enzyme was inactivated by DMEM supplemented with 10% FBS and 1% antibiotics-antimycotics. Single cell suspensions were
15
seeded and maintained in αMEM supplemented with 10% FBS and 1%
antibiotics-antimycotics. The medium was changed every two days. When colonies were observed, the mesenchymal cells were isolated by trypsinization with 0.05% trypsin-EDTA (Gibco) and the remaining cells in the culture plates were washed once with Dulbecco’s Phosphate-Buffered Saline (DPBS; Welgene) and maintained in serum-free keratinocyte growth medium-2 (KGM-2; Lonza Rockland, Rockland, ME, USA). Cells were sub- cultured at 70% confluence.
Preparation and application of CM
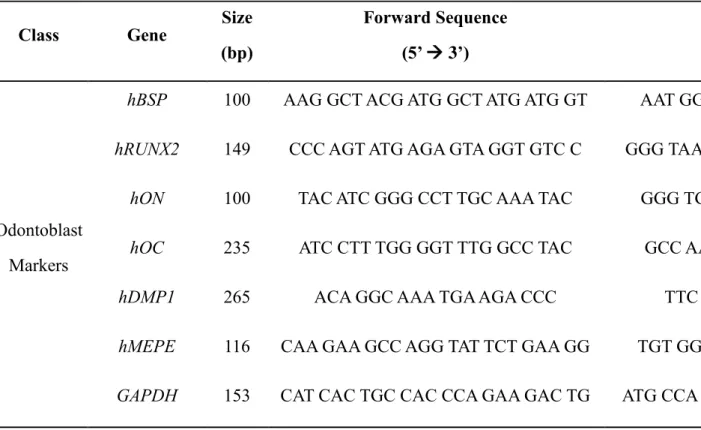
To prepare human epithelial CM, HERS-SV40/hTERT cell line was counted and cultured in vitro for 24 hours in serum-free growth medium, KGM-2. After washing with DPBS, the cells were cultured in fresh KGM-2 for 48 hours. The conditioned medium was collected, centrifuged at 2000 x g for 10 minutes at 4°C, then filtered by 0.22 μm filter membrane (Millipore, Billerica, MA, USA) to eliminate cell debris before aliquoting and freezing at -80°C. Frozen CM was lyophilized using a freeze-dryer (IlShin BioBase, Dongducheon, South Korea) at -80°C and 5 mTorr for a week (Figure 1A).
The lyophilized powder was dissolved in a known volume of distilled water to concentrate the CM by a factor of 16.
In cell culture, the culture media was switched to either non-induction control media (αMEM supplemented with 5% FBS) or induction media (αMEM supplemented with 5% FBS, 50 μg/ml ascorbic acid, 10 mM β-
16
glycerophosphate, and 0.1 μM dexamethasone) supplemented with or without HERS/ERM CM or freeze-dried CM (Figure 1B) when SHED reached confluence. HERS/ERM CM was added to constitute 10% of the medium, while HERS/ERM freeze-dried CM was supplemented at 4X and 8X concentrations, which are equivalent to 40% and 80% constitution, respectively.
Cell Counting Kit-8 (CCK-8) cytotoxicity assay
Cells were counted and dispensed (5000 cells/100 μl/well) in a 96-well plate. The plate was incubated for 24 hours in a humidified incubator (5%
CO2, 37°C), and CCK-8 solution was added to each well of the plate. After two hours of incubation, the absorbance of the wells was measured at 450 nm using a microplate reader (BMG LABTECH, Ortenberg, Germany). Media was changed every day, and the absorbance was measured at the same time each day for six days.
Alizarin red S staining
Primary SHED was cultured to confluence and cultured for 8, 12, 16, and 20 days in differentiation induction media, αMEM supplemented with 5%
FBS, 50 μg/ml ascorbic acid, 10 mM β-glycerophosphate, and 0.1 μM dexamethasone with varying CM and freeze-dried CM concentrations. The medium was changed every other day. To visualize the accumulation of mineralized nodules, the differentiated SHED were stained with 2% Alizarin
17
red S solution (Sigma-Aldrich, St. Louis, MO, USA). The cells were washed with DPBS and fixed with 4% paraformaldehyde (T&I Biotechnology, Seoul, Korea) at room temperature. After washing with DPBS, the cells were stained with 2% Alizarin red S solution (Sigma-Aldrich). The remaining Alizarin solution was washed with distilled water.
DAPI staining
Primary SHED was cultured to confluence in 12-well plates (SPL, Pocheon, Korea) and cultured for 10, 12, 14, 16, or 18 days in differentiation induction media. On the day of DAPI staining, cells were fixed with 4%
paraformaldehyde at room temperature. After washing with DPBS, DAPI (Sigma) diluted (1:1000) in DPBS was added in the dark and incubated at room temperature for 10 minutes. The remaining DAPI was washed with DPBS and pictures were taken using an inverted microscope (Nikon, Melville, NY, USA). All immunofluorescence staining were conducted more than three times.
Reverse transcription-polymerase chain reaction (RT-PCR)
Cell pellets in RNAlater (Ambion, Austin, TX, USA) were washed with DPBS supplemented with 2% FBS. Total RNA isolation was performed using the RNeasy mini kit (Qiagen, Hilden, Germany). An additional DNase I treatment from the RNase-free DNase set (Qiagen) was done for removal of genomic DNA contamination. Total RNA (1 μg) was reverse transcribed
18
using the amfiRivert cDNA synthesis Platinum Master Mix (GenDEPOT, Barker, TX, USA). The following RT conditions were used: annealing at 25°C for 5 min, followed by extension at 42°C for 60 min, and heat inactivation of the enzyme at 70°C for 15 min. The RT products were stored at -20°C until quantitative analysis.
Real-time quantitative PCR (RT-qPCR)
The cDNA reverse transcribed from SHED were amplified using Thunderbird SYBR qPCR Mix (Toyobo, Osaka, Japan) and specific primers for human BSP, Runx2, ON, OC, DMP1, MEPE, and GAPDH listed in Table 1. Real-time PCR was performed by CFX CONect Real-Time PCR Detection System (Bio-Rad, Hercules, CA, USA). The cycle threshold (CT) values of each gene were standardized to those of the housekeeping gene, glyceraldehyde-3-phosphate dehydrogenase (GAPDH). The following PCR conditions were used: initializing at 95°C for 30 sec, denaturing at 95°C for 10 sec, annealing at 60°C for 30 sec, followed by elongation at 72°C for 33 sec, and heat inactivation of the enzyme at 70°C for 15 min.
