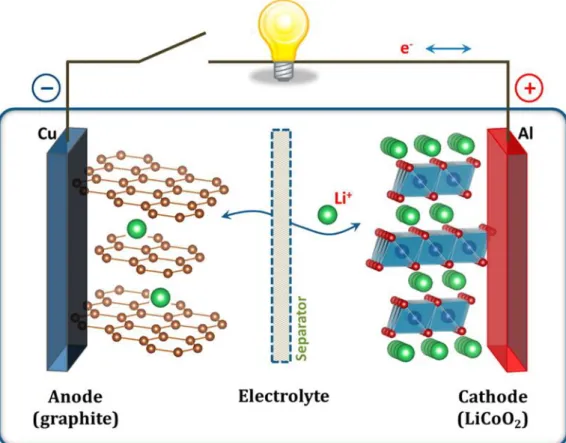
However, the future expansion of LIB technology is limited due to the high cost and scarcity of both core elements lithium and cobalt. In view of the rigidity of the solid electrolyte, the new cell model is intended to have sufficient flexibility to be easily transported and practically used. In parallel with the development of the cell platform, energy efficiency was also improved by improving the materials and assembly methods for each part of the seawater battery, which will be an indicator of future battery development.
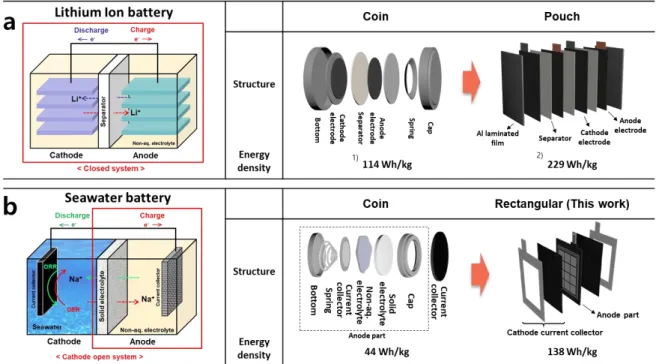
In addition, the increased efficiency of the parallel stacked modules indicates the capability of this cell in practical use. Schematic figure showing the working mechanism of (a) Li-ion battery and (b) seawater battery and corresponding unit cells. Initial rectangular model (a) photograph showing the electrical resistance of the unit cell and cathode current collector and (b) EIS versus the coin-shaped unit cell.
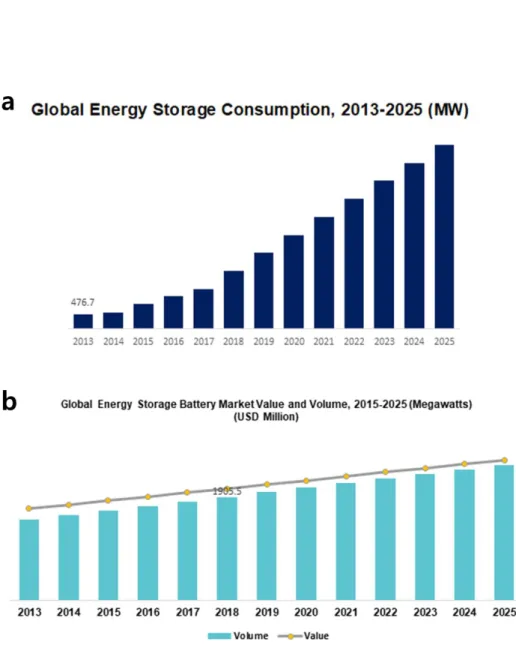
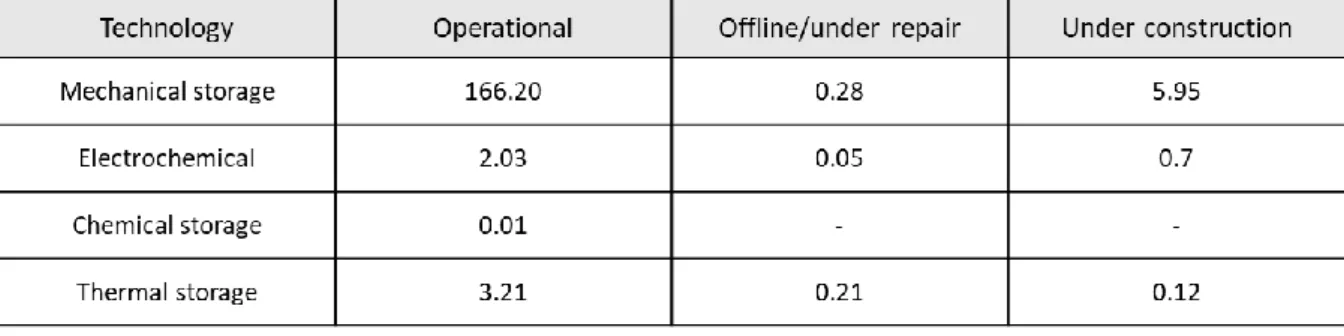
Schematic of a rectangular unit cell and detailed compartment structure .. a, c, e) Charge voltage profiles measured at a constant current of 0.28 mA/cm2 followed by a constant voltage of 3.9 V (for e) and (b, d, f ) Discharge voltage profiles measured at a constant current rate of −0.28 mA/cm2 and corresponding energy (discharge energy and capacitance density are calculated and indicated). Nominal installed capacity for different categories of energy storage worldwide (Md Mustafizur Rahman, 2020).
