G ENERAL I NTRODUCTION
A TOMICALLY D ISPERSED C ATALYSTS
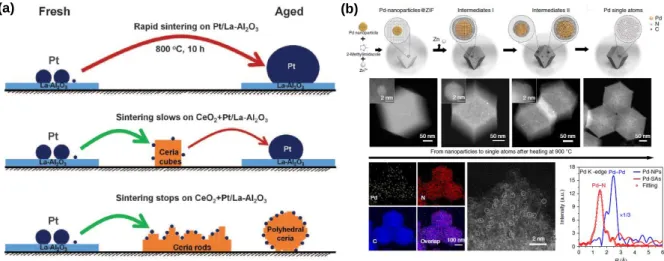
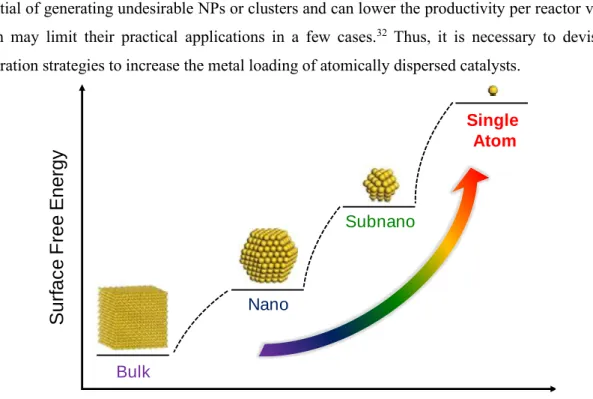
- Introduction to Atomically Dispersed Catalysts
- Synthetic Strategy for Atomically Dispersed Catalysts
- Characterization of Atomically Dispersed Catalysts
- Catalytic Properties of Atomically Dispersed Catalysts
Zeng and colleagues exploited electrochemical deposition as a universal route to prepare atomically dispersed catalysts61. Chemical etching of the metallic NPs or bulk metals on the support can also be used to fabricate atomically dispersed catalysts.
O XYGEN R EDUCTION R EACTION
- Introduction to Oxygen Reduction Reaction
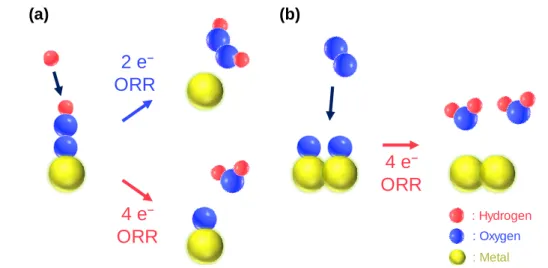
- Electrocatalysts for 2 e − Pathway Oxygen Reduction Reaction
- Electrocatalysts for 4 e − Pathway Oxygen Reduction Reaction
- Atomically Dispersed Catalysts for Oxygen Reduction Reaction
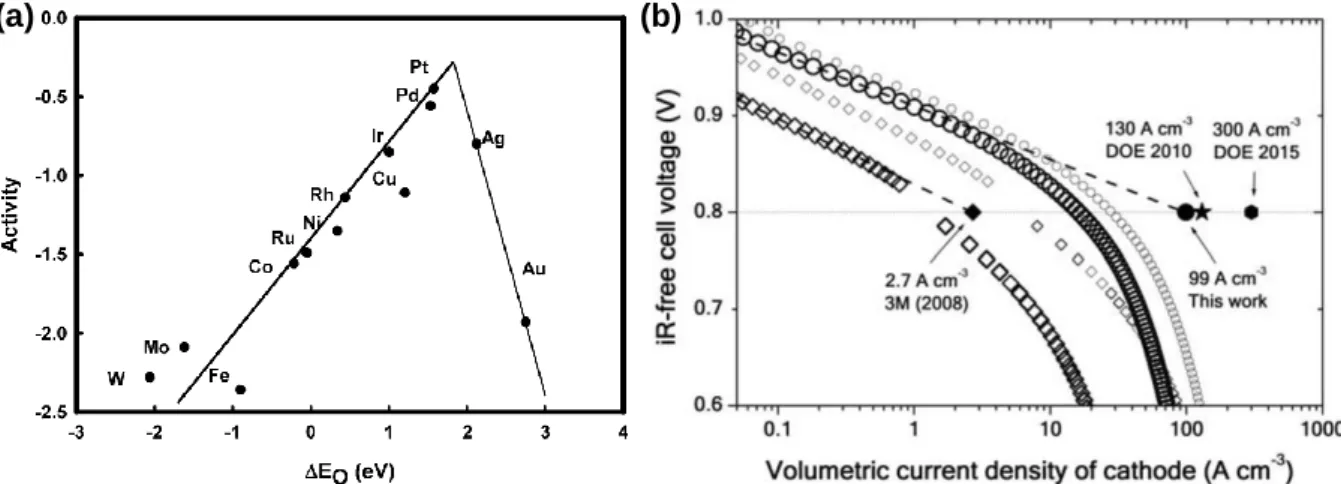
A volumetric current density of the best non-precious metal-based catalysts for the ORR at the time. This thesis presents our efforts to reveal the origin of the unique catalytic behavior of atomically dispersed catalysts in the ORR.