Western blot analysis
Primary SHED was differentiated for 16 and 20 days with CM and freeze- dried CM supplements, then collected in 1.5 ml tubes and pelleted by centrifugation at 13,200 rpm, 4°C, for 3 minutes. Pellets were re-suspended in RIPA lysis buffer (RKMB-030-0050, Rockland Immunochemicals,
19
Limerick, PA, USA) supplemented with Halt Protease and Phosphatase Inhibitor Cocktail (78440, ThermoFisher Scientific, Waltham, MA, 1:100) and incubated on ice. Proteins were separated by 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE) and transferred to immunoblot PVDF membrane (Bio-Rad). Membranes were blocked with 5%
non-fat dry milk (Bio-Rad) in TBS-T [20 mM Tris base, 137 mM NaCl, 0.1%
Tween 20 (pH 7.6)], and incubated overnight at 4°C with primary antibody diluted in TBS-T buffer (1:1000). Monoclonal antibody against DSPP (sc- 73632, Santa Cruz), binding to the DPP domain CSRGDASYNSDESKDNG [76], was purchased from Santa Cruz Biotechnology (Santa Cruz Biotechnology, CA, USA). After washing with TBS-T buffer, membranes were incubated with goat anti-mouse IgG secondary antibodies conjugated to horseradish peroxidase (BR170-6516, Bio-Rad). Labeled protein bands were detected using a bio-image analyzer (Bio-Rad).
Statistical analysis
The data were presented as means ± standard deviations of three replicates for each treatment. Data analysis was performed using analysis of variance (ANOVA) and post hoc LSD t-test and Duncan for pair-wise comparisons.
The differences were considered to be significant when p < 0.05. All statistical analyses were conducted using SAS software (version 9.4).
20
Results
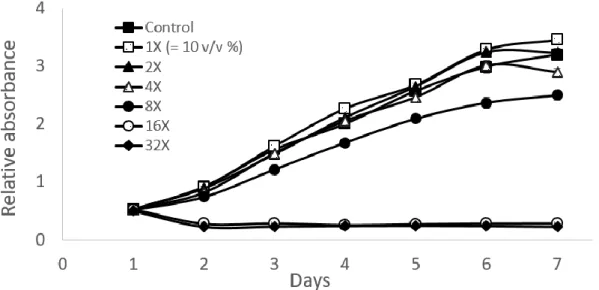
The viability of SHED in HERS/ERM freeze-dried CM treatment A toxicological test was performed for a week to examine the effects of HERS/ERM freeze-dried CM on the viability of SHED at different concentrations in vitro. The CCK-8 assay indicated that cell count was not greatly altered from the control up to 8X concentration, but the absorbance measured dramatically dropped at 16X and 32X concentrations after just one day of incubation. At 8X concentration, the absorbance measurement was visibly lower than the control, but cells still had no difficulty reaching confluence by the end of the week. The 1X, 2X and 4X freeze-dried CM treatments seemed to follow the same trend as the control (Figure 2).
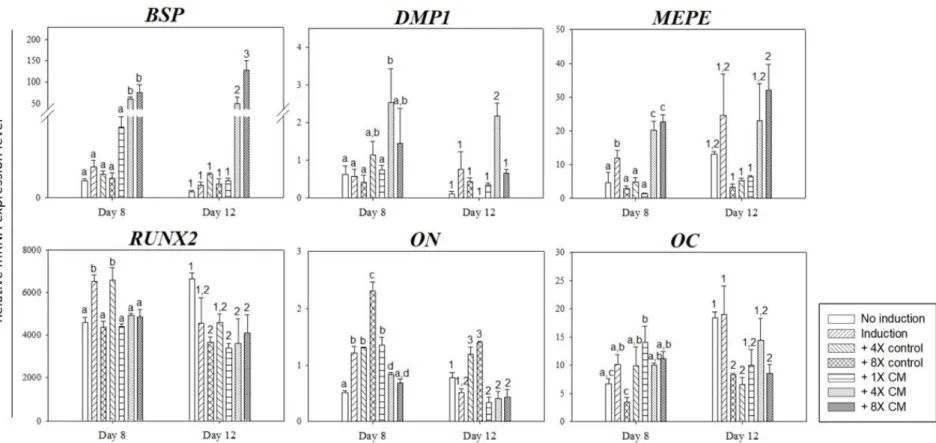
HERS/ERM CM induces odontogenic differentiation of SHED in vitro After 8 and 12 days of CM treatment, differentiated SHED was analyzed by RT-qPCR to determine whether CM enhanced odontogenesis and mineralization (Figure 3). The mineralization-related BSP expression was significantly increased in freeze-dried CM treatments on both day 8 and 12, while DMP1 was significantly enhanced in 4X CM treatment on both days compared to the controls and in 8X CM treatment on day 12. MEPE expression was also significantly increased in freeze-dried CM groups on day 8 and 12 when compared to the freeze-dried basal media controls. On the other hand, early mineralization markers like RUNX2 showed a decreasing
21
trend in all treatment on day 12 compared to the non-induction control. Other early markers, such as ON and OC, also showed a similar trend as RUNX2.
Treatment groups had relatively higher gene expression levels on day 8 but declined on day 12. The relative ON expression in basal media control groups was higher than CM treatment on both days, but the 4X control showed significantly less OC expression on both days.
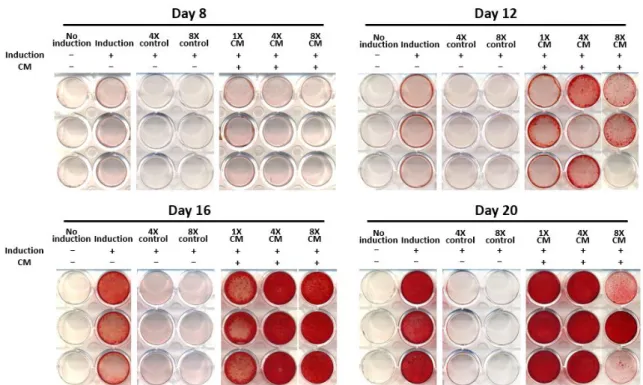
Alizarin red S staining was also performed to compare the calcification nodule formation of CM-treated groups to the non-induction control. Staining results exhibited no staining of nodules on day 8. The first signs of mineralized nodule formation were observed on day 12 in CM-treated groups.
