Lithium ion batteries (LIBs) have captured the market for energy storage devices due to their high energy densities. Volumetric EDs should be emphasized in space-constrained situations, even if gravimetric EDs are more considered in literature. The high volumetric EDs were achieved by spray deposition which we highlight as a novel electrode fabrication process in this work.
The droplet solvent rapidly evaporated during the time of flight, as well as when they encountered hot stream collectors. The electrode densities achieved by sputter deposition were several times higher than those of conventional sputtering methods. Therefore, several times higher EDs were obtained from our lithium metal | redox-active polymer cells when compared to cells prepared by scavenging methods.
The volumetric ED gains overwhelmed the loss of porosity, leading to mass transfer problems, such that the volumetric EDs of spray-deposited electrodes were higher even at high rates than those of bladed electrodes. We expect that the spray deposition method is suitable for the production of flexible batteries of all polymers, while conventional inorganic materials can be deposited with the method.
Introduction
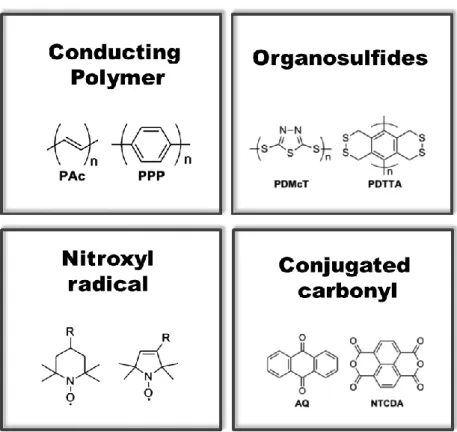
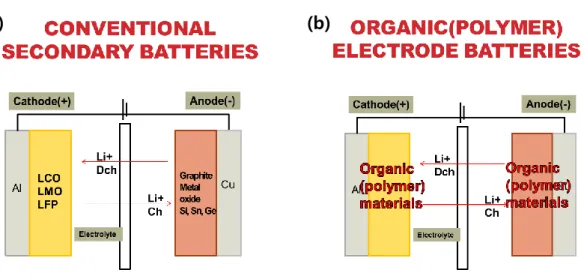
An LIB cell consists of two electrodes (a cathode and an anode) with electrolyte and separator (Figure 1.2a). As organic counterparts to the conventional inorganic materials for cathodes, redox-active organic materials can only be used in the same configuration as active materials if they have a different reduction potential than that of the other electrode (Figure 1.2b). And there are four main types of structures and redox reaction mechanisms (Figure 1.4).18 The first is to conduct polymers that have electrical conductivity in conjugated polymer structures.
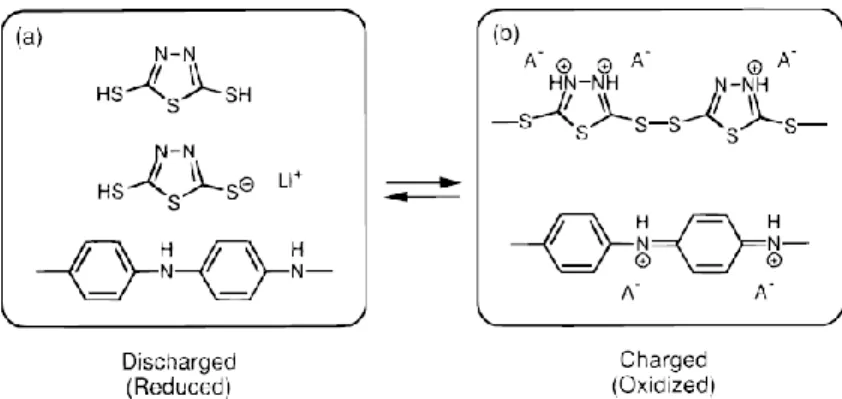
This is a cleavage/bond formation mechanism that could gain two electrons in a redox reaction. 14, 19. As a result, DTA or side chain type organosulfur compounds showed higher retention capacity than GTA or main chain type (Figure 1.10). One of them, Shirakawa, synthesized polyacetylene, and the other, Heeger and MacDiarmid, added iodine halogen to polyacetylene (Figure 1.11). Then polyacetylene became conductive as an insulator.35-42 Figure 1.13 shows the conductivity of materials, which as an insulator is divided into three parts. , semiconductor and metal.