O UTLINE OF T HIS D ISSERTATION
Thus, depending on the type of metal and ligand used, the oxygen binding energy of atomically dispersed catalysts can be modulated. Therefore, relevant experimental studies are needed to resolve the discrepancies between previous results and understand the different catalytic trends of atomically dispersed catalysts.
R EFERENCES
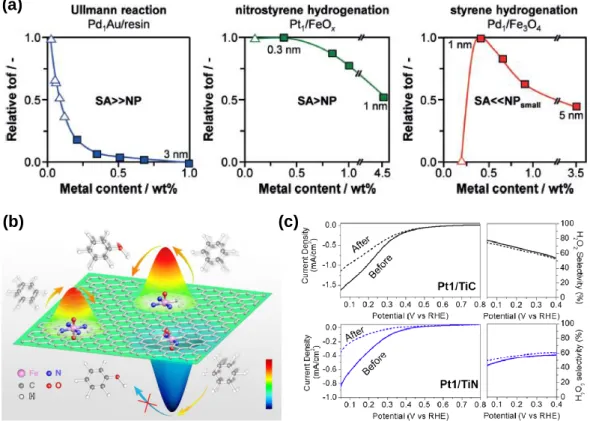
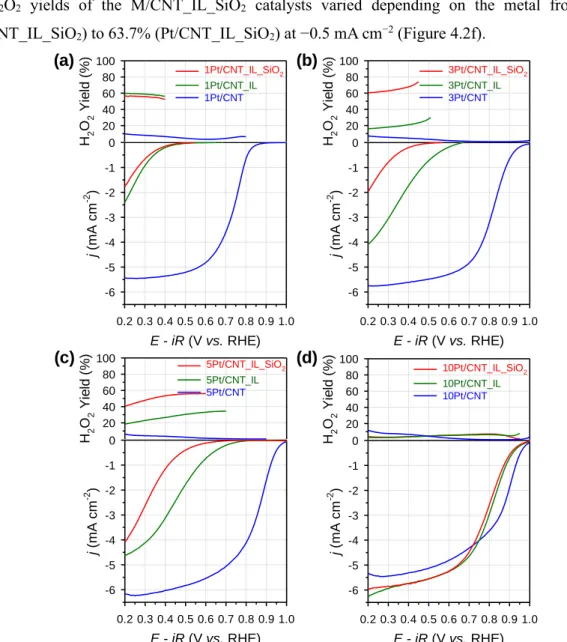
In the case of Pt/CNT_IL catalysts, atomically distributed Pt sites were created at 1 wt%. We anticipate that this work will provide insights into the preparation of atomically distributed catalysts. These results convinced the clear difference of ORR catalytic trends between NP-based catalysts and atomically distributed catalysts.
Relationship between the DFT-calculated (b) OOH and (c) O2 binding energies and the experimentally determined H2O2 yields of the atomically dispersed catalysts.
E XPERIMENTAL M ETHODS
- Synthesis of Fe 3 O 4 /CNT
- Synthesis of model catalysts
- Characterization Methods
- Electrochemical Characterization
- Analysis of ORR Selectivity
Transmission electron microscope (TEM) images were obtained by a JEOL 2100 instrument under an accelerating voltage of 200 kV. Prior to ORR performance tests, the catalysts were cleaned by cycling the potential between 0.05 and 1.2 V (vs. the reversibly hydrogen electrode, RHE) for 50 cycles at a scan rate of 100 mV s−1 (500 mV s−1 for Pt / C) in an electrolyte saturated with N2. Linear sweep voltammetry (LSV) polarization curves for the ORR were obtained by sweeping the potential from 1.2 V to 0.2 V (from -0.01 V to 1.1 V for Pt/C), in an electrolyte of saturated with O2 at the electrode rotation speed of 1600 rpm.