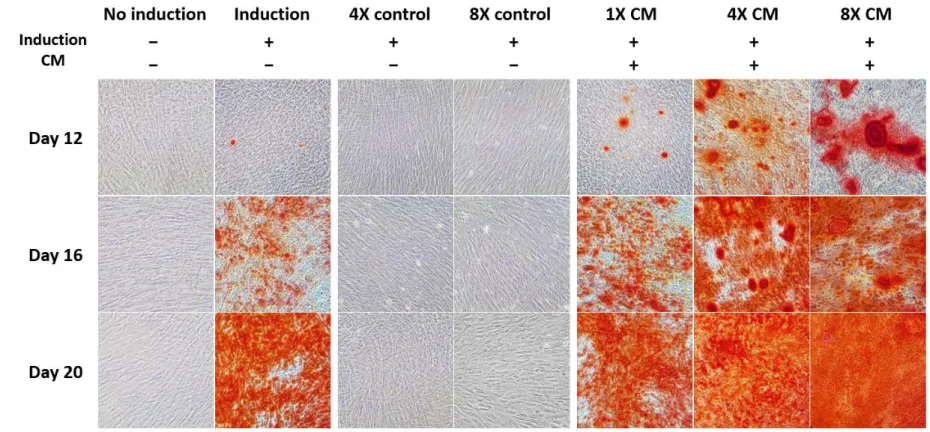
Odontogenic induction without CM exhibited mineralized nodules on day 16 and increased onwards, while CM-treated groups showed a notable increase in nodule formation from day 12 to day 20 (Figure 4A). All induction groups excluding basal media controls were stained thoroughly on day 20, but in earlier days, the difference between the amounts of nodule formation was prominent among these treatment groups. In contrast, the non-induction group and basal media control groups were not stained for the duration of 20 days. At 200X magnification, SHED had larger Alizarin stained portions as the concentration of CM treatment was increased (Figure 4B). The addition of 1X CM also increased the number of nodule formation compared to the induction group without CM, but not more than the freeze-dried CM treatments. A similar pattern was also observed in unstained morphology of differentiated SHED, where the freeze-dried CM-treated cells had
22
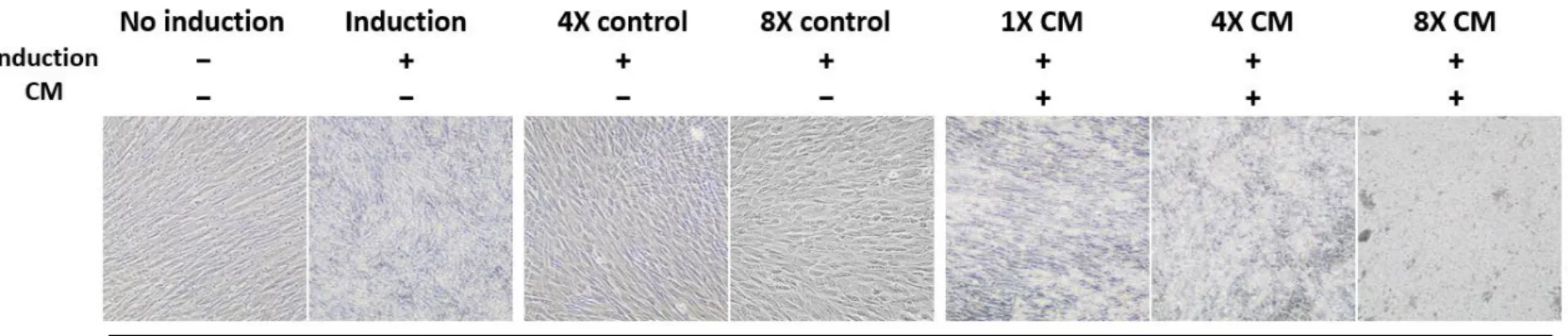
significantly more visible mineralized aggregates compared to the non- induction and basal media controls. The aggregates also appeared to be more densely formed in higher concentrations of CM compared to the 1X CM group (Figure 6A).
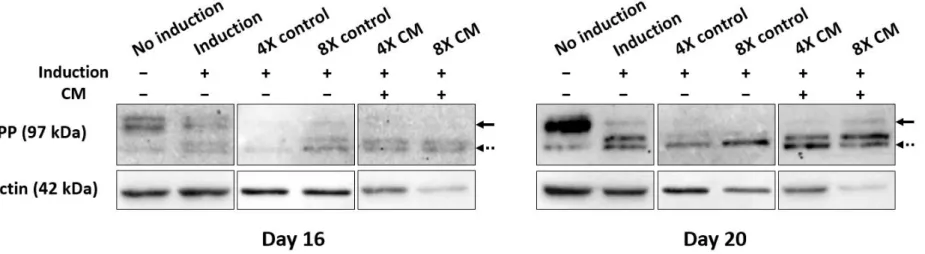
Furthermore, Western blot was run to verify that mineralization in vitro occurred as a result of odontogenic differentiation of SHED. The immunoblots demonstrated that the relative amount of DPP proteins was increased in freeze-dried CM treatment groups on day 16 compared to the non-induction and induction controls. On day 20, the DPP expression increased in all treatments compared to the non-induction control (Figure 5).
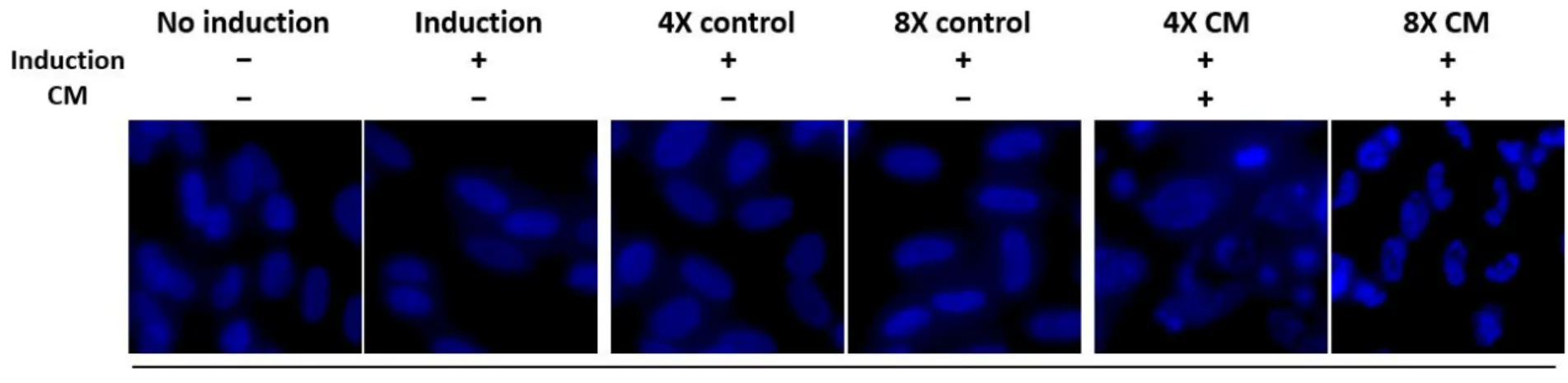
Terminal differentiation of SHED when treated with HERS/ERM freeze- dried CM
To test the viability of SHED under the influence of freeze-dried CM over a longer period, DAPI staining experiment was conducted on day 18. The non-induction and induction controls showed consistent morphology of SHED nuclei throughout 18 days. On the other hand, 4X and 8X CM-treated nuclei began to alter and showed similar signs to DNA fragmentation on day 18. This data conforms to the expression of β-actin in western blotting, in which 4X and 8X CM-treated cells exhibited less amount of β-actin, despite loading the equal quantity of proteins for all treatments. By day 16 and 20, both freeze-dried CM-treated groups had significantly less expression of β- actin proteins compared to the controls (Figure 5).