Introduction
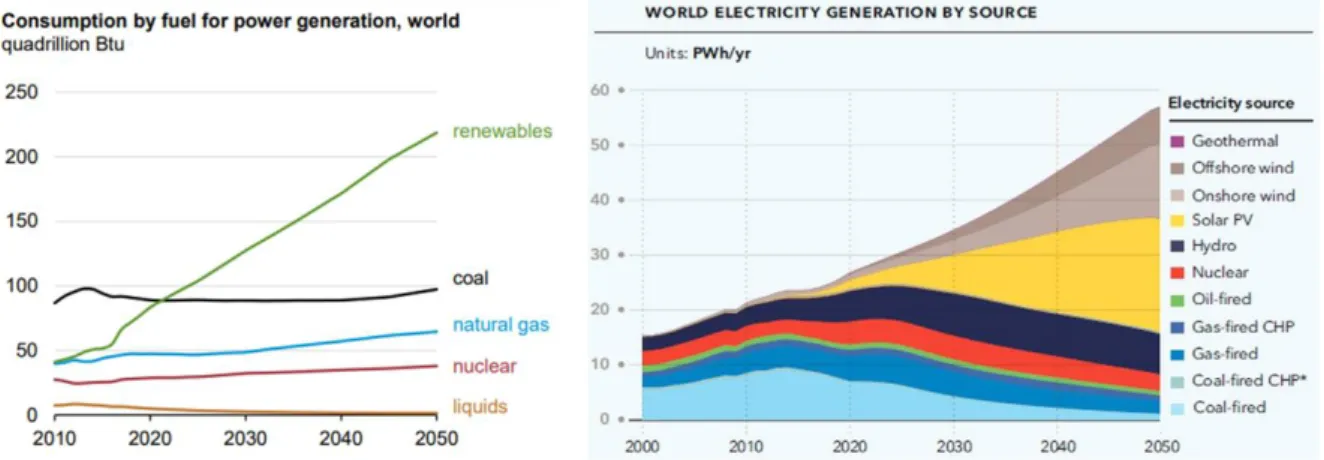
Need for electrochemical energy storage devices
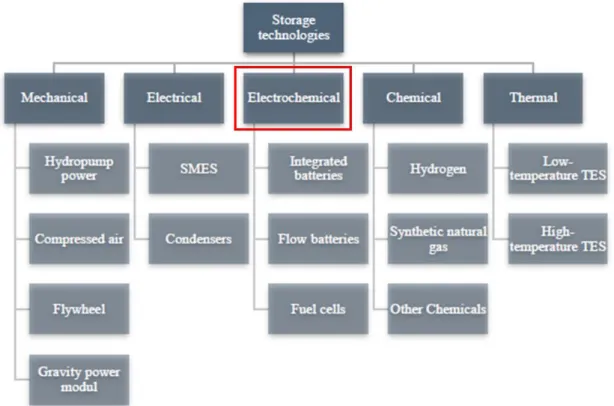
Type of energy storage systems
Second, it is necessary to maximize the area of the solid electrolyte region for output and voltage efficiency. Therefore, for the overall cell performance, both the characteristics of the cathode and the anode must be considered. In cathode part, the electrochemical properties of the cathode part have a great influence on the output and voltage efficiency of the seawater battery.
Since the anode part of the seawater battery is separated from the cathode by a solid electrolyte, sodium metal can be directly used as an active material. In the case of a coin cell, since the anode part is independent of the cathode part, a custom-made fixture was required for operation. The original rectangular seawater battery was made by simply attaching a cathode current collector to the surface of the finished anode part.
Increasing the electrical resistance of the cathode current collector is one of the major problems other than ceramics in increasing the area of the unit cell. Third external process 2, Assembling the cathode current collector on the surface of the anode cell and fixing it by spot welding the titanium mesh.