To evaluate the ORR selectivity of the catalysts, the yield of HO2- during ORR was obtained by the following equations using RRDE measurements.
R ESULTS AND D ISCUSSION
- Synthesis of model catalysts
- Physicochemical Characterizations
- Investigating Catalytic Role of Active Species for ORR
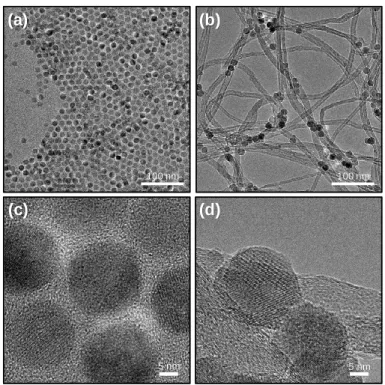
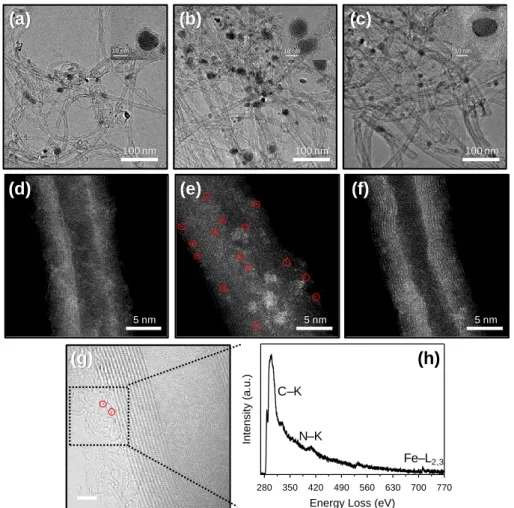
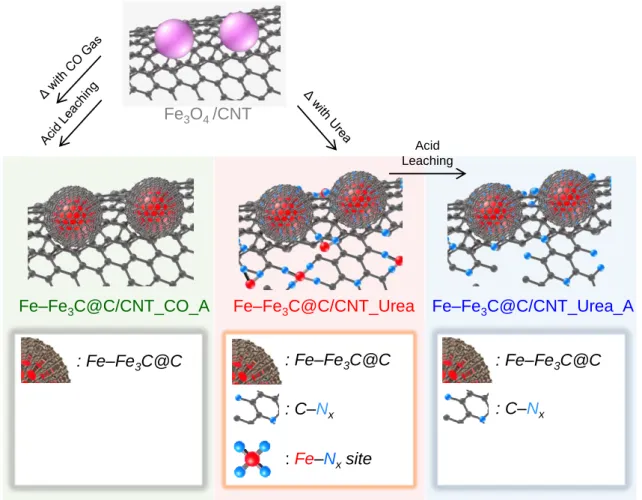
The TEM image of Fe–Fe3C@C/CNT_CO_A (Figures 2.4a) also shows that the Fe–Fe3C@C faces were supported on the CNTs. The XRD pattern of Fe–Fe3C@C/CNT_Urea (Figure 2.3) shows that the Fe3O4 phase was completely transformed into Fe and Fe3C phases. The HAADF-STEM image of Fe–Fe3C@C/CNT_Urea (Figure 2.4e) shows numerous sub-nanometer bright spots.
Summary illustration of the role of Fe-Nx sites and Fe-Fe3C@C sites for ORR.
C ONCLUSION
In addition, to further investigate the role of the Fe–Fe3C@C sites, the ORR activity of acid-treated CNT with hollow graphitic carbon layers was measured and compared with Fe–. These results may indicate that the Fe-Fe3C particles in the graphite layers promote easy 2 e− × 2 e− oxygen reduction. As a result, Fe–Fe3C@C/CNT_CO_A showed higher reduction current than bare CNT regardless of H2O2 concentrations (Figures 2.8c), indicating that peroxide reduction occurred more favorably at Fe–Fe3C@C sites than at the hollow carbon layers of the CNT.
In an early report, the Bao group suggested that electron transfer from the inner metallic Fe particles to the surface carbon layer can activate the carbon surfaces by reducing a local work function, thereby increasing the catalytic ORR activity.18 In our model catalysts, such a phenomenon is where the unique core-shell structure promotes 2 e− × 2 e− ORR was experimentally demonstrated.