23
Table 1. Sequences of primers used for RT-PCR
Class Gene Size
(bp)
Forward Sequence (5’ 3’)
Reverse Sequence (5’ → 3’)
Odontoblast Markers
hBSP 100 AAG GCT ACG ATG GCT ATG ATG GT AAT GGT AGC CGG ATG CAA AG hRUNX2 149 CCC AGT ATG AGA GTA GGT GTC C GGG TAA GAC TGG TCA TAG GAC C
hON 100 TAC ATC GGG CCT TGC AAA TAC GGG TGA CCA GGA CGT TCT TG
hOC 235 ATC CTT TGG GGT TTG GCC TAC GCC AAT AGG GCG AGG AGT G
hDMP1 265 ACA GGC AAA TGA AGA CCC TTC ACT GGC TTG TAT GG
hMEPE 116 CAA GAA GCC AGG TAT TCT GAA GG TGT GGT TGA AAT GTT GGT GCT GAPDH 153 CAT CAC TGC CAC CCA GAA GAC TG ATG CCA GTG AGC TTC CCG TTC AG
24
A
B
Figure 1. Procedure outline for establishing the HERS/ERM freeze-dried conditioned medium (CM) used in the following experiments.
(A) The HERS/ERM cell line was seeded 60 x 104 cells per 90 mm dish in serum-free growth medium and the CM was collected after 48 hours of incubation. The CM was centrifuged at 2000 x g for 10 minutes, filtered with a 0.22-μm membrane filter, and frozen at -80°C before lyophilization. By adding a known volume of distilled water to the freeze-dried CM powder, the CM was concentrated by a factor of 16. (B) The SHED was cultured in induction medium supplemented (c) with or (b) without CM, or (e) with freeze-dried CM obtained from the HERS/ERM cells, and compared to the SHED cultured in (a) non-induction medium, or in (d) induction medium supplemented with freeze-dried basal medium (BM).
25
Figure 2. Toxicological test of freeze-dried conditioned medium (CM) on the SHED in vitro. The SHED was treated with growth medium supplemented with the HERS/ERM CM at six different concentrations, 1X, 2X, 4X, 8X, 16X, and 32X, and compared to the control; the value of 1X represents the addition of 10% (v/v) CM. The SHED was seeded at Day 0, and the first absorbance was measured at Day 1, the day on which the CM were added. Cell viability was assessed every 24 hours for a week by CCK-8 assay. The concentration of 16X and 32X led to immediate cell death, while all other treatment groups eventually reached cell confluence by the last day. The 8X group exhibited a slower growth rate compared to lower concentration groups, but reached confluence by the end of the week. Data are mean ± SD of three replicates.
26
Figure 3. Relative gene expression of differentiated SHED in vitro. The SHED was treated with induction medium supplemented with different concentrations of HERS/ERM conditioned medium (CM) for 8 and 12 days, where the value of 1X represents the addition of CM at 10% (v/v) of the total volume. The relative mRNA expression levels of BSP, DMP1, MEPE, RUNX2, ON and OC were
27
measured by quantitative RT-PCR analysis and the data were normalized by GAPDH levels. The HERS/ERM freeze-dried CM-treated groups showed a significant increase in BSP, DMP1, and MEPE expression compared to the controls, while early mineralization markers, RUNX2, ON and OC, showed significant decreases on day 12. Data are mean ± SD of three replicates. Bars labeled with different letters or numbers are significantly different from the non-induction control (1,2,3p < 0.05 and a,b,cp < 0.05). BSP: bone sialoprotein; DMP1: dentin matrix acidic phosphoprotein 1; MEPE: matrix extracellular phosphoglycoprotein; RUNX2: Runt-related transcription factor 2; ON: osteonectin; OC: osteocalcin.
28
A
Figure 4. Mineralized nodule formation of differentiated SHED in vitro. The SHED was cultured in induction medium supplemented with different concentrations of HERS/ERM conditioned medium (CM), where the value of 1X represents the addition of 10% (v/v) CM. The label indicates the presence of induction and/or CM. The calcium nodule formation was observed through (A) Alizarin red S stained cells after 8, 12, 16 and 20 days of culture. Differentiated SHED produced more mineralized nodules when supplemented with CM, and mineralization density increased as CM concentration was increased. All experiments were conducted in three replicates.
29
B
Figure 4. (B) Alizarin red S stained cells after 12, 16 and 20 days of culture, photographed at 200X magnification. Mineralized nodules were observed 12 days after the start of odontogenic induction, and accumulated until all differentiated groups reached mineralization equilibrium after 20 days. None of the controls were Alizarin red S stained. All experiments were conducted in three replicates.
30
Figure 5. Western blot analysis of differentiated SHED in vitro. The SHED was cultured in induction medium supplemented with different concentrations of HERS/ERM conditioned medium (CM) for 16 and 20 days, where the value of 1X represents the addition of CM at 10% (v/v) of the total volume. The label indicates the presence of induction and/or CM. The expression levels of DPP and β-actin proteins were analyzed by western blotting. DPP was more strongly expressed in CM-treated groups compared to the non- induction and induction controls. The disappearance of DSPP bands (solid arrows) corresponded with the appearance and the thickness of DPP bands (dotted arrows). The β-actin protein bands were thinner in freeze-dried CM-treated groups compared to the basal media control bands. Lane 1: non-induced SHED; lane 2: odontogenic induced SHED; lane 3: odontogenic induced SHED treated with 4X freeze-dried basal media (BM); lane 4: odontogenic induced SHED treated with 8X freeze-dried BM; lane 5: odontogenic induced SHED treated with 4X freeze-dried CM; lane 6: odontogenic induced SHED treated with 8X freeze-dried CM.
31
A
Figure 6. Cell morphology of differentiated SHED in vitro. The SHED was cultured in differentiation medium supplemented with different concentrations of HERS/ERM conditioned medium (CM), where the value of 1X represents the addition of CM at 10% (v/v) of the total volume. The label indicates the presence of induction and/or CM. (A) Cell morphology was observed 20 days after odontogenic induction. Cells treated with freeze-dried CM was highly mineralized after 20 days of odontogenic induction, compared to the basal media controls which showed no mineral nodule formation.