The structure of the conducting polymer is conjugated, which is alternating a single bond and a double bond (Figure 1.12). Radical electron is initially reactive, so among them, less reactive appendages should be used to control the degree of it, such as nitroxyl and phenoxyl group (Figure 1.15). Usually, nitroxyl radical compounds are more commercialized today in papers than Figure 1.15 explains the redox mechanism in it.
Among the group of pendants, TEMPO (Poly 2,2,6,6-tetramethylpeperidinyloxy-4-yl methacrylate) was widely used as a nitroxoxide group (Figure 1.17). In 2002, using TEMPO with a PMA backbone, Nakahara produced organic batteries.50 The theoretical capacity of TEMPO was 111 mAh g-1 and the redox reaction plateau was 3.5 V (vs. li/Li-1). Each carbonyl group is respectively subjected to the redox reaction.58-59 The improvement of structural stability for the addition of the aromatic molecule, the quinone structure has been known in electrode materials of carbonyl compounds.60-68.
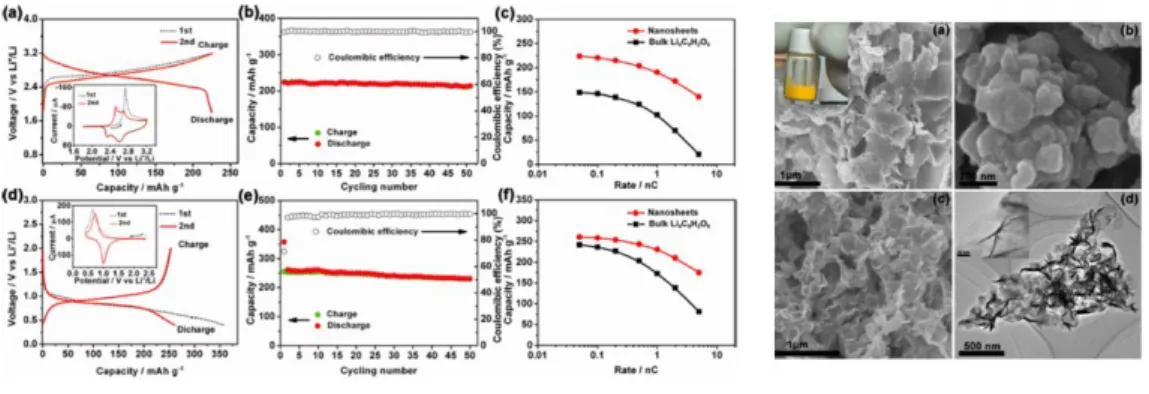
Among the quinone-type structures, anthraquinone-based polymer was applied to cathode materials for rechargeable lithium batteries (Figure 1.19). In molecular engineering, Yanliang Liang put other heteroatoms such as oxygen, sulfur, nitrogen atoms into anthraquinone materials (Figure 1.20).69 This heteroatom molecular engineering changed the LUMO and HOMO energy, causing the difference in redox voltage, morphology and ion diffusion. Carbonyl molecules were made into nano-sized structures such as nanoplates and nanoparticles.70 The organic molecules, DHTPA, had redox reaction of both negatively charged state and positively charged state at 0.8V and 2.6V respectively with 4 electron movement.
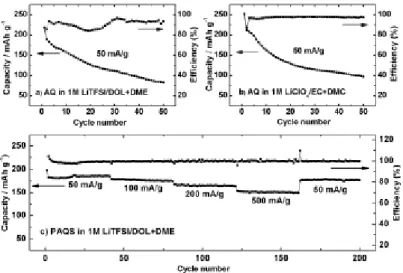
Liquid electrolytes in carbonyl cathode electrode batteries were replaced with polymer gel electrolytes (Figure 1.22).71 Calix materials that were phenol-based oligomers were used. When oxidized, 8-electrons have completely redox reaction with low barrier of chionone molecules.
Experiment
Result & Discussion
Gravimetric capacity of (a) Blade electrode, (b) spray electrode using PVdF and (c) knife electrode, (d) spray electrode using PEDOT. The rate capability of the sheet electrode was also better than that of the spray electrode using PEDOT:PSS. The spray electrode composites were triple dense so they had small pores in the composites.
For the PVDF bonding electrodes, the volume capacity of the blade electrode was about 20 mAh cm-3 at 1C while the sputter electrode was 64 mAh cm-3 at 1C. The volumetric capacitance performance of the spray electrode was three times that of the blade electrode at 1C. PEDOT:PSS, the blade electrode volume capacity was close to 20mAh cm-3 at 1C while the sputter electrode was 38mAh cm-3 at 1C.