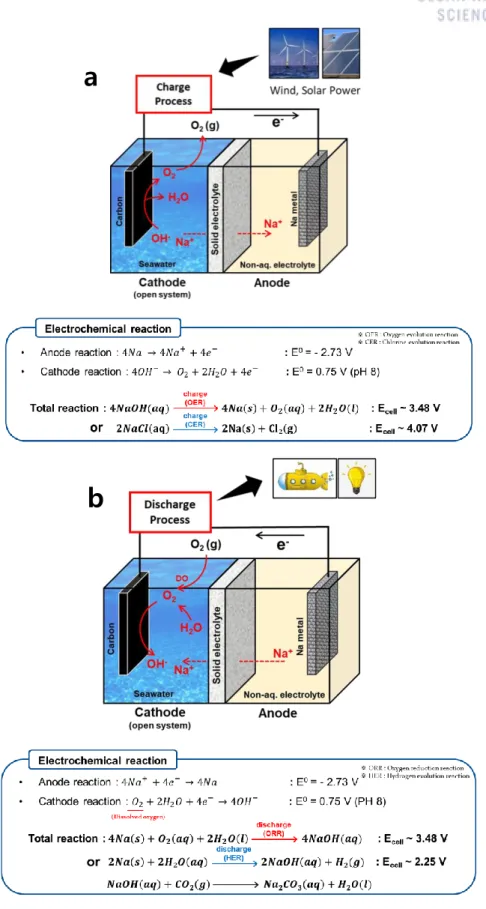
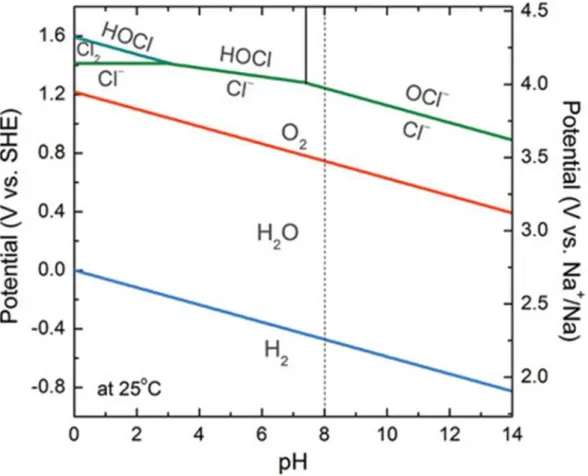
Principle of Seawater batteries
Study of Seawater battery unit cell design
Need for the unit cell development
In the case of lithium-ion batteries, starting with the development of closed-system button cells, prismatic and pouch-shaped unit cells with improved energy density for ESS or EV have recently been developed (Figure 10 a). Therefore, not only the operating mechanism, but also the customized design according to the target application is important. Since the seawater battery has an open anode structure, it requires a different structure from other electrochemical cell platforms as follows.
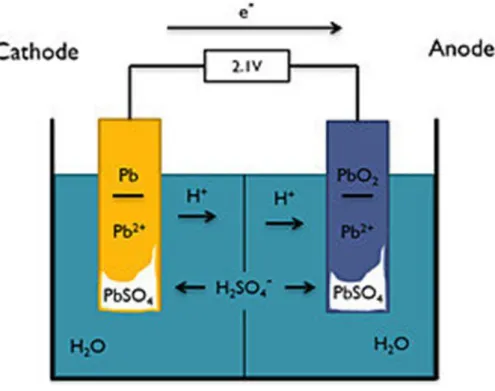
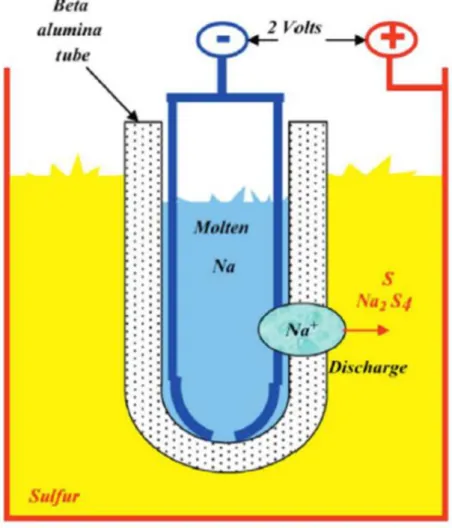
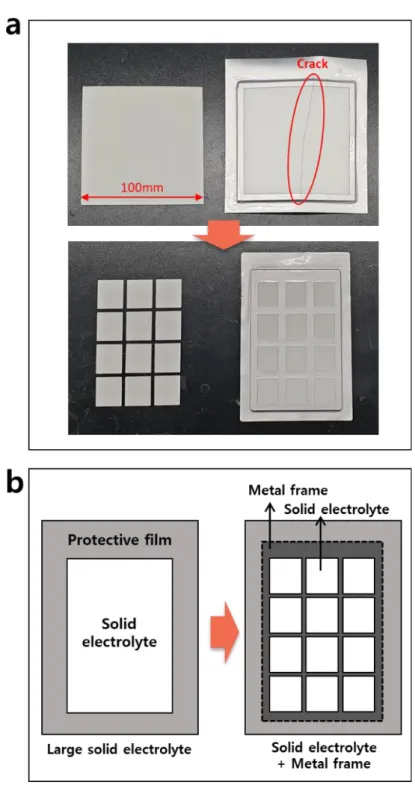
First, the seawater battery is directly immersed in seawater, so this should be taken into account in the design. Lithium and lead-acid batteries have low ohmic resistance due to the optimized cell platform (Figure 11 a).86,87 However, the seawater battery has a low power density due to the ohmic resistance generated from the cathode current collector and the solid electrolyte ( Figure 11 b). From a design point of view, expanding the area of a solid electrolyte is not good in terms of durability and requires a lot of technology.