32
B
Figure 6. (B) Cell nuclei were stained by DAPI after 18 days of induction and photographed at 600X magnification to observe nuclear alterations. Microscopic images showed signs of DNA fragmentation in freeze-dried CM-treated cells, while the non-induction and induction control groups maintained smooth, oval-shaped nuclei. The pictures are representative of three independent experiments.
33
Discussion
Tooth development is a complex, regulated series of reciprocal communication between the epithelium and mesenchyme, which eventually differentiate into ameloblasts and odontoblasts, respectively, to form hard- tissue [2]. To reproduce cellular interaction, stem cells have been used for in vitro co-culture systems [8] or bioengineering for tooth regeneration [77].
However, the invasiveness of stem cells has made the transition to bedside difficult, as well as other issues including the expenses and maintenance of cell culture. CM, on the contrary, are proven to have regenerative effects and are easily obtained from cell cultures.
CM has been established from various cell types and culture conditions, and have been utilized in the induction of cell differentiation in vitro [8, 78]
and in vivo [11, 79, 80]. It is said to contain soluble factors [81-83] that create an odontogenic microenvironment favorable for cell differentiation [13].
However, the effect of cytokines and other paracrine factors on the regulation of cell function is complex, and thus, the results are not always dose- dependent. For instance, one study found that the concentration of CM supplements did not correlate with cell proliferation [84]. Others found that varying concentrations of CM had no effect on the cell behavior [40, 85].
Interestingly, the CM in our study had concentration-dependent enhancing effects on the odontogenic capacity of SHED. This could be due to our method of concentrating the CM by lyophilization. After collecting and
34
freezing the CM from HERS/ERM cell line, the frozen CM was freeze-dried, and the resultant powder was dissolved in a known volume of distilled water to concentrate the CM by a factor of 16. The rationale behind this was to increase the concentration of CM supplement in differentiation medium without constituting a larger proportion of the total volume. Thus, I was able to add 40% and 80% of CM (v/v) to the differentiation medium by adding only 25 and 50 μl of CM, respectively, per milliliter of odontogenic induction medium.
In vitro differentiation of SHED with the supplementation of freeze-dried CM was examined by mineralization-related gene expression levels, namely BSP, DMP1, MEPE, RUNX2, ON, OC, and as well as the expression of DPP proteins. Previous studies state that BSP is expressed in mature osteoblasts [86, 87], and its appearance correlates to calcified nodule formation [88]. On both day 8 and 12, BSP was significantly increased in higher concentrations of CM (Figure 3), and Alizarin red S staining exhibited increased formation of mineralized nodules in differentiated SHED with 4X and 8X freeze-dried CM supplements on day 12 (Figure 4B). Similar to BSP, DMP1 is also associated with the start of mineralization [89]. As expected, the mRNA expression of DMP1 was enhanced significantly in 4X freeze-dried CM treatment; but data was not significant in 8X freeze-dried CM treatment (Figure 3A). This may be due to inconsistent effects of 8X freeze-dried CM on SHED differentiation, as observed by the slight variations between replicates in Alizarin red S staining on day 12 and 20 (Figure 4A). Also,
35
MEPE is reported to be present in mineralizing tissues as its cleaved form, acidic-serine-aspartate-rich-MEPE-associated motif [90, 91]. Unlike BSP, DMP1, and MEPE, the expression of RUNX2, ON, and OC was significantly downregulated in CM treatments on day 12, which is consistent with the claim that RUNX2 expression declines in more mature odontoblasts [92], while ON and OC are inversely correlated with calcium deposition [93, 94].
Lastly, DPP proteins that are closely related to odontogenesis and mineralization in vitro [95] exhibited thicker bands in differentiated SHED compared to the non-induction control on immunoblot, and bands at higher sizes around 131 kDa seemed to disappear in correspondence to the appearance and the thickness of lower DPP bands at 97 kDa [96]. Because the monoclonal DSPP (LFMb-21) antibody binds to the DPP domain CSRGDASYNSDESKDNG, both cleaved DPP and the remaining uncleaved DSPP form should be detected on Western blot. However, there is still no accepted explanation for the multiple bands detected by DSPP (LFMb-21) antibody. Additionally, DPP bands were expressed highly in all differentiated SHED on day 20 (Figure 5). This result was also consistent with Alizarin red staining, which displayed significant levels of calcification nodules on day 20 (Figure 4A).
To confirm that the increased odontogenic effect on SHED was due to the conditioning of medium by epithelial cells for 48 hours, the unconditioned basal medium was added to the induction medium as a control variable. The basal medium was freeze-dried and used at 4X and 8X concentrations to
36
compare directly with the freeze-dried CM treatments. As a result, the lack of significant differences in relative gene levels, mineralized nodule deposits, and Western blot in the basal media controls verified that the enhancement of odontogenic capacity was due to the HERS/ERM cell line conditioning of the basal medium, and not its original components.
However, it appeared that long-term treatment of freeze-dried CM resulted in cell death, and the nuclei appeared to disintegrate, as determined by DAPI staining (Figure 6B) and the decreasing β-actin expression in Western blotting (Figure 5B). This could be due to the accumulation of freeze- dried CM toxicity in long-term cell cultures, which was also observed at higher concentrations of freeze-dried CM according to the CCK-8 assay. Cell growth and viability were not greatly affected up to 8X concentration but resulted in immediate cell death at 16X and 32X concentrations on the first day of absorbance measurement (Figure 2). However, freeze-dried basal media did not result in cell death on day 18, indicating that concentrated levels of basal media components were not the cause of cellular toxicity. Thus, it could also be explained by the nature of the cytodifferentiation stage in tooth development. At terminal differentiation of odontoblasts, the cell cycle stops, and the cells elongate, polarize and secrete a dentin matrix [5]. Smooth, oval shapes of the nuclei were maintained until day 14 in 4X freeze-dried CM treatment and day 16 in 8X freeze-dried CM treatment (data not shown).
Therefore, cell death naturally occurs as cells are terminally differentiated by CM, and without further cell proliferation due to confluence, only the secreted
37
pre-dentin matrix remains as asserted by the strong expression of DPP proteins even when beta-actin was reduced.
Our results suggest that the odontogenic differentiation effects of CM derived from HERS/ERM cell line on SHED were concentration-dependent up to 8X concentration factor in vitro. In-depth studies are needed to reveal the specific composition of the CM and its involvement in the odontogenic differentiation mechanism of mesenchymal cells.
38
References
1. Thesleff, I., et al., Epithelial-mesenchymal signaling during tooth development. CONect Tissue Res, 1995. 32(1-4): p. 9-15.