R EFERENCES
Due to their extraordinary catalytic properties, atomically dispersed catalysts have received tremendous attention recently as a research front in the field of catalysis. The most straightforward and widespread method to synthesize atomically dispersed noble metal catalysts is an impregnation-thermal activation method. The SiO2 protective layer could mitigate agglomeration of the isolated metal sites during the thermal activation step and thus maximize the density of atomically scattered sites.
Through this “trapping and immobilization” strategy, a variety of atomically dispersed precious metals (Os, Ru, Rh, Ir, and Pt) were prepared.
E XPERIMENTAL M ETHODS
- Synthesis of M/CNT, M/CNT_IL and M/CNT_IL_SiO 2 Catalysts
- Characterization Methods
- XAS Experiments
- Mass Spectrometry
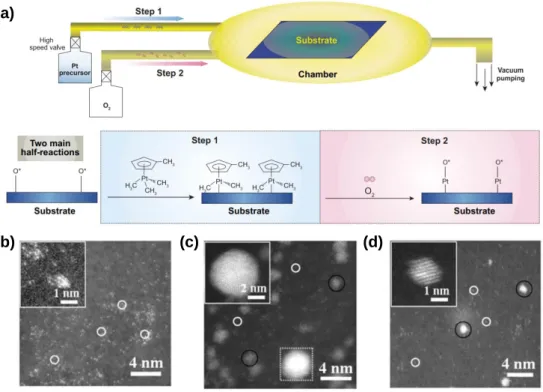
Using a combination of X-ray absorption spectroscopy (XAS) and mass spectrometry (MS) during the thermal activation processes, we found that the strong interaction between the metal precursors and the support provides a stable, isolated anchoring of the metal precursors during the impregnation step and inhibits the degradation of the precursors during the activation step, which helps in conversion of the precursor to an isolated single-atom site. Elemental analyzes for C, H, N, O, and S were performed using an elemental analyzer (Thermo Scientific, Flash 2000). Background removal and absorption coefficient normalization and adjustment for k3 Fourier transform extended X-ray absorption fine structure (EXAFS) spectra were performed using Athena and Artemis software.54 The amplitude reduction factor (S02) of Pt was fixed at 0.85, as was found by fitting the EXAFS spectrum of the Pt foil.
The activation of the metal precursor-impregnated samples was analyzed in a quartz flow reactor coupled to a mass spectrometer (Pfeiffer Vacuum GSD320).
R ESULTS AND D ISCUSSION
- Trapping-and-Immobilizing Strategy to Atomically Dispersed Catalysts
- Role of IL and SiO 2 Coatings
- General Applicability of the Developed Strategy
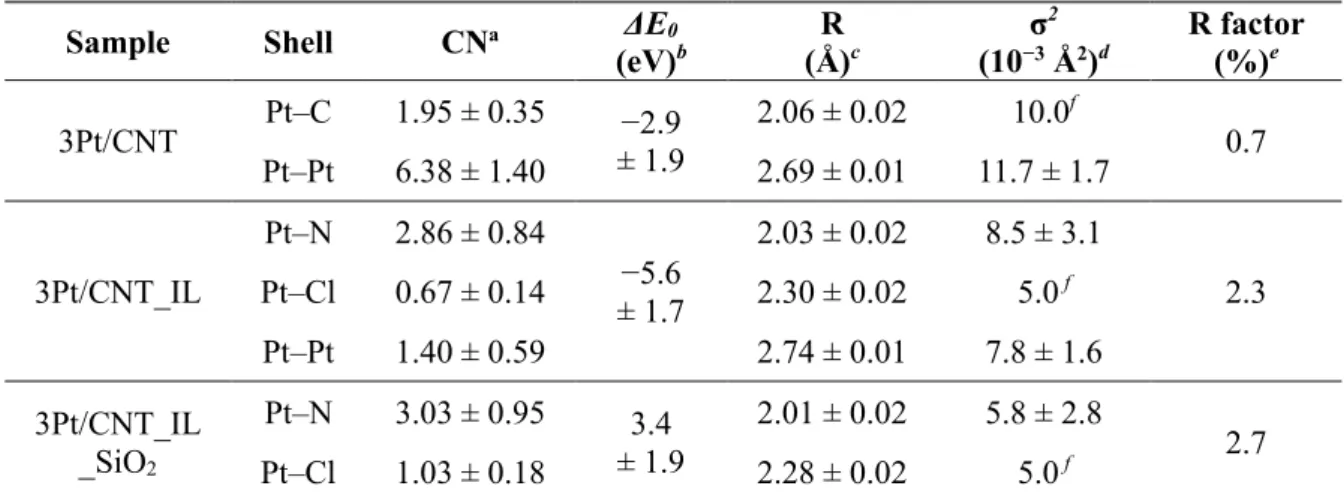
Fitting parameters for Pt L3-edge k3-weighted EXAFS spectra of 3Pt/CNT, 3Pt/CNT_IL and 3Pt/CNT_IL_SiO2. For the Pt/CNT_IL_SiO2 catalysts, the formation of Pt NPs was further hindered. Fitting parameters for Pt L3-edge k3-weighted EXAFS spectra of H2PtCl6, 3Pt/CNT_Imp and 3Pt/CNT_IL_Imp.