The thickness of the spray electrode was three times thinner than the one blade, and these differences in thickness value affected the overall volumetric capacity in cell performance. Using PVdF binder, the capacity retention of the spray electrode was 90%, while the blade electrode was 84%. Also for PEDOT:PSS, the performance of the spray electrode was better than the capacity retention of the blade electrode.
When looking at the interface between Al current collector and electrode composites, the amount of red line in blade electrode was more than spray electrode because spray electrode had greater density than blade one. The red line indicated interfacial contact areas between current collector and electrode assembly, which means that the interfacial contact area of spray electrode was higher than blade one. These good adhesive properties bonded electrode components well, then caused good capacity retention with long cycle life in spray electrode.
Previous data already showed that less developed ionic path in spray electrode composites caused fading ability at a high rate. In this graph, the characteristic of ionic path in spray electrode is properly affected from 20C rate point compared to the value of decreasing rate in blade one. In volumetric capacity, the value of syringe electrode was four times greater than blade electrodes calculating each density to gravimetric capacity.
Due to the low porosity, the sputtering electrode had a higher density, which was an additional factor for the resistance of the sheet. In the sputtering electrode composites, the PEDOT:PSS content per surface area tripled due to the dense packing, which made the resistance plate performance worse compared to the cutting electrode.
Conclusion
Hu, Y.; Sun, X., Flexible Rechargeable Lithium Ion Batteries: Advances and Challenges in Materials and Process Technology. Oyama, N.; Tatsuma, T.; Sato, T.; Sotomura, T., Dimercaptan-polyaniline composite electrodes for high energy density lithium batteries. Liu, L.; Tian, F.; Wang, X.; Yang, Z.; Zhou, M.; Wang, X., Porous polythiophene as a cathode material for lithium batteries with high capacity and good cycling stability.
Suga, T.; Sugita, S.; Ohshiro, H.; Oyaizu, K.; Nishide, H., P- and n-Type Bipolar Redox-Polymer Active Radical: Towards Fully Organic Polymer-Based Rechargeable Devices with Variable Configuration. Suga, T.; Pu, Y.-J.; Kasatori, S.; Nishide, H., Cathode- and anode-active poly(nitroxylstyrene) for rechargeable batteries: p- and n-type redox switching via substitution effects. Oyaizu, K.; Suga, T.; Yoshimura, K.; Nishide, H., Synthesis and characterization of radical-bearing polyethers as an active electrode material for organic secondary batteries.
Oyaizu, K.; Ando, Y.; Konishi, H.; Nishide, H., Nernstian adsorbate-like bulk layer of organic radical polymers for high-density charge storage. Suga, T.; Ohshiro, H.; Sugita, S.; Oyaizu, K.; Nishide, H., Emerging N-type redox-active radical polymer for a fully organic polymer-based rechargeable battery. Oyaizu, K.; Kawamoto, T.; Suga, T.; Nishide, H., Synthesis and charge transport properties of redox-active nitroxide polyethers with large site density.
Oyama, N.; Sarukawa, T.; Mochizuki, Y.; Shimomura, T.; Yamaguchi, S., Significant effects of poly(3,4-ethylenedioxythiophene) additive on redox reactions of poly(2,5-dihydroxy-1,4-benzoquinone-3,6-methylene) cathode for rechargeable Li batteries. Song, Z.; Zhan, H.; Zhou, Y., Anthraquinone-based polymer as high-performance cathode material for rechargeable lithium batteries. Choi, W.; Harada, D.; Oyaizu, K.; Nishide, H., Aqueous electrochemistry of poly(vinylanthraquinone) for anode active materials in high density rechargeable polymer/air batteries.
L.; Jiang, Y.-B.; Bae, I.-T.; Xiao, Q.; Zhan, H.; Liu, J.; Wang, D., Polymer-graphene nanocomposites as ultrafast charge and discharge cathodes for rechargeable lithium batteries. Liang, Y.; Zhang, P.; Yang, S.; Tao, Z.; Chen, J., Fused heteroaromatic organic compounds for high power electrodes of rechargeable lithium batteries. Y.; Song, H.-K., An entangled network of redox-active and conducting polymers as a cathode for ultrafast rechargeable batteries.
Acknowledgements