2. Thesleff, I., Epithelial-mesenchymal signalling regulating tooth morphogenesis. J Cell Sci, 2003. 116(Pt 9): p. 1647-8.
3. Zhang, Y.D., et al., Making a tooth: growth factors, transcription factors, and stem cells. Cell Res, 2005. 15(5): p. 301-16.
4. Aberg, T., et al., Runx2 mediates FGF signaling from epithelium to mesenchyme during tooth morphogenesis. Dev Biol, 2004. 270(1): p. 76-93.
5. Gritli-Linde, A., et al., Shh signaling within the dental epithelium is necessary for cell proliferation, growth and polarization. Development, 2002.
129(23): p. 5323-5337.
6. Nakashima, M. and A. Akamine, The application of tissue engineering to regeneration of pulp and dentin in endodontics. J Endod, 2005.
31(10): p. 711-8.
7. Smith, A.J. and H. Lesot, Induction and regulation of crown dentinogenesis: embryonic events as a template for dental tissue repair? Crit Rev Oral Biol Med, 2001. 12(5): p. 425-37.
8. Arakaki, M., et al., Role of epithelial-stem cell interactions during dental cell differentiation. J Biol Chem, 2012. 287(13): p. 10590-601.
9. Yu, J., et al., Epithelial-mesenchymal cell ratios can determine the crown morphogenesis of dental pulp stem cells. Stem Cells Dev, 2008. 17(3):
39
p. 475-82.
10. Pawitan, J.A., Prospect of Stem Cell Conditioned Medium in Regenerative Medicine. Biomed Research International, 2014.
11. Choung, H.W., et al., The role of preameloblast-conditioned medium in dental pulp regeneration. Journal of Molecular Histology, 2013. 44(6): p.
715-721.
12. Lee, J.H., et al., Odontogenic differentiation of human dental pulp stem cells induced by preameloblast-derived factors. Biomaterials, 2011.
32(36): p. 9696-706.
13. Yu, J.H., et al., Differentiation of dental pulp stem cells into regular- shaped dentin-pulp complex induced by tooth germ cell conditioned medium.
Tissue Engineering, 2006. 12(11): p. 3097-3105.
14. Ohshima, H., et al., The eternal tooth germ is formed at the apical end of continuously growing teeth. Archives of Oral Biology, 2005. 50(2): p.
153-157.
15. Klein, O.D., et al., Developmental disorders of the dentition: an update. Am J Med Genet C Semin Med Genet, 2013. 163C(4): p. 318-32.
16. Nam, H., et al., Expression profile of the stem cell markers in human Hertwig's epithelial root sheath/Epithelial rests of Malassez cells. Mol Cells, 2011. 31(4): p. 355-60.
17. Nam, H., et al., Establishment of Hertwig's epithelial root sheath/epithelial rests of Malassez cell line from human periodontium. Mol Cells, 2014. 37(7): p. 562-7.
40
18. Jussila, M. and I. Thesleff, Signaling networks regulating tooth organogenesis and regeneration, and the specification of dental mesenchymal and epithelial cell lineages. Cold Spring Harb Perspect Biol, 2012. 4(4): p.
a008425.
19. Jernvall, J. and I. Thesleff, Reiterative signaling and patterning during mammalian tooth morphogenesis. Mech Dev, 2000. 92(1): p. 19-29.
20. Wang, Y.X., et al., Porcine tooth germ cell conditioned medium can induce odontogenic differentiation of human dental pulp stem cells. Journal of Tissue Engineering and Regenerative Medicine, 2011. 5(5): p. 354-362.
21. Du, Z., et al., Mesenchymal stem cell-conditioned medium reduces liver injury and enhances regeneration in reduced-size rat liver transplantation. J Surg Res, 2013. 183(2): p. 907-15.
22. van Poll, D., et al., Mesenchymal stem cell-derived molecules directly modulate hepatocellular death and regeneration in vitro and in vivo.
Hepatology, 2008. 47(5): p. 1634-43.
23. Ionescu, L., et al., Stem cell conditioned medium improves acute lung injury in mice: in vivo evidence for stem cell paracrine action. Am J Physiol Lung Cell Mol Physiol, 2012. 303(11): p. L967-77.
24. Monsel, A., et al., Mesenchymal stem cell derived secretome and extracellular vesicles for acute lung injury and other inflammatory lung diseases. Expert Opin Biol Ther, 2016. 16(7): p. 859-71.
25. Cantinieaux, D., et al., Conditioned medium from bone marrow- derived mesenchymal stem cells improves recovery after spinal cord injury in
41
rats: an original strategy to avoid cell transplantation. PLoS One, 2013. 8(8):
p. e69515.
26. Tajiri, N., et al., Intravenous transplants of human adipose-derived stem cell protect the brain from traumatic brain injury-induced neurodegeneration and motor and cognitive impairments: cell graft biodistribution and soluble factors in young and aged rats. J Neurosci, 2014.
34(1): p. 313-26.
27. Yamagata, M., et al., Human dental pulp-derived stem cells protect against hypoxic-ischemic brain injury in neonatal mice. Stroke, 2013. 44(2):
p. 551-4.
28. Potapova, I.A., et al., Mesenchymal stem cells support migration, extracellular matrix invasion, proliferation, and survival of endothelial cells in vitro. Stem Cells, 2007. 25(7): p. 1761-8.
29. Bhang, S.H., et al., Efficacious and clinically relevant conditioned medium of human adipose-derived stem cells for therapeutic angiogenesis.
Mol Ther, 2014. 22(4): p. 862-72.
30. Chen, L., et al., Conditioned medium from hypoxic bone marrow- derived mesenchymal stem cells enhances wound healing in mice. PLoS One, 2014. 9(4): p. e96161.
31. Li, H.S., et al., Cytokine profiles in conditioned media from cultured human intervertebral disc tissue - Implications of their effect on bone marrow stem cell metabolism. Acta Orthopaedica, 2005. 76(1): p. 115-121.
32. Kapiteijn, K., et al., Human embryo-conditioned medium stimulates
42
in vitro endometrial angiogenesis. Fertil Steril, 2006. 85 Suppl 1: p. 1232-9.
33. Moore, M.A.S., Cytokine and chemokine networks influencing stem cell proliferation, differentiation, and marrow homing. Journal of Cellular Biochemistry, 2002: p. 29-38.
34. Liu, H., et al., MEPE is downregulated as dental pulp stem cells differentiate. Archives of Oral Biology, 2005. 50(11): p. 923-928.
35. Nakashima, M., Bone morphogenetic proteins in dentin regeneration for potential use in endodontic therapy. Cytokine & Growth Factor Reviews, 2005. 16(3): p. 369-376.