The HAADF-STEM images of 3Pt/CNT_IL_SiO2 before HF etching showed that the SiO2 protective layer covered the atomically dispersed Pt supported on CNT_IL (Figure 3.11a–c).
C ONCLUSION
In contrast to the rapid activation observed in the generation of Pt NPs, this process was slowed down on Pt precursors supported on CNT_IL. The strong anchoring of the Pt precursors on the CNT_IL seems to inhibit its degradation, allowing stable isolation of the generated atomically dispersed sites, while inhibiting agglomeration into NPs. Interestingly, the SiO2 layer only delayed the formation of Cl2, indicating that the SiO2 coating can further mitigate the collision of mobile Pt precursors.
R EFERENCES
DFT calculations of (a) O, (b) OOH and (c) O2 binding energies of model systems for atomically dispersed catalysts and nanoparticle catalysts. Oxygen binding energies of models for atomically dispersed catalysts and nanoparticle catalysts with multiple oxygen species. The atomically dispersed catalysts and NP-based catalysts are designated as M1 and MNP, respectively (M = Os, Ru, Rh, Ir and Pt).
Relationship between the DFT-calculated (a) O, (b) OOH and (c) O2 binding energies and the experimentally determined H2O2 yields of atomically dispersed catalysts and nanoparticle catalysts. This apparent relationship between the electronic structure of atomically dispersed catalysts and the catalytic activity may be related to oxygen-binding energy. We propose some future works to more deeply understand the catalytic properties of atomically dispersed catalysts.
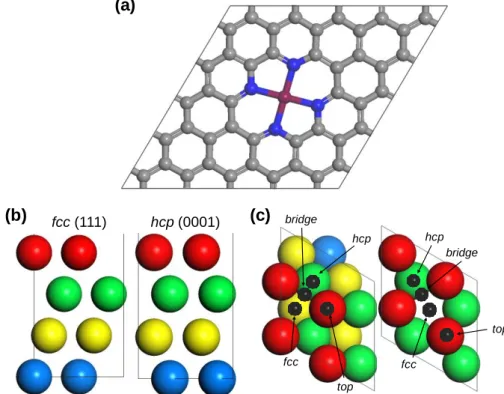
U NRAVELING C ATALYTIC T RENDS OF A TOMICALLY D ISPERSED C ATALYSTS FOR
E XPERIMENTAL M ETHODS
- Electrochemical Characterization
- O 2 Temperature Programmed Desorption
- Density Functional Theory Calculations
R ESULTS AND D ISCUSSION
- ORR Activity and Selectivity Trends
- Origin of the ORR Selectivity Trends
- O 2 Temperature-Programmed Desorption
- Origin of the ORR Activity Trends
To unravel the origin of the catalytic properties manifested by the atomically dispersed M/CNT_IL_SiO2 catalysts, we focused on the selectivity trends for these catalysts. Relationships between oxygen binding energies and ORR selectivities and activities of model catalysts. a) Linear relationship between the thermodynamic propensity for O–O bond preservation (GO − GOOH) and the H2O2 yields of model systems for atomically distributed catalysts. We also tried to obtain a general selectivity trend that includes both the NP-based and atom-dispersed catalysts (Figure 4.10).
Therefore, there may be a trade-off between activity and selectivity in the 2 e -ORR pathway, which should be considered when designing atomically dispersed catalysts for the ORR.
C ONCLUSION
Volcanic relationship between the O bond energies and the input potential of the (a) 4 and (b) 2 e− ORR pathway for M1 and MNP catalysts. The onset potentials for the 4 e- ORR pathway were obtained from the disc current modified by the normalized ring current, while the onset potentials for the 2 e- ORR pathway were obtained from the ring current normalized by the ring efficiency. In general, the oxygen binding energy can serve as a descriptor for both the selectivity and activity of the 2 e-ORR pathway.