Additionally, a design that complements this is required because the contact resistance between the cathode current collectors of a seawater battery has the largest proportion of the total cell resistance.
Previous research of Seawater battery unit cell development
Unit cell structure and considerations
Purpose of research
Experimental
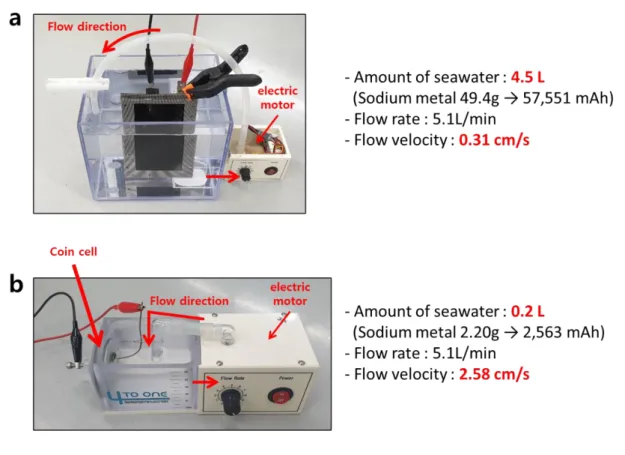
Cell tester simulating the marine environment for (a) rectangular (including 4.5 L seawater, flow rate 5.1 L/min, flow velocity 0.31 cm/s using electric motor) and (b) coin cell. including 0.2 L of seawater, flow velocity 2.58 cm/s using electric motor).
Result & discussion
If a sodium-impregnated carbon current collector is cut to the desired size and used as the current collector and anode active material, a unit cell with a certain capacity can be formed. In the newly designed rectangular cell, a carbon current collector impregnated with metallic sodium was used, just like a coin. When a metal-impregnated carbon current collector is used, the rectangular cell has an initial capacity of about 3 Ah, and more metallic sodium can be charged by charging.
However, the anode part swelled over time despite using the same electrolyte and current collector as the coin cell (Figure 22a). However, when the liquid electrolyte and the carbon current collector were used together, it started to swell right after the cell was made. To suppress the swelling of the anode, a metal current collector in the form of a grid was used instead of a carbon-based current collector that reacts with the liquid electrolyte to generate gas (Figure 23a).
Unlike the previous case, only a metal current collector and a liquid electrolyte were included without introducing sodium metal during cell assembly. Seawater battery using a sodium metal impregnated carbon current collector (a) swollen anode part after operation, (b) Performance decreased due to swelling of the cell and (c) the cell. The reason for using the carbon cloth material as a current collector is as follows.
The seawater battery cathode current collector is not consumed or increased during the electrochemical reaction. The carbon-based material has a specific surface area of 1500 m2 or more per gram, so it is suitable as a current collector. As a disadvantage, the resistance increases significantly as the area increases due to the high electrical resistance of the carbon dust (Figure 24a).
To reduce the electrical resistance of these carbon-based materials, titanium was added as a current collector and applied to the surface. However, if the contact between the carbon jet manifold and the seawater is disturbed, it can lead to performance degradation. Compared with the original model, the maximum resistance of the carbon current collector was reduced from 75 ohms to 24 ohms by 1/3 (Figure 26 a), and the maximum output power increased three times accordingly (Figure 26 b).