36. Di Santo, S., et al., Novel Cell-Free Strategy for Therapeutic Angiogenesis: In Vitro Generated Conditioned Medium Can Replace Progenitor Cell Transplantation. Plos One, 2009. 4(5).
37. Lee, M.J., et al., Enhancement of wound healing by secretory factors of endothelial precursor cells derived from human embryonic stem cells.
Cytotherapy, 2011. 13(2): p. 165-78.
38. Sze, S.K., et al., Elucidating the secretion proteome of human embryonic stem cell-derived mesenchymal stem cells. Mol Cell Proteomics, 2007. 6(10): p. 1680-9.
39. Nishimura, I., et al., Precursor tissue analogs as a tissue-engineering strategy. Tissue Eng, 2003. 9 Suppl 1: p. S77-89.
40. Gharaei, M.A., et al., Human dental pulp stromal cell conditioned medium alters endothelial cell behavior. Stem Cell Research & Therapy, 2018.
9.
43
41. Dowling, P. and M. Clynes, Conditioned media from cell lines: a complementary model to clinical specimens for the discovery of disease- specific biomarkers. Proteomics, 2011. 11(4): p. 794-804.
42. Thesleff, I. and T. Aberg, Molecular regulation of tooth development.
Bone, 1999. 25(1): p. 123-5.
43. Tompkins, K., Molecular mechanisms of cytodifferentiation in mammalian tooth development. CONect Tissue Res, 2006. 47(3): p. 111-8.
44. Thesleff, I., et al., Interference of tooth differentiation with interposed filters. Dev Biol, 1977. 58(1): p. 197-203.
45. Jiang, N., et al., Exosomes Mediate Epithelium-Mesenchyme Crosstalk in Organ Development. ACS Nano, 2017. 11(8): p. 7736-7746.
46. Huang, C.C., et al., Exosomes as biomimetic tools for stem cell differentiation: Applications in dental pulp tissue regeneration. Biomaterials, 2016. 111: p. 103-115.
47. Narayanan, R., C.C. Huang, and S. Ravindran, Hijacking the Cellular Mail: Exosome Mediated Differentiation of Mesenchymal Stem Cells. Stem Cells International, 2016.
48. Ueno, A., et al., MC3T3-E1-conditioned medium-induced mineralization by clonal rat dental pulp cells. Matrix Biol, 2001. 20(5-6): p.
347-55.
49. Shapiro, I.M., W.J. Landis, and M.V. Risbud, Matrix vesicles: Are they anchored exosomes? Bone, 2015. 79: p. 29-36.
50. Anderson, H.C., R. Garimella, and S.E. Tague, The role of matrix
44
vesicles in growth plate development and biomineralization. Front Biosci, 2005. 10: p. 822-37.
51. Bernard, G.W. and D.C. Pease, An electron microscopic study of initial intramembranous osteogenesis. Am J Anat, 1969. 125(3): p. 271-90.
52. Golub, E.E., Role of matrix vesicles in biomineralization. Biochim Biophys Acta, 2009. 1790(12): p. 1592-8.
53. Hayashi, Y. and H. Nagasawa, Matrix vesicles isolated from apical pulp of rat incisors: crystal formation in low Ca x Pi ion-product medium containing beta-glycerophosphate. Calcif Tissue Int, 1990. 47(6): p. 365-72.
54. Kirsch, T., Determinants of pathological mineralization. Curr Opin Rheumatol, 2006. 18(2): p. 174-80.
55. Golub, E.E., et al., Induction of chondrocyte vesiculation in vitro. J Biol Chem, 1983. 258(1): p. 616-21.
56. Colombo, M., G. Raposo, and C. Thery, Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol, 2014. 30: p. 255-89.
57. Akisaka, T. and C.V. Gay, The plasma membrane and matrix vesicles of mouse growth plate chondrocytes during differentiation as revealed in freeze-fracture replicas. Am J Anat, 1985. 173(4): p. 269-86.
58. Hale, J.E. and R.E. Wuthier, The mechanism of matrix vesicle formation. Studies on the composition of chondrocyte microvilli and on the effects of microfilament-perturbing agents on cellular vesiculation. J Biol Chem, 1987. 262(4): p. 1916-25.
45
59. Dahbour, S., et al., Mesenchymal stem cells and conditioned media in the treatment of multiple sclerosis patients: Clinical, ophthalmological and radiological assessments of safety and efficacy. CNS Neurosci Ther, 2017.
23(11): p. 866-874.
60. Fukuoka, H. and H. Suga, Hair Regeneration Treatment Using Adipose-Derived Stem Cell Conditioned Medium: Follow-up With Trichograms. Eplasty, 2015. 15: p. e10.
61. Sohn, S.J., et al., Anti-aging Properties of Conditioned Media of Epidermal Progenitor Cells Derived from Mesenchymal Stem Cells.
Dermatol Ther (Heidelb), 2018. 8(2): p. 229-244.
62. Doseff, A.I., Apoptosis: the sculptor of development. Stem Cells Dev, 2004. 13(5): p. 473-83.
63. Matalova, E., A.S. Tucker, and P.T. Sharpe, Death in the life of a tooth.
Journal of Dental Research, 2004. 83(1): p. 11-16.
64. Matalova, E., E. Svandova, and A.S. Tucker, Apoptotic signaling in mouse odontogenesis. OMICS, 2012. 16(1-2): p. 60-70.
65. Fleischmannova, J., et al., Mouse models of tooth abnormalities. Eur J Oral Sci, 2008. 116(1): p. 1-10.
66. Kim, J.Y., et al., Inhibition of apoptosis in early tooth development alters tooth shape and size. J Dent Res, 2006. 85(6): p. 530-5.
67. An, S., et al., Short-term effects of calcium ions on the apoptosis and onset of mineralization of human dental pulp cells in vitro and in vivo. Int J Mol Med, 2015. 36(1): p. 215-21.
46
68. Joo, K.H., et al., Cytokine Expression of Stem Cells Originating from the Apical Complex and Coronal Pulp of Immature Teeth. J Endod, 2018.
44(1): p. 87-92 e1.
69. Yang, X., et al., Pro-inflammatory cytokines induce odontogenic differentiation of dental pulp-derived stem cells. J Cell Biochem, 2012. 113(2):
p. 669-77.
70. Fujita, H., et al., Necrotic and apoptotic cells serve as nuclei for calcification on osteoblastic differentiation of human mesenchymal stem cells in vitro. Cell Biochemistry and Function, 2014. 32(1): p. 77-86.
71. Kirsch, T., W. Wang, and D. Pfander, Functional differences between growth plate apoptotic bodies and matrix vesicles. Journal of Bone and Mineral Research, 2003. 18(10): p. 1872-1881.