Our results suggest that the unique ORR selectivity and activity trends of the atomically dispersed noble metal catalysts originate from their anomalously weak oxygen binding energies associated with their geometric configurations.
R EFERENCES
By exploiting the ligand-substituted Rh catalysts, we investigated the ligand effects of atom-dispersed catalysts on their ORR catalytic properties. These results clearly demonstrated that the reduced oxidation state of atom-dispersed catalysts by CO ligand is favorable for the ORR performance. It is therefore essential to understand the optimal coordination structure of atomically dispersed catalysts at a molecular level.
In fact, the metal sites of atomically dispersed catalysts can be considered as solutes in solid solution.
L IGAND E XCHANGE R EACTION OF A TOMICALLY D ISPERSED C ATALYSTS TO
E XPERIMENTAL M ETHODS
- Synthesis of M/CNT_IL_G
- Characterization Methods
- XAS Experiments
- Electrochemical Characterizations
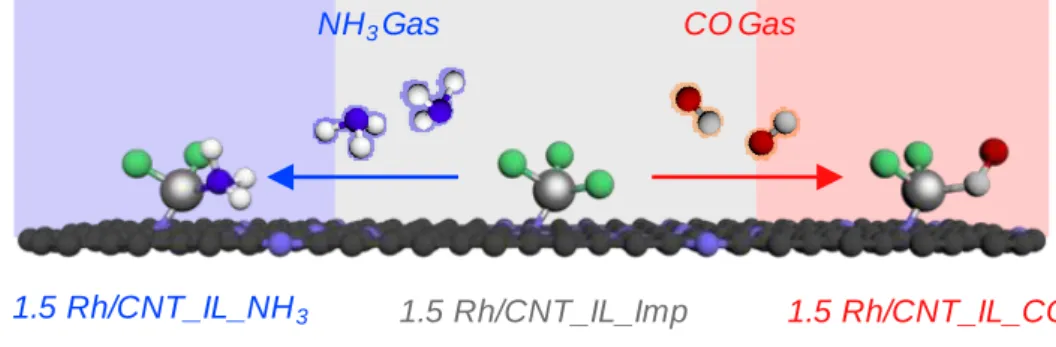
The resulting CNT was treated in the same manner as described above with 720 g of 6 M HCl (diluted from 36% HCl, Samchun Chemicals) and then dried overnight at 60 °C. When performing successive ligand exchange reactions, all the gases (G) used are written in the order of use. The ORR activities and selectivities of the catalysts were measured by linear sweep voltammetry (LSV) at a scan rate of 5 mV s−1 with an electrode rotation.
The resistivity of the solution was determined from the intersection of the x-axis in the high-frequency region of the Nyquist plot.
R ESULTS AND D ISCUSSION
- Synthesis and Characterization of 1.5Rh/CNT_IL_G Catalysts
- Reversible Ligand Exchange
- Electrochemical Properties of Ligand-Modulated Catalysts
Due to the changes in the coordination structure of Rh, distinguishable XANES spectra of 1.5Rh/CNT_IL_NH3 and 1.5Rh/CNT_IL_CO can be observed. It is analogous to 1.5Rh/CNT_IL_CO, demonstrated by the change of XANES and EXAFS spectra of subsequent NH3. The XPS peaks of 1.5Rh/CNT_IL_NH3 are shifted to higher binding energy by 0.4 eV compared to 1.5Rh/CNT_IL_CO.
The ORR polarization curves and H2O2 yields exhibit that large difference in ORR activity and selectivity between 1.5Rh/CNT_IL_NH3 and 1.5Rh/CNT_IL_CO (Figure 5.8a).
C ONCLUSION
This thesis shows the development of preparation methods for the atomically distributed catalysts and investigates their catalytic tendencies and origins for the oxygen reduction reaction (ORR). Compared with non-noble metal-based atomically dispersed catalyst, usually M-N/C catalyst, it has been more difficult to produce noble metal-based atomically dispersed catalysts. For these reasons, the development of the general preparation strategy for noble metal-based atom-dispersed catalysts has been challenging.
A general trapping-and-immobilizing strategy for atopically dispersed noble metal catalysts to unravel their catalytic propensities for the oxygen reduction reaction”.