In addition, thanks to the titanium metal, the fabric-based current collector's loosening problem was solved, and the physical durability was greatly improved. In addition, holes were drilled in the electrode tab and cathode current collector to enable collection.
Summary
Seawater battery module, application & poilot system
- Design of Seawater battery module
- Electrochemical characteristics of Seawater battery module
- Application operation using Seawater battery module
- Design of automation pilot system
- Summary
Since the seawater battery module is a parallel module, the current also increases proportionally as the number of cells increases. According to Ohm's law, the reduction in power is proportional to the square of the current, so it is clear that the power loss of a seawater battery module increases with increasing power consumption. Based on the main concept of rechargeable SWBs operating with an open cathode in seawater, the application of these systems in marine, coastal and offshore energy sources or energy storage is expected to be suitable.
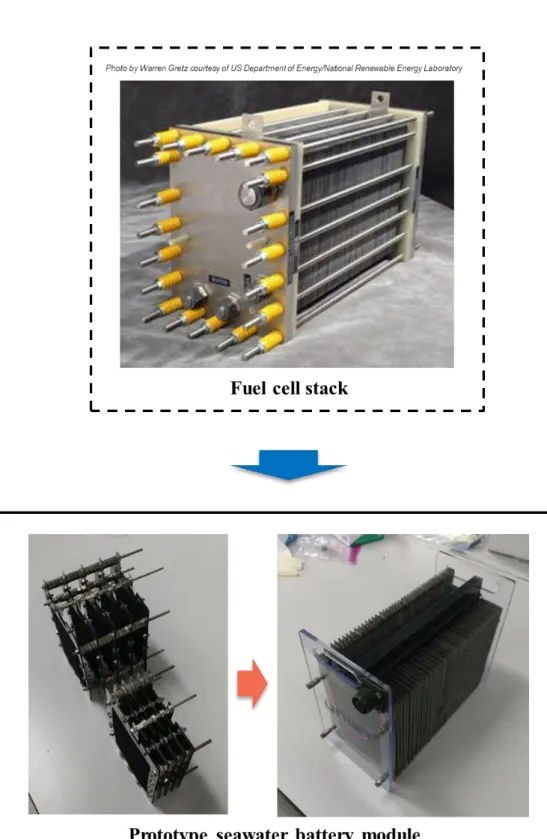
The seawater battery unit cell has strict sealing requirements because it has to bond three materials (metal, polymer and ceramic). In this study, we fabricated a prototype seawater battery module using the developed large-area rectangular cells. In addition, the designed seawater battery module managed to operate the light buoy for 9 days in a real marine environment.
In line with the increasing demand for energy storage devices, various electrochemical battery systems are being actively developed. In this study, we proposed a seawater battery as an alternative to lithium-ion batteries and supplemented the insufficient cell system. In addition, through the development of a prototype seawater battery module and pilot system, it announced the starting point of a seawater battery system that will be improved in the future.
In Chapter 3, a prototype seawater battery module and a pilot system capable of producing it are discussed. The seawater battery module can be easily mounted through the hole in the cathode part. Starting with the realization of the seawater battery concept as a coin cell system in the previous study, this study took it a step further and proved its value as an energy storage device.
The cheapest combinations of solar power, wind power and energy storage system for large scale power grid. Frequency control of isolated power system with wind farm using flywheel energy storage system. Voltage and frequency control in smart distribution systems in presence of DER using flywheel energy storage system.
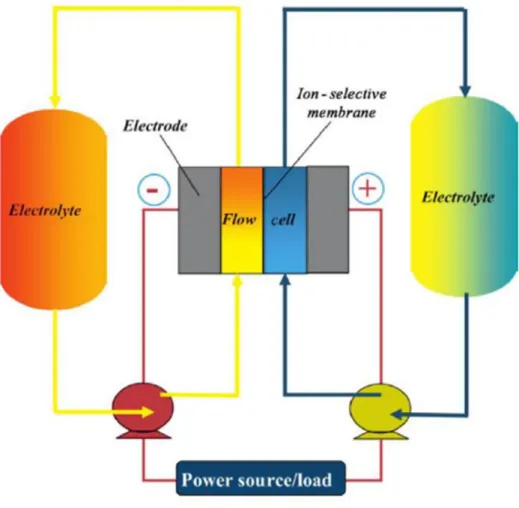
Pumped hydroelectric energy storage and spatial diversity of wind resources as methods to improve the utilization of renewable energy sources (Doctoral dissertation, University of Colorado at Boulder). A Chemistry and Materials Perspective on Lithium Redox Flow Batteries Towards High Density Electrical Energy Storage.