72. Wuthier, R.E. and G.F. Lipscomb, Matrix vesicles: structure, composition, formation and function in calcification. Frontiers in Bioscience- Landmark, 2011. 16: p. 2812-+.
73. Hatori, M., et al., End labeling studies of fragmented DNA in the avian growth plate: evidence of apoptosis in terminally differentiated chondrocytes. J Bone Miner Res, 1995. 10(12): p. 1960-8.
74. Mansfield, K., R. Rajpurohit, and I.M. Shapiro, Extracellular phosphate ions cause apoptosis of terminally differentiated epiphyseal chondrocytes. J Cell Physiol, 1999. 179(3): p. 276-86.
75. Keklikoglu, N. and S. Akinci, ATF-2 immunoreactivity in post- mitotic and terminally differentiated human odontoblasts. Med Mol Morphol,
47
2015. 48(3): p. 164-8.
76. Ogbureke, K.U.E. and L.W. Fisher, Expression of SIBLINGs and their partner MMPs in salivary glands. Journal of Dental Research, 2004.
83(9): p. 664-670.
77. Ikeda, E., et al., Fully functional bioengineered tooth replacement as an organ replacement therapy. Proc Natl Acad Sci U S A, 2009. 106(32): p.
13475-80.
78. Lee, H.K., et al., Odontoblastic inductive potential of epithelial cells derived from human deciduous dental pulp. J Mol Histol, 2016. 47(3): p. 345- 51.
79. Kawamura, R., et al., EDTA soluble chemical components and the conditioned medium from mobilized dental pulp stem cells contain an inductive microenvironment, promoting cell proliferation, migration, and odontoblastic differentiation. Stem Cell Res Ther, 2016. 7(1): p. 77.
80. Yang, Z.H., et al., Apical tooth germ cell-conditioned medium enhances the differentiation of periodontal ligament stem cells into cementum/periodontal ligament-like tissues. J Periodontal Res, 2009. 44(2):
p. 199-210.
81. Al-Sharabi, N., et al., Bone marrow stromal cell paracrine factors direct osteo/odontogenic differentiation of dental pulp cells. Tissue Eng Part A, 2014. 20(21-22): p. 3063-72.
82. Oh, H.J., et al., CPNE7, a preameloblast-derived factor, regulates odontoblastic differentiation of mesenchymal stem cells. Biomaterials, 2015.
48
37: p. 208-17.
83. Osugi, M., et al., Conditioned media from mesenchymal stem cells enhanced bone regeneration in rat calvarial bone defects. Tissue Eng Part A, 2012. 18(13-14): p. 1479-89.
84. Wu, J., et al., Conditioned medium from periapical follicle cells induces the odontogenic differentiation of stem cells from the apical papilla in vitro. J Endod, 2013. 39(8): p. 1015-22.
85. Kang, K.J., et al., Indirect co-culture of stem cells from human exfoliated deciduous teeth and oral cells in a microfluidic platform. Tissue Engineering and Regenerative Medicine, 2016. 13(4): p. 428-436.
86. Bianco, P., et al., Bone sialoprotein (BSP) secretion and osteoblast differentiation: relationship to bromodeoxyuridine incorporation, alkaline phosphatase, and matrix deposition. J Histochem Cytochem, 1993. 41(2): p.
183-91.
87. Zhu, X.L., et al., Synthesis and processing of bone sialoproteins during de novo bone formation in vitro. Biochem Cell Biol, 2001. 79(6): p.
737-46.
88. Gordon, J.A., et al., Bone sialoprotein expression enhances osteoblast differentiation and matrix mineralization in vitro. Bone, 2007.
41(3): p. 462-73.
89. Hao, J., et al., Differential expression patterns of the dentin matrix proteins during mineralized tissue formation. Bone, 2004. 34(6): p. 921-32.
90. Cho, Y.D., et al., Molecular Regulation of Matrix Extracellular
49
Phosphoglycoprotein Expression by Bone Morphogenetic Protein-2. Journal of Biological Chemistry, 2009. 284(37): p. 25230-25240.
91. Staines, K.A., et al., MEPE is a novel regulator of growth plate cartilage mineralization. Bone, 2012. 51(3): p. 418-430.
92. Miyazaki, T., et al., Inhibition of the terminal differentiation of odontoblasts and their transdifferentiation into osteoblasts in Runx2 transgenic mice. Arch Histol Cytol, 2008. 71(2): p. 131-46.
93. Doi, Y., et al., Immobilized Dpp and Other Proteins Modify Ocp Formation. Calcified Tissue International, 1993. 52(2): p. 139-145.
94. Sodek, K.L., et al., Relationships between bone protein and mineral in developing porcine long bone and calvaria. Bone, 2000. 26(2): p. 189-198.
95. Prasad, M., W.T. Butler, and C. Qin, Dentin sialophosphoprotein in biomineralization. CONect Tissue Res, 2010. 51(5): p. 404-17.
96. Joshi, R., et al., Dentin sialophosphoprotein (DSPP) gene-silencing inhibits key tumorigenic activities in human oral cancer cell line, OSC2.
PLoS One, 2010. 5(11): p. e13974.
50
국문초록
사람 치아 상피세포에 의한 치수 줄기세포의 상아모세포 분화 유도
채 근 영
서울대학교 대학원 치의과학과 분자유전학 전공 (지도교수 이 진)
치아 발생과 재생 과정은 상피와 외배엽성 간엽 줄기세포 간의 다양한 상호작용으로 이루어지며 치아를 구성하는 법랑질 및 상아질은 이러한 상피-간엽 상호작용을 통해 분화된 법랑모세포와 상아모세포로부터 형성된다. 현재까지의 치아재생 연구는 분리 및 유지가 용이한 치수 간엽줄기세포를 중심으로 이루어졌는데, 반면에, 치아 상피세포는 치아 발생이 끝나고 나면 대부분 소실되기 때문에 확보 및 활용이 어려워 관련 연구가 많이 이루어지지 못하였다. 최근 선행 연구를 통해 사람 치주조직으로부터 치아 상피세포인 Hertwig’s epithelial root sheath/epithelial rests of Malassez (HERS/ERM)가 확보되었으며 HERS/ERM 세포주 또한 확립되었으나 아직까지 치아조직재생에 대한 HERS/ERM의 역할에 대해서는 충분한 검증이 이루어지지 않았다. 본 연구에서는 사람 유치 치수줄기세포(stem cells from exfoliated deciduous teeth, SHED)에 HERS/ERM 세포주의 조건배지를 처리한 후 SHED의 상아모세포 분화 능력을 관찰함으로서 치아재생 과정의 